Abstract
Accumulation of aggregation-prone misfolded proteins disrupts normal cellular function and promotes ageing and disease. Bacteria, fungi and plants counteract this by solubilizing and refolding aggregated proteins via a powerful cytosolic ATP-dependent bichaperone system, comprising the AAA+ disaggregase Hsp100 and the Hsp70-Hsp40 system. Metazoa, however, lack Hsp100 disaggregases. We show that instead the Hsp110 member of the Hsp70 superfamily remodels the human Hsp70-Hsp40 system to efficiently disaggregate and refold aggregates of heat and chemically denatured proteins in vitro and in cell extracts. This Hsp110 effect relies on nucleotide exchange, not on ATPase activity, implying ATP-driven chaperoning is not required. Knock-down of nematode Caenorhabditis elegans Hsp110, but not an unrelated nucleotide exchange factor, compromises dissolution of heat-induced protein aggregates and severely shortens lifespan after heat shock. We conclude that in metazoa, Hsp70-Hsp40 powered by Hsp110 nucleotide exchange represents the crucial disaggregation machinery that reestablishes protein homeostasis to counteract protein unfolding stress.
Keywords: chaperones, disaggregation, Hsp70, Hsp110, protein folding
Introduction
A broad range of intrinsic and extrinsic stress conditions perturbs protein homeostasis resulting in the accumulation of misfolded and aggregated proteins (Tyedmers et al, 2010; Vabulas et al, 2010). Aggregation and loss of function of the misfolded proteins is accompanied by destabilization and sequestration of bystander proteins, endangering cell physiology and promoting disease and ageing (Gidalevitz et al, 2006, 2009). To counteract deleterious consequences of aggregation, cells harbour elaborate protein quality control machineries that eliminate aggregates by chaperone-mediated disaggregation and refolding or degradation. Disaggregation and refolding predominates in bacteria, yeast and plants and is essential for thermotolerance and resistance to severe protein folding stress in these organisms (Parsell et al, 1994; Queitsch et al, 2000; Weibezahn et al, 2004). For this, an Hsp100 chaperone (termed Hsp104 in S. cerevisiae and ClpB in E. coli) that belongs to the family of AAA+-ATPases cooperates with the Hsp70-Hsp40 system (termed Ssa1-Ydj1 in S. cerevisiae and DnaK-DnaJ in E. coli) (Parsell et al, 1994; Glover and Lindquist 1998; Goloubinoff et al, 1999; Weibezahn et al, 2004; Tessarz et al, 2008). The Hsp100 acts as a disaggregase that extracts individual polypeptides from the aggregate by threading them through the central pore of the ATPase hexamer (Barends et al, 2010; Haslberger et al, 2010). However, Hsp104/ClpB in the absence of the partner Hsp70 system does not resolve protein aggregates (Glover and Lindquist, 1998; Goloubinoff et al, 1999; Weibezahn et al, 2004; Zietkiewicz et al, 2004; Winkler et al, 2012). This defines the bichaperone system as the functional unit required for disaggregation and refolding activity. For thermally aggregated proteins, the disaggregation activity of the bichaperone systems of E. coli and S. cerevisiae is facilitated by the presence of small Hsps (sHsps) during the aggregation process which intercalate into the aggregates and probably affect the aggregate structure (Ehrnsperger et al, 1997; Lee et al, 1997; Mogk et al, 2003a; Ratajczak et al, 2009).
Although animal cells lack Hsp100 chaperones in the cytosol and nucleus, cell extracts show a disaggregation activity of unknown identity (Cohen et al, 2006; Bieschke et al, 2009; Murray et al, 2010). To identify this disaggregation activity, we considered an involvement of the Hsp70-Hsp40 system for several reasons. First, members of the Hsp70 and Hsp40 families are central components of the cellular protein quality control system that prevent aggregation and promote refolding of misfolded proteins (Young, 2010; Hartl et al, 2011). Second, Hsp70s colocalize with protein aggregates in animal cells, hinting at an activity associated with aggregates (Garcia-Mata et al, 2002; Kim et al, 2002). Third, the yeast and bacterial Hsp70-Hsp40 systems directly interact with protein aggregates to initiate the disaggregation process by the Hsp100-dependent bichaperone system (Weibezahn et al, 2004; Zietkiewicz et al, 2006). Fourth, Hsp70-Hsp40 without Hsp100 has a limited capacity to solubilize aggregates in vitro; this activity seems tightly restricted to aggregates that are small or have low β-sheet content (Diamant et al, 2000; Lewandowska et al, 2007). In other studies, no such activity was detected (Glover and Lindquist, 1998; Goloubinoff et al, 1999; Weibezahn et al, 2004; Cashikar et al, 2005; Haslberger et al, 2008; Miot et al, 2011). In light of these data, we surmised that cooperation partner(s) of Hsp70 confer disaggregation activity to Hsp70.
Hsp70s perform their various tasks by interacting with assorted co-chaperones that stimulate ATP-dependent folding by Hsp70 and direct the chaperone to substrates or specific cellular sites (Kabani, 2009; Kampinga and Craig, 2010; Young, 2010). Hsp40 co-chaperones stimulate ATP hydrolysis by Hsp70, resulting in an Hsp70 conformation with high affinity for substrate. Nucleotide exchange factors (NEFs) stimulate ADP release that enables rebinding of ATP to Hsp70, restoring the low affinity conformation and triggering substrate release. Hsp110 proteins represent a distinct eukaryotic branch of the Hsp70 chaperone superfamily that lack substrate refolding activity but instead act as NEFs for Hsp70s (Dragovic et al, 2006; Raviol et al, 2006b). Although passive substrate binding has been attributed to Hsp110s (Oh et al, 1999; Polier et al, 2010), it remains unclear whether this is relevant to Hsp70-mediated folding events. However, mammalian Hsp110s associate with disease-related amyloidogenic proteins and protein aggregates such as mutant SOD1 or tau (Yamashita et al, 2007; Wang et al, 2009; Eroglu et al, 2010; Olzscha et al, 2011). This prompted us to test whether the Hsp110s enable Hsp70-mediated metazoan disaggregation.
We show a novel activity for Hsp110 functionally associated with the metazoan Hsp70 system in protein disaggregation. This primarily involves Hsp110 nucleotide exchange activity rather than canonical ATPase-dependent chaperone function. Further, we show that this Hsp110 activity is required for protein aggregate solubilization in the nematode Caenorhabditis elegans and that downregulation of Hsp110 greatly reduces worm lifespan after heat shock.
Results
Test system for in vitro analysis of protein disaggregation
We addressed the disaggregation potential of the human Hsp70 system by employing well-established in vitro chaperone assays using firefly luciferase and malate dehydrogenase (MDH) as model thermolabile substrates, and two different aggregation methods, to span a wide spectrum of potential aggregate size, structure and chaperone stringency requirements for solubilization. In one assay, luciferase was chemically denatured by urea and diluted from denaturant into chaperone-free buffer, resulting in the formation of stable aggregates (Schröder et al, 1993; Glover and Lindquist, 1998). In the other assay, luciferase and MDH were aggregated by heating in the presence or absence of the heat-activatable small heat-shock protein Hsp26 that co-aggregates with proteins, rendering aggregates more amenable to chaperone action (Goloubinoff et al, 1999; Cashikar et al, 2005; Haslbeck et al, 2005). In addition, we tested the effect of substrate concentration during thermal aggregation, thus exploring the importance of a parameter which affects aggregation rates and perhaps aggregate structure. We applied two criteria to establish that chemical or thermal treatments caused aggregation of luciferase and MDH rather than misfolding. First, the efficient reactivation of treated proteins by the Hsp70-Hsp40 systems of S. cerevisiae (Ssa1-Ydj1) requires an Hsp100 disaggregase (Hsp104) (Figures 1C, 2C and E). Second, the treated proteins become insoluble as judged by behaviour in centrifugation assays (Figures 1A and 2A).
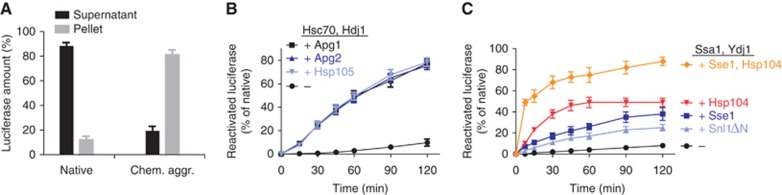
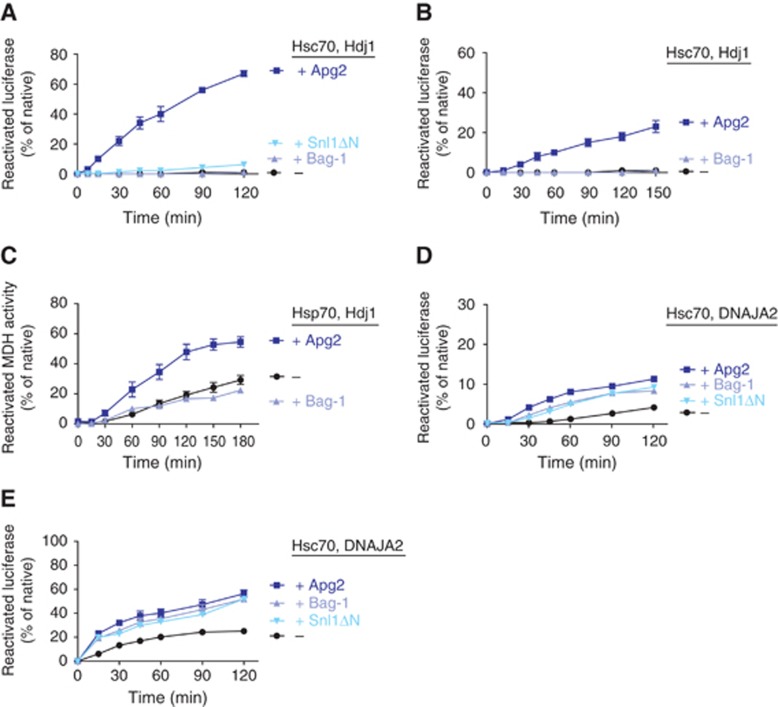
Figure 1.
Chemically aggregated luciferase is efficiently reactivated by the human Hsp70 system including Hsp110 as NEF. (A) Luciferase levels in supernatants and pellets of native or chemically aggregated luciferase samples. Data shown represent the average of at least three experiments±s.e. (B) Reactivation of 20 nM luciferase from urea-induced aggregates was monitored upon addition of the human Hsp70-Hsp40 (2 μM Hsc70, 1 μM Hdj1) or Hsp70-Hsp40-Hsp110 system (Hsc70, Hdj1 and 0.2–0.4 μM Hsp105, Apg2 or Apg1). (C) Reactivation of chemically aggregated luciferase by the yeast Hsp70-Hsp40-NEF system (2 μM Ssa1, 1 μM Ydj1±0.1 μM Sse1 or 2 μM Snl1ΔN) or bichaperone system including the disaggregase Hsp104 (Ssa1, Ydj1, 1 μM Hsp104±Sse1). Reactivation data shown represent the average of at least three experiments±s.e.
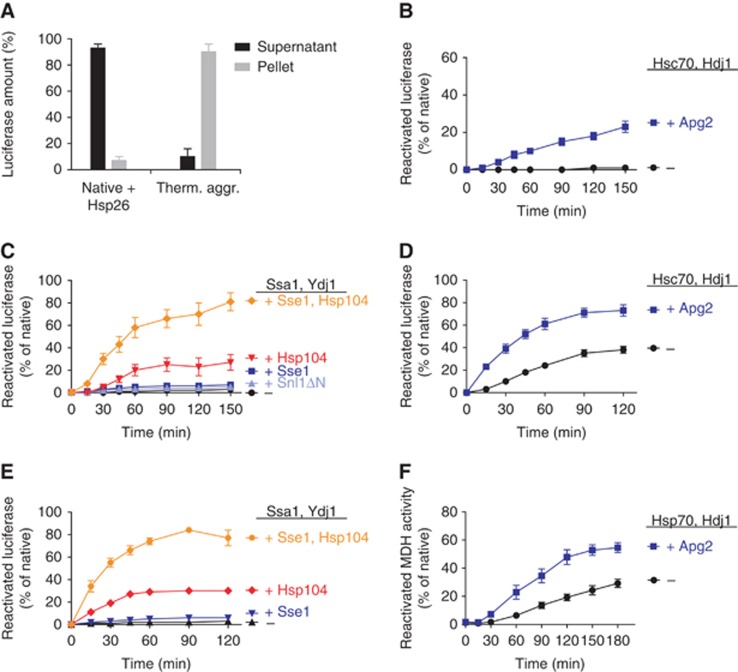
Figure 2.
The disaggregation activity of the human Hsp70-Hsp40-Hsp110 extends to heat-aggregated luciferase as well as MDH. (A) Luciferase levels in supernatants and pellets of native or thermally aggregated luciferase samples. (B) Luciferase (2 μM) was heat aggregated in the presence of Hsp26, diluted to 20 nM luciferase and subsequently, reactivation was monitored upon addition of the human Hsp70 system±Hsp110 (2 μM Hsc70, 1 μM Hdj1 and 0.4 μM Apg2). (C) Reactivation of heat-aggregated luciferase treated as in (B) was monitored upon addition of the yeast Hsp70-Hsp40-NEF system (2 μM Ssa1, 1 μM Ydj1±0.1 μM Sse1 or 2 μM Snl1ΔN) or bichaperone system including the disaggregase Hsp104 (Ssa1, Ydj1, 1 μM Hsp104±Sse1). (D) Luciferase (20 nM) was heat aggregated in the presence of Hsp26 and reactivation was monitored upon addition of the human Hsp70 system (2 μM Hsc70, 1 μM Hdj1±0.2 μM Apg2). (E) Luciferase (20 nM) was heat aggregated in the presence of Hsp26 and reactivation was monitored upon addition of the yeast Hsp70 or bichaperone system (2 μM Ssa1, 1 μM Ydj1±0.2 μM Sse1, ±1 μM Hsp104). (F) MDH (0.5 μM) was thermally aggregated and, after four-fold dilution, reactivation was monitored upon addition of the human Hsp70-Hsp40-NEF system (4 μM Hsp70, 2 μM Hdj1±0.4 μM Apg2). Reactivation data shown represent the average of at least three experiments±s.e.
We tested the disaggregation activity of the major human cytosolic Hsp70 system, consisting of the constitutively expressed Hsc70 (HSPA8) and the Hsp40 co-chaperone Hdj1 (DNAJB1), supplemented with one of the three human Hsp110s Apg2 (HSPH2), Hsp105 (HSPH1) or Apg1 (HSPH3). The nucleotide exchange activity of Apg1 and Apg2 has not been previously tested, so we used stopped flow kinetic measurements of nucleotide exchange for these proteins on Hsc70, and found that both were similarly potent NEFs for Hsc70 (Supplementary Figure 1A). Both were equally effective in promoting nucleotide exchange from Hsp70 (HSPA1), suggesting no Hsp70 specificity. For comparison, we employed the human NEF Bag-1 or the soluble, catalytically active fragment of the yeast Bag-1-homologue Snl1 (Snl1ΔN). These are structurally unrelated to Hsp110s but stimulate nucleotide release by a similar mechanism (Sondermann et al, 2001; Andreasson et al, 2008a). Both Bag-1 homologues functioned as NEFs for Hsc70 (Supplementary Figure 1B), but were less potent than Apg1 and Apg2. Because the Hsp110s displayed higher activities than Bag-1 or Snl1ΔN, and also because excessive NEF concentrations inhibit Hsp70-mediated substrate reactivation (Dragovic et al, 2006; Tzankov et al, 2008), we titrated all NEFs employed in this study to achieve optimal stimulation of protein disaggregation (Supplementary Figure 1C).
To account for potential Hsp40-specific effects, we compared type I versus type II Hsp40 proteins. Both type I and type II Hsp40s stimulate the Hsp70 ATPase and have similar potential for promiscuous substrate interactions (Kampinga and Craig, 2010). However, the specificities and kinetics for substrate interactions differ (Lu and Cyr, 1998; Fan et al, 2004; Cintron and Toft, 2006). Therefore, we performed our experiments using the predominant representatives, human Hdj1 (type II) and yeast Ydj1 (type I) and alternatively also human DNAJA2 (type I) and yeast Sis1 (type II) (Kampinga and Craig, 2010).
Hsp110 enables human Hsp70 system to efficiently reactivate chemically aggregated luciferase
When luciferase was aggregated by urea treatment and dilution into refolding buffer, followed by the addition of chaperones, human Hsc70 with Hdj1 did not support luciferase reactivation. In contrast, addition of the Hsp110 Apg2 stimulated reactivation of luciferase to ∼70% of native control over a 2-h period (Figure 1B). Apg2 alone was not effective. Under these experimental conditions, protein disaggregation and refolding activity for the human Hsc70-Hdj1-Apg2 system is comparable, albeit slower, to the activity of the yeast bichaperone system consisting of Ssa1, Ydj1 and the disaggregase Hsp104 (Figure 1C). Consistent with previous reports (Glover and Lindquist, 1998; Tessarz et al, 2008) and similar to the human Hsc70-Hdj1 system, the yeast Ssa1-Ydj1 system alone did not support reactivation of aggregates of chemically denatured luciferase (Figure 1C), providing important testimony to the aggregated state of luciferase.
We then tested whether the critical role of Apg2 for protein disaggregation is a general feature of mammalian Hsp110 proteins. For the Hsc70-Hdj1 system, inclusion of any one of the three human Hsp110 homologues Hsp105, Apg2 and Apg1 was equally effective in luciferase solubilization and reactivation (Figure 1B). Together, these data demonstrate that with an Hsp110-type NEF, the human Hsp70 system efficiently reactivates luciferase from urea-induced aggregates, confirming a disaggregation activity for the Hsp110 chaperone system.
For the yeast Ssa1-Ydj1 system, inclusion of the Hsp110-type NEF Sse1 also increased the final yield and rate of luciferase reactivation from aggregates obtained from chemical denaturation, although to a lesser extent as compared to the human Hsp110-Hsc70-Hdj1 system (Figure 1B and C). The unrelated NEF Snl1ΔN also stimulated reactivation by Ssa1 and Ydj1. These findings demonstrate that upon addition of Sse1 (or to a lesser extent Snl1ΔN), the yeast Ssa1-Ydj1 system on its own is more effective in disaggregating luciferase than previously suspected since we observe substantial luciferase reactivation from chemically induced aggregates in absence of Hsp104. However, Hsp104 remains essential for fast and efficient solubilization of aggregates; addition to Ssa1-Ydj1-Sse1 profoundly increased both the yield and the kinetics of luciferase reactivation (Figure 1C). Our data furthermore show that the well-established bichaperone system of yeast, consisting of Ssa1, Ydj1 and Hsp104 (Glover and Lindquist, 1998; Cashikar et al, 2005; Haslbeck et al, 2005; Tessarz et al, 2008) is incomplete, since only the inclusion of an NEF for Ssa1 develops the full disaggregation potential of the yeast Hsp70 and Hsp100 chaperone machineries.
Hsp110 enables the human Hsp70 system to reactivate thermally aggregated luciferase and MDH
The physiological significance of reversible protein aggregation is particularly striking during conditions that lead to thermal denaturation of proteins (Parsell et al, 1994). Here, the supportive role of sHsps is well documented (Ehrnsperger et al, 1997; Lee et al, 1997; Mogk et al, 2003a; Ratajczak et al, 2009). Therefore, we assayed the reactivation of luciferase heated to 45°C in the presence of a five-fold molar excess of the heat-activatable Hsp26 of S. cerevisiae. Luciferase was heat aggregated at a high concentration (2 μM) and diluted 100-fold for the chaperone assays. Under these aggregation conditions, Hsc70, Hdj1 and Apg2 reactivated a lower amount of heat-aggregated luciferase (25%, Figure 2B) compared to chemically aggregated luciferase (70%, Figure 1B), but reached a level comparable to that achieved by the yeast bichaperone system composed of Ssa1, Ydj1 and Hsp104 (Figure 2C). In stark contrast to the human chaperones, the yeast Ssa1-Ydj1 system with or without Sse1 was incapable of promoting any reactivation in absence of the disaggregase Hsp104. These results suggest that the employed thermal aggregation procedure has more stringent chaperone requirements than is the case with chemical aggregation of luciferase, since the yeast Ssa1-Ydj1-NEF system was able to reactivate 30–40% of chemically aggregated luciferase (Figure 1C). Only upon further addition of the disaggregase Hsp104 did the yeast Ssa1-Ydj1 system show disaggregation activity towards heat-induced aggregates, albeit with low rates and yields (Figure 2C). However, upon additional inclusion of the NEF Sse1, luciferase disaggregation rate and yields increased strongly as compared to Ssa1, Ydj1 and Hsp104 without Sse1. This demonstrates that previous in vitro studies (Glover and Lindquist, 1998; Cashikar et al, 2005; Haslbeck et al, 2005; Tessarz et al, 2008) may have underestimated the disaggregation capacity of the yeast chaperones.
To test aggregation conditions that might resemble protein aggregation in vivo more closely, we heat-aggregated luciferase at a 100-fold lower concentration (20 nM), again in the presence of a five-fold molar excess of Hsp26, resulting in insolubility of the luciferase. Under these conditions, human Hsc70 and Hdj1, with or without Apg2, were significantly faster and more potent in reactivating luciferase, resulting in ca. 40% reactivation without or 70% reactivation with Apg2 (Figure 2D). The efficiency of yeast chaperones Ssa1, Ydj1 and Sse1 with or without Hsp104 was largely unchanged and Hsp104 was still required for disaggregation (Figure 2E). We conclude that the human Hsp70-Hsp40-Hsp110 system consisting of Hsc70, Hdj1 and Apg2 reactivates luciferase from heat-induced aggregates obtained under two different conditions. Aggregates from either condition were completely inert to the action of the yeast complement of chaperones comprised Ssa1, Ydj1 and Sse1. In contrast to the human system, the yeast disaggregation machinery required Hsp104.
Thus, we observed a striking increase in efficiency for the human Hsp70 system when luciferase was heat aggregated at a low concentration (Figure 2B and D), although not for the yeast Hsp70 system (Figure 2C and E). To test whether the aggregates obtained at the two conditions (2 μM versus 20 nM luciferase) differed structurally, we monitored their density by glycerol gradient centrifugation. We observed that aggregates obtained at 2 μM migrated more deeply into the gradient than those obtained at 20 nM (Supplementary Figure 2A), suggesting that the more chaperone-resistant aggregates are larger or more densely packed than the more amenable ones. Aggregates obtained at 20 nM displayed a small, but reproducible shift by approximately two fractions (Supplementary Figure 2A). This experimental set-up does not provide information on the exact size of these aggregates, but the protein complexes formed under these conditions clearly qualify as aggregates on the basis of their insolubility, their dependence on the yeast bichaperone system (i.e., Hsp104) for reactivation, and the observed shift in glycerol gradient centrifugation. Interestingly, Hsp26 co-migrated with both types of aggregates in the glycerol gradient, however the observed patterns likely reflect both substrate binding and substrate-independent changes in the oligomeric status of Hsp26 (Haslbeck et al, 1999). To investigate the effects of substrate concentration during aggregation as well as the contribution of sHsps in more detail, we monitored reactivation of luciferase heat-aggregated at both concentrations, but in the absence of Hsp26. Strikingly, Hsp26 was absolutely required for luciferase reactivation from aggregates obtained at the higher 2 μM luciferase concentration. Even the well-established yeast bichaperone system was incapable of reactivating luciferase aggregated in the absence of Hsp26 (Supplementary Figure 2B), underscoring the severity of the aggregation regime. In contrast, heat-aggregation of luciferase at the lower concentration of 20 nM abolished the Hsp26 inclusion requirement for disaggregation. The human Hsc70-Hdj1-Apg2 system was only slightly less efficient for reactivation of luciferase that was heat-aggregated alone versus in the presence of Hsp26 (Supplementary Figure 1C; Figure 2D). Reactivation of luciferase aggregates by the yeast chaperones was also marginally lower in the absence of Hsp26 (Supplementary Figure 2D). Therefore, aggregation in the presence of Hsp26 at low substrate concentration represents a less stringent condition with respect to chaperone requirements, which may explain the relatively high activity of Hsc70 with Hdj1 even in the absence of Apg2.
Hence, while the Hsp70-Hsp40-Hsp110 system of yeast, Ssa1, Ydj1 and Sse1, invariably fails to disaggregate heat-treated luciferase efficiently, the corresponding human system, Hsc70, Hdj1 and Apg2, can act even on aggregates obtained under severe conditions and efficiently solubilizes aggregates formed under more permissive conditions such as chemical aggregation or thermal aggregation at low substrate concentration. Hsp26 increases solubilization under our permissive conditions and renders otherwise inert aggregates amenable to chaperones under our more drastic conditions, effectively enhancing disaggregation. In conclusion, we demonstrate that the human Hsp70 system in conjunction with Hsp110 possesses remarkable disaggregation activity towards both chemically and thermally aggregated luciferase.
These findings were further corroborated using the alternative substrate MDH that formed aggregates by heat treatment (47°C) in the absence of a small Hsp. Consistent with previous reports (Goloubinoff et al, 1999), we found that the yeast Ssa1-Ydj1 system absolutely required the disaggregase Hsp104 for MDH reactivation (Supplementary Figure 2E). In contrast, human Hsp70 with Hdj1 already displayed some disaggregation activity, which was significantly stimulated by Apg2 (Figure 2F). These results together establish that human Hsp110 NEFs enable the Hsp70 system to acquire substantial disaggregation activity. This activity of the human Hsp110-Hsc70-Hdj1 system contrasts sharply with the yeast Sse1-Ssa1-Ydj1 system that is largely inactive in MDH disaggregation and requires the disaggregase Hsp104 for similar yields of refolded substrate.
Solubilization of mixed ex vivo aggregates
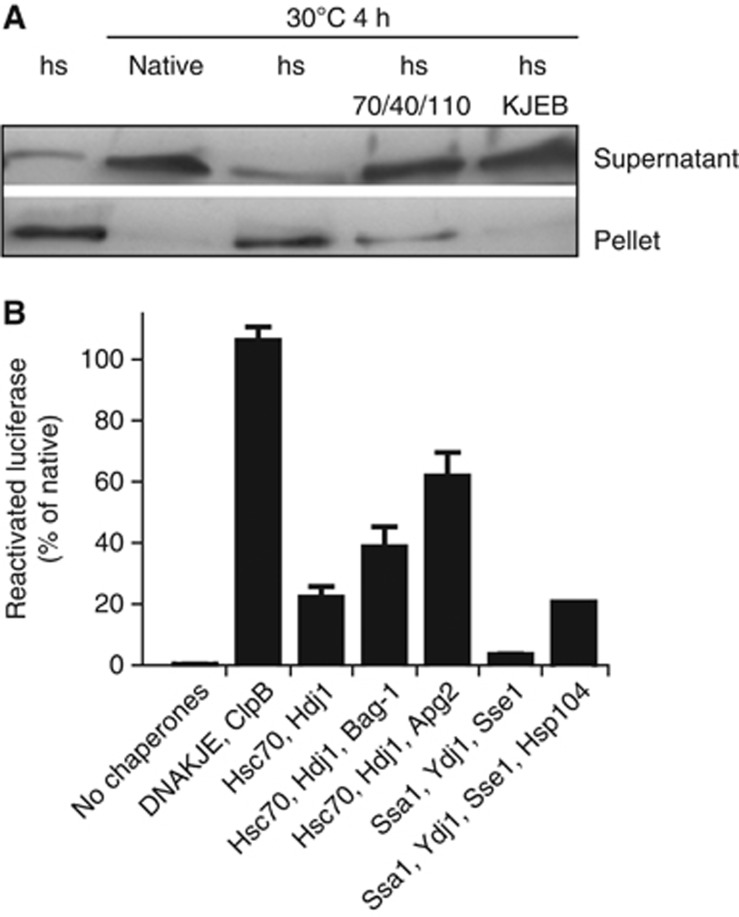
We next investigated whether disaggregation by the human Hsp70 system extends to protein aggregates generated within the complex milieu in heat-treated lysates of human U2OS cells. To have a read-out for reactivation of aggregated proteins as well as to monitor luciferase aggregation in vivo by fluorescence microscopy (see below), we expressed the heat-labile EGFP-luciferase fusion in the U2OS cells. After heat shock at 45°C, most of the EGFP-luciferase was insoluble (Figure 3A), with no spontaneous solubilization or reactivation observed over the course of a 4-h incubation (Figure 3B). Addition of human Hsc70, Hdj1 and Apg2 to heat-shocked lysate, however, resulted in almost quantitative solubilization of EGFP-luciferase (Figure 3A). Importantly, ca. 60% of the luciferase reached the native state after disaggregation with human Hsc70, Hdj1 and Apg2, whereas only 20% luciferase was reactivated by Hsc70 and Hdj1 alone (Figure 3B). The yeast Ssa1-Ydj1-Sse1 system was unable to reactivate aggregated luciferase, and even with Hsp104 additionally present, only 20% luciferase was reactivated (Figure 3B). For this reason, the bacterial bichaperone system comprising DnaK (Hsp70), DnaJ (Hsp40), GrpE (NEF) and the disaggregase ClpB served as a positive control. DnaKJE with ClpB quantitatively solubilized and reactivated EGFP-luciferase (Figure 3A and B). In conclusion, we demonstrate that the human Hsp70-Hsp40-Hsp110 potently disaggregates luciferase from aggregates in human cell lysate.
Figure 3.
The human Hsp70-Hsp40-Hsp110 system resolubilizes aggregates from cell lysates containing EGFP-luciferase. (A) EGFP-luciferase levels in supernatants and pellets of native or heat-shocked cell lysates after incubation at 30°C in the absence of chaperones or in the presence of 2 μM Hsc70, 1 μM Hdj1 and 0.2 μM Apg2 or the E. coli bichaperone system consisting of Hsp70, Hsp40, NEF and Hsp100-type disaggregase (2 μM DnaK, 0.4 μM DnaJ, 0.2 μM GrpE (DnaKJE) and 1 μM ClpB). (B) Reactivation of heat-aggregated EGFP-luciferase after 4 h at 30°C in the absence or presence of the indicated chaperones. Concentrations were as indicated above, the yeast chaperones were added at the following concentrations: Ssa1 2 μM, Ydj1 1 μM, Sse1 0.2 μM, Hsp104 1 μM. Aside from the last combination for which one representative data set out of two is shown, reactivation data shown represent the average of at least four experiments±s.e.
Protein disaggregation requires Hsp110 NEF activity
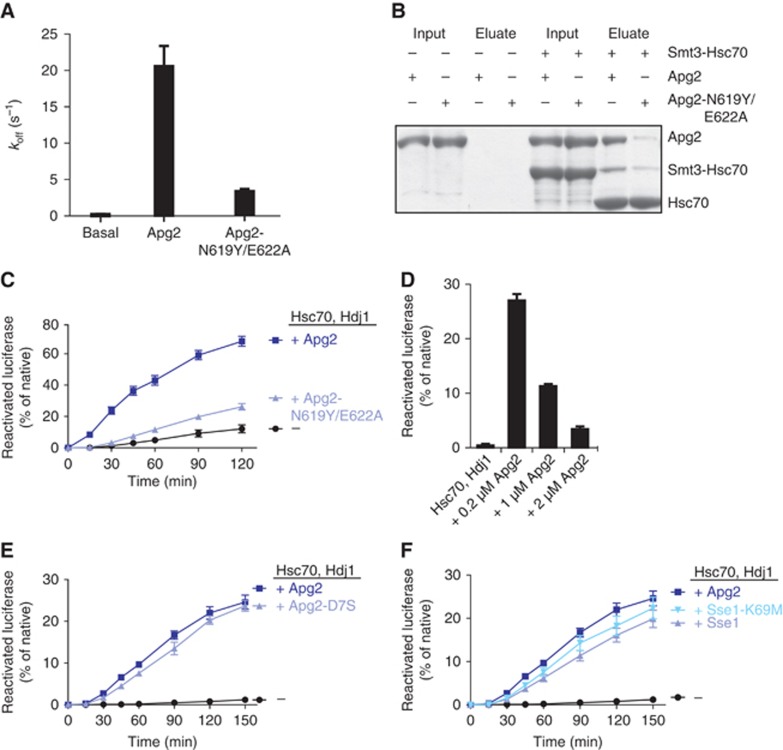
To determine which properties of Apg2 stimulate protein disaggregation, we first examined the NEF activity. The Sse1 mutant protein Sse1-2 carrying the point mutations N572Y and E575A does not interact with Hsp70 and lacks NEF activity (Polier et al, 2008). These residues are conserved in the human Hsp110 homologues, allowing us to generate the corresponding Apg2 variant, Apg2-N619Y/E622A (Supplementary Figure 3A). We determined by stopped flow kinetic measurements of MABA-ADP dissociation from human Hsc70 that Apg2-N619Y/E622A, similar to Sse1-2, displayed strongly impaired NEF activity (Figure 4A) and was deficient in interacting with human Hsc70 (Figure 4B). This deficiency in promoting nucleotide exchange resulted in drastically reduced reactivation of heat-aggregated luciferase (Figure 4C), indicating that NEF activity of Apg2 or its interaction with Hsc70 is vital for its role in disaggregation.
Figure 4.
(A) The Apg2 mutant Apg2-N619Y/E622A is NEF deficient. Dissociation rates of MABA-ADP from 0.5 μM Hsc70 were determined upon addition of 1 μM Apg2 or Apg2-N619Y/E622A. Data represent the average of six experiments±s.d. (B) The Apg2 mutant Apg2-N619Y/E622A is deficient in complex formation with Hsc70. Interaction between mutant or WT Apg2 and His6-Smt3-Hsc70 was tested by a pulldown on Co-IDA, followed by elution with Ulp1 protease. (C) The Apg2 variant Apg2-N619Y/E622A does not support efficient disaggregation of chemically aggregated luciferase. Reactivation of 20 nM luciferase from urea-induced aggregates was monitored upon addition of 2 μM Hsc70 and 1 μM Hdj1 in the presence or absence of 0.2 μM Apg2 or Apg2-N619Y/E622A. Reactivation data represent the average of at least four experiments±s.e. (D) The stimulatory effect of Apg2 on disaggregation is strongly concentration dependent. Reactivation of 20 nM luciferase from heat-induced aggregates obtained at a concentration of 2 μM luciferase was monitored upon addition of 2 μM Hsc70, 1 μM Hdj1 and varying concentrations of Apg2. The values shown represent the final yield of reactivated luciferase after 120 min. Reactivation data represent the average of three experiments±s.d. (E) The ATPase-deficient Apg2 mutant Apg2-D7S supports luciferase disaggregation equally well as Apg2. Reactivation of 20 nM luciferase from heat-induced aggregates obtained at a concentration of 2 μM luciferase was monitored upon addition of 2 μM Hsc70 and 1 μM Hdj1 in the presence or absence of 0.2μM Apg2 or Apg2-D7S. Reactivation data represent the average of at least three experiments±s.e. (F) Sse1 as well as the ATPase-deficient mutant Sse1-K69M support disaggregation together with Hsc70 and Hdj1. Reactivation of luciferase from heat-induced aggregates obtained at a concentration of 2 μM luciferase was monitored upon addition of 2 μM Hsc70, 1 μM Hdj1 and 0.2 μM Apg2 or Sse1 or Sse1-K69M. Reactivation data represent the average of six experiments±s.e.
In line with the notion that NEF activity represents an essential contribution of Apg2 to the disaggregation activity of the Hsp70 system, we observed a strong inhibition of luciferase disaggregation with increasing Apg2 concentrations. This was already manifest at sub-stoichiometric Apg2-Hsc70 ratios (Figure 4D). Inhibition of Hsp70 functions has been observed for other NEFs (Packschies et al, 1997; Bimston et al, 1998; Gassler et al, 2001; Kabani et al, 2002; Yamagishi et al, 2004; Dragovic et al, 2006; Raviol et al, 2006b; Polier et al, 2008) and in the case of Hsp110s reflects the increasing disruption of the Hsp70 ATPase cycle due to release of ATP prior to hydrolysis (Yamagishi et al, 2004; Raviol et al, 2006b). In contrast, increasing Apg2 levels would not be expected to decrease disaggregation if substrate binding or holdase activity was the major contribution of Apg2.
Hsp110 does not require an ATPase-dependent, chaperone-like activity
We next addressed the possibility that Apg2 might also support disaggregation by a chaperone-like mechanism. Apg2 can interact with substrates as evidenced by a partial prevention of thermal aggregation (Gotoh et al, 2004; Supplementary Figure 3B). ATP hydrolysis is a central regulatory feature in the canonical chaperone function of Hsp70, and a recent study reported that Hsp110s also have decreased affinities towards peptides in the presence of ATP (Xu et al, 2012). We therefore investigated whether Apg2 ATPase activity is important for protein disaggregation. Based on a known Hsc70 ATPase-deficient mutation (Wilbanks et al, 1994) we generated the Apg2 variant Apg2-D7S, corresponding to Hsc70-D10S, which is mutated in the catalytic centre of the ATPase domain. The steady-state ATPase activity of wild-type Apg2 (0.0025 ATP hydrolysed per second) agreed very well with values determined earlier (Raviol et al, 2006a). In contrast, Apg2-D7S had a significantly reduced steady-state ATPase activity with 0.0007 ATP hydrolysed per second (Supplementary Figure 3C). Still, Apg2-D7S supported disaggregation to the same level as wild-type Apg2 (Figure 4E). These results demonstrate that the ATPase activity of Apg2, and consequently nucleotide regulation of potential substrate interactions of Apg2, is not required for the protein disaggregation function.
These results indicate that Apg2-mediated stimulation of protein disaggregation depends mainly on functional nucleotide exchange. A novel activity specific to human Hsp110s (versus yeast Hsp110, Sse1) therefore seemed unlikely. We then directly tested whether the protein disaggregation function is specific to human Hsp110s or can also be fulfilled by yeast Sse1, provided the appropriate Hsp70-Hsp40 partner system is present. For this, we monitored disaggregation of thermally aggregated luciferase by Hsc70 and Hdj1 with either human Apg2 or yeast Sse1 (Figure 4F). Strikingly, Sse1 supported luciferase reactivation almost as effectively as the human Apg2, implying the powerful disaggregation activity of the human Hsp70 system requires a function provided by Hsp110 but not unique to human Hsp110.
Yeast Sse1 fully supports human Hsc70/Hdj1 in protein disaggregation, allowing us to test Sse1 mutant proteins as substitutes for human counterparts. We monitored disaggregation with Sse1-K69M, a well-established Hsp110 mutant that completely lacks ATPase activity (Raviol et al, 2006a), replacing Apg2 in the human Hsp70 system. We found that Apg2 and Sse1-K69M proteins support luciferase disaggregation equally well (Figure 4F), confirming our conclusion that Hsp110 does not power disaggregation through an ATP-driven chaperone-like mechanism.
Efficient disaggregation depends on all components of the Hsp70 system
Since NEF activity appears to be an essential contribution of Hsp110s towards efficient protein disaggregation, we tested whether a human NEF unrelated to Hsp110 (Bag-1) could promote disaggregation. Strikingly, Bag-1 did not aid the human Hsp70-Hdj1 system in the disaggregation of chemically aggregated luciferase (Figure 5A), although it can stimulate luciferase refolding by Hsc70 and Hdj1 and although we took into account the comparatively lower NEF activity of Bag-1 (Gassler et al, 2001; Supplementary Figure 1) by testing a range of concentrations (Supplementary Figure 1C). Similarly, employing the yeast Bag-1 homologue Snl1ΔN instead of Bag-1 did not facilitate luciferase reactivation by Hsc70 and Hdj1 (Figure 5A). Furthermore, Bag-1 was completely unable to stimulate disaggregation of thermally aggregated luciferase (Figure 5B) or MDH (Figure 5C) by human Hsc70/Hsp70 and Hdj1. These findings are particularly puzzling in light of the fact that Hsp110s and Bag-1-type NEFs employ a similar nucleotide exchange mechanism (Sondermann et al, 2001; Andreasson et al, 2008a). One possible explanation for this apparent paradox is that the Hsc70 ATPase cycle might proceed with different kinetics depending on the NEF present, resulting in vastly different disaggregation efficiencies. Alternatively, passive substrate interactions of Hsp110 might contribute to efficient disaggregation.
Figure 5.
Efficient disaggregation depends on all components of the Hsp70 system. (A) Reactivation of 20 nM luciferase from urea-induced aggregates was monitored upon addition of the human Hsp70 system (2 μM Hsc70, 1 μM Hdj1±0.4 μM Apg2 or 0.4 μM Bag-1 or 2 μM Snl1ΔN). (B) Luciferase (2 μM) was heat aggregated in the presence of Hsp26, diluted to 20 nM luciferase and subsequently, reactivation was monitored upon addition of the human Hsp70 system (2 μM Hsc70, 1 μM Hdj1±0.4 μM Apg2 or 0.4 μM Bag-1). (C) MDH (0.5 μM) was thermally aggregated and, after four-fold dilution, reactivation was monitored upon addition of the human Hsp70 system (4 μM Hsp70, 2 μM Hdj1±0.4 μM Apg2 or 0.4 μM Bag-1). For Bag-1, one representative data set out of two is shown. (D) Luciferase (2 μM) was heat aggregated in the presence of Hsp26, diluted to 20 nM luciferase and subsequently, reactivation was monitored upon addition of the human Hsp70 system (2 μM Hsc70, 1 μM DNAJA2 and 0.2 μM Apg2 or 0.8 μM Bag-1 or 1 μM Snl1ΔN). One representative data set out of two is shown. (E) Luciferase (20 nM) was heat aggregated in the presence of Hsp26 and reactivation was monitored upon addition of the human Hsp70 system (2 μM Hsc70, 1 μM DNAJA2 and 0.2 μM Apg2 or 0.4 μM Bag-1 or 1 μM Snl1ΔN). For Snl1ΔN, one representative data set out of two is shown. Reactivation data shown in this figure represent the average of at least three experiments±s.e. except where stated otherwise.
Although it is unclear which parameter of the cycle is affected differently by the two NEFs, it is evident that Hsp70 functional cycling equally depends on the Hsp40 protein present. In addition, substrate targeting to Hsp70 depends on Hsp40. For these reasons, we addressed the specific contribution of the Hsp40 component by replacing Hdj1, a type II Hsp40, with the type I Hsp40 DNAJA2 (Kampinga and Craig, 2010). We then tested disaggregation of thermally aggregated luciferase by this alternative Hsp70 system comprising Hsc70, DNAJA2 and an NEF. When luciferase was aggregated at the higher concentration of 2 μM, the combination of Hsc70, DNAJA2 and Apg2 reactivated 10% of the luciferase (Figure 5D) versus 25–30% for Hsc70, Hdj1 and Apg2 (Figure 2B). The relatively low efficiency indicates that only a minor sub-population of inactivated luciferase, possibly even a soluble fraction (see also Figure 2A), is amenable to the Hsc70-DNAJA2-Apg2 chaperone system. The observation that the reaction appears almost completed after 2 h supports this interpretation. This relatively low level of reactivation by Hsc70, DNAJA2 and Apg2 was also achieved by replacing Apg2 with Bag-1 (Figure 5D). When luciferase was heat aggregated at the lower concentration of 20 nM luciferase, a substantial fraction of luciferase was disaggregated by the Hsc70-DNAJA2-Apg2 combination (Figure 5E). Moreover, Apg2 and Bag-1 were equally effective in supporting luciferase reactivation (Figure 5E). To exclude the possibility that non-NEF activities of Bag-1, exerted by any one of its several subdomains, might contribute to disaggregation, we tested the yeast Bag-1 homologue Snl1ΔN that comprises only the BAG domain responsible for nucleotide exchange. This NEF supported disaggregation together with Hsc70 and DNAJA2 as well as did Bag-1 (Figure 5D and E), indicating that only nucleotide exchange is required under these conditions. Unlike Bag-1, Apg2 supported disaggregation irrespective of the J-protein employed to levels dependent on the Hsp40 present.
These results demonstrate that the Hsp40 plays an important role in disaggregation. We therefore asked whether disaggregation of permissively heat-aggregated luciferase by the yeast chaperones might require a type II Hsp40 protein. We replaced Ydj1 with Sis1 in the yeast system and observed only marginal reactivation in the absence of Hsp104 (Supplementary Figure 3D). We conclude the yeast Hsp70 is unable to reactivate heat-aggregated luciferase with an alternative Hsp40.
Supporting the inference that Bag-1 has a limited capacity to drive disaggregation, we noted that Hsc70 and Hdj1 with Bag-1 added exogenously to heat-shocked human cell lysates reactivated a fraction of endogenously expressed luciferase (ca. 40% of luciferase reactivated) (Supplementary Figure 3E), unlike our results using purified systems where the Hsc70-Hdj1-Bag-1 system was completely inactive (Figure 5A–C). This partial activity could be due to additional factors in the lysates or may reflect differences in the structure and amenability of the aggregates resulting from differences in their composition and formation.
In summary, our results indicate that Apg2 enables efficient disaggregation in vitro primarily via NEF activity. Under aggregation conditions with less stringent chaperone requirements, NEF activity is sufficient for protein disaggregation by Hsc70 with DNAJA2 since BAG-domain NEFs can substitute for Hsp110 (Figure 5E).
Hsp110 proteins colocalize with EGFP-luciferase foci after heat shock
Our in vitro results with chemically and thermally aggregated proteins indicate that an Hsp110 NEF is mandatory for efficient disaggregation by Hsc70 together with Hdj1, the Hsp40 homologue that is by far predominant after heat shock (Hageman et al, 2011) and required for survival after heat shock (Uchiyama et al, 2006). Therefore, we asked whether endogenous Apg2 in human U2OS cells colocalizes in vivo with EGFP-luciferase after heat shock. Heat shock at 45°C for 30 min caused accumulation of fluorescent foci containing both EGFP-luciferase and Apg2 with partially overlapping localization (Supplementary Figure 4, middle panels). This partial colocalization was evident also after heat-shocked cells were shifted back to 37°C for 30 min, with aggregates of EGFP-luciferase and Apg2 sometimes coalescing in the perinuclear region (Supplementary Figure 4, lower panels). We also saw this perinuclear focal pattern of Apg2 in heat-shocked cells not expressing EGFP-luciferase, suggesting that Apg2 may colocalize with aggregated endogenous proteins in these cells. Whether protein aggregation during heat shock at 45°C for 30 min can be reversed by human chaperones is still unclear. However, our observations suggest that aggregated EGFP-luciferase and human Hsp110s are localized to the same subcellular structures. This implicates human Hsp110s in protein aggregate management. Our observation that human Hsp110 colocalizes with heat-induced aggregates extends previous findings demonstrating Hsp105 colocalization with amyloid inclusions (Ishihara et al, 2003; Wang et al, 2009; Olzscha et al, 2011).
Hsp110 is required in vivo for solubilization of aggregates
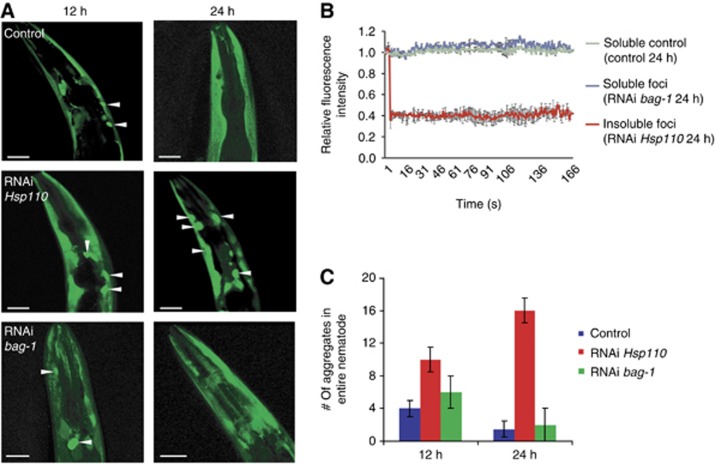
To confirm our findings in vivo, we analysed the role of Hsp110s in aggregation and disaggregation in transgenic C. elegans expressing luciferase-YFP in muscle cells as a sensor for protein aggregation. The C. elegans genome encodes three Hsp70-related putative NEFs. Two are ER localized and belong to the Grp170 protein family (Easton et al, 2000), and there is a single Hsp110 member, C30C11.4 (Nikolaidis and Nei, 2004). We assayed the contribution of the Hsp110 to disaggregation activity in worms maintained at either normal growth conditions (20°C) or exposed to a heat shock at 35°C. We used RNAi knock-down after heat shock to follow the effect of Hsp110 on protein disaggregation as opposed to prevention of aggregation. Large foci of aggregated luciferase-YFP appeared after a 1 h heat shock at 35°C; these disappeared during 24 h recovery in control animals fed with E. coli expressing only the empty RNAi vector L4440. (Figure 6A, upper panels; Figure 6C). However, in animals fed with E. coli expressing Hsp110 dsRNA post-heat shock, the luciferase foci persisted and accumulated during recovery (Figure 6A, lower panels; Figure 6C).
Figure 6.
Knock-down of Hsp110 results in the persistence of heat shock-induced luciferase. (A) Luciferase-YFP expressing nematodes were heat-shocked at 35°C for 1 h and then transferred onto RNAi plates (C30C11.4 (Hsp110), bag-1 or empty vector (L4440) as control) and returned to 20°C. The aggregation propensity of luciferase-YFP was monitored during the indicated recovery time 12 h (left) and 24 h (right) post heat shock. For each condition, 20 nematodes were analysed. Representative images of the foci (white arrows) of the head region are shown. The two different time points depict different animals. The aggregation propensity however was indistinguishable between animals treated with the same RNAi and heat-shock conditions. The scale bars are 25 μM. (B) FRAP analysis demonstrates that foci of the Hsp110 knock-down are immobile aggregates (red), whereas the luciferase-YFP of the control (blue) or upon knock-down of bag-1 is soluble (green). FRAP was performed on three different animals of each genetic background. The graphs represent the average recovery of fluorescence and the variations are depicted by the error bars. (C) Quantification of luciferase-YFP foci of (A) of the whole nematode 12 and 24 h post heat shock.
To differentiate between a specific role in protein disaggregation versus a more general role as an NEF, we compared disaggregation of heat-aggregated luciferase upon knock-down of the C. elegans bag-1 homologue. Upon knock-down of bag-1, protein aggregates similar to those in control animals appeared within 12 h. However, in contrast to Hsp110-depleted animals and similar to control animals, bag-1-depleted animals completely recovered and no further accumulation of insoluble luciferase species could be detected after 24 h. This suggests that depletion of Hsp110 within the cell, but not of Bag-1, interferes with the solubilization of heat-aggregated proteins (Figure 6A, lower panels; Figure 6C). This could explain the reduced luciferase reactivation in heat-shocked Hsp105−/− cells recently observed (Yamagishi et al, 2011). Fluorescence recovery after photobleaching (FRAP) analysis performed on animals 24 h after the heat shock showed the luciferase-YFP in controls or upon knock-down of bag-1 was soluble and mobile. In contrast, foci formed in animals with reduced Hsp110 did not recover any fluorescence signal after photobleaching, reflecting complete insolubility of the luciferase-YFP aggregates (Figure 6B). These results demonstrate that absence of Hsp110 in vivo renders C. elegans unable to resolubilize luciferase-YFP aggregates. These data provide evidence that Hsp110 is an essential component of the protein disaggregation machinery in C. elegans.
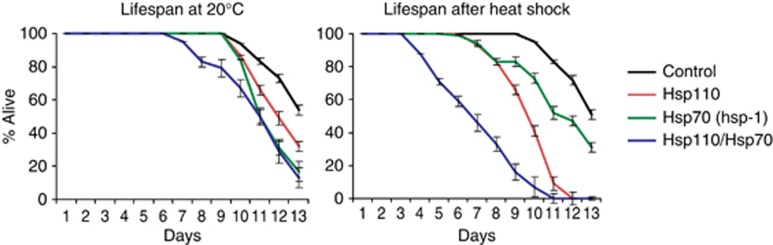
To investigate whether interfering with protein disaggregation is detrimental on an organismal level, we examined the nematode lifespan with reduced expression of Hsp110 (C30C11.4), or the Hsp70 homologue hsp-1, or both. The knock-down of hsp-1 led to a developmental delay of about 1 day in 80% of the analysed animals. However, all animals grown on RNAi against hsp-1 and hsp-1/C30C11.4 reached adulthood and did not display any other obvious phenotypes. For animals grown at 20°C, RNAi against either Hsp110 or Hsp70 led to a slight reduction in lifespan of 1–2 days (Figure 7 left panel). This phenotype was only slightly aggravated in the double knock-down of Hsp110 and Hsp70 with the lifespan reduced by about 3 days. This probably reflects the housekeeping role of Hsp110 in Hsp70-mediated protein folding. The limited phenotypes for single knock-downs of either Hsp110 or Hsp70 are consistent with redundant chaperone roles under unstressed control conditions. Strikingly, however, when animals were exposed to a 1 h heat shock (35°C) on day 1 of their lives and subsequently shifted back to 20°C, knock-down of Hsp110 alone caused a drastic lifespan reduction of about 4.5 days (Figure 7, right panel). This suggests that the other two cytosolic C. elegans NEFs (bag-1 and unc-23) do not efficiently substitute for Hsp110 in cells encountering a high load of protein aggregation. This effect was exacerbated upon double knock-down of Hsp110 and Hsp70. These results demonstrate that a decline in protein disaggregation capacity due to reduction of Hsp110 causes long-term imbalance in proteostasis when combined with increased levels of protein aggregation, resulting in detrimental consequences for the organism as evidenced by the severely shortened lifespan.
Figure 7.
Knock-down of Hsp110 decreases lifespan particularly upon heat shock. Left panel: Lifespan analysis of C. elegans upon knock-down of C30C11.4 (Hsp110), hsp-1 (Hsp70) and double knock-down of Hsp110/Hsp70 by RNAi at 20°C. Knock-down of Hsp110 or hsp-1 slightly decreases lifespan at 20°C. The double knock-down of Hsp110 and hsp-1 however reduces lifespan by ∼3 days. Right panel: Lifespan analysis of C. elegans upon knock-down of Hsp110, Hsp70 and double knock-down of Hsp110/Hsp70 by RNAi after heat shock at 35°C for 1 h on day 1 show an aggravated shortening of the lifespan. Knock-down of Hsp110 shortens lifespan by ∼4.5 days compared to the control and is further exacerbated upon additional knock-down of Hsp70. Data represent the average of 100 animals each.
Discussion
We report that metazoans have evolved a disaggregation activity exerted by Hsp70, Hsp40 and Hsp110. Hsp110 empowers the Hsp70-Hsp40 system to efficiently solubilize and reactivate substrates in vitro from various protein aggregates, including those with stringent chaperone requirements (Figure 1B; Figure 2B, D and F). Moreover, we show that Hsp110 is required for solubilization of protein aggregates at an organismic level in vivo, since knock-down of the only Hsp110 homologue in C. elegans after a short heat shock causes increased formation and persistence of aggregates (Figure 6). It is worth noting that human Hsc70 and Hdj1 display higher intrinsic disaggregation capacity than the yeast homologues Ssa1 and Ydj1 or Sis1 (Figure 2D–F; Supplementary Figure 2C and D), suggesting that the gain in disaggregation capacity of the human Hsp70 chaperone system may not be attributable simply to Hsp110. This adaptation of the Hsp70 system in metazoans compensates for the absence of Hsp104, the potent AAA+-ATPase in yeast and bacteria that fulfills this disaggregase function in protein homeostasis.
We demonstrate that Hsp110 stimulates disaggregation principally via NEF activity and that ATPase-dependent chaperone-like activity is not required (Figure 4). Further, with Hsc70 and the alternative Hsp40 DNAJA2, the NEFs Bag-1 or Snl1ΔN can substitute for Hsp110 in vitro provided the aggregation conditions are mild, showing that stimulation of nucleotide exchange is sufficient under these conditions. Our results contradict a recent study claiming an essential role for Hsp110 ATP hydrolysis and substrate interactions in protein disaggregation in vitro (Shorter, 2011). Our data are, however, consistent with earlier findings that an ATPase-deficient Sse1 mutant retains functional activity in vivo and suppresses lethality of yeast Δsse1,sse2 mutations (Shaner et al, 2004; Raviol et al, 2006b).
The critical role acquired by the animal Hsp70-Hsp40-Hsp110 system in disaggregation is emphasized by our observation that knock-down of Hsp110 in briefly heat-shocked C. elegans (on day 1 of their lives) results in persistent aggregates and a drastically reduced lifespan. Recent studies further support our conclusion ascribing the previously enigmatic disaggregation activity in animal cells to the cooperation between the Hsp70 system and Hsp110. Deletion of Hsp105, the most abundant heat stress-inducible Hsp110 homologue (Yasuda et al, 1995; Vos et al, 2008), causes accumulation of hyperphosphorylated tau and neurofibrillary tangles in mice (Eroglu et al, 2010). In support of our results, reactivation of luciferase is impaired in Hsp105−/− murine cells after heat shock (Yamagishi et al, 2011). Furthermore, we find that small Hsps facilitate the disaggregation process and are essential under some thermal stress conditions (Figure 2B–E; Supplementary Figure 2B–D), indicating that the repertoire of the metazoan Hsp70 system in protein disaggregation can be expanded by the multitude of sHsps present in vivo.
The overall efficiency we measured for the Hsp70-Hsp40-Hsp110 system under various aggregation conditions remained slightly below that for the complete yeast bichaperone system (including Hsp104). Possibly, fast aggregate removal may be less critical in animal cells compared to bacteria, plants and fungi which cannot escape if exposed to stressful environmental conditions. An Hsp70 system with expanded chaperoning repertoire including a disaggregation activity could supersede a dedicated disaggregation factor. The animal Hsp70 system has adapted to solubilizing aggregates by employing Hsp110s, but may also be networked with additional, specialized co-chaperones. We have addressed the contribution of sHsps by employing the well-characterized yeast Hsp26 chaperone, which is activated by elevated temperatures (Cashikar et al, 2005; Haslbeck et al, 2005). While MDH aggregates obtained in the absence of an sHsp could be resolubilized efficiently by the human Hsp70 system (Figure 2F) or the yeast bichaperone system (Supplementary Figure 2E), luciferase aggregates formed under similarly high substrate concentration (0.5 μM MDH versus 2 μM luciferase) required Hsp26 for disaggregation by the human or yeast chaperones (Figure 2B and C; Supplementary Figure 2B). This requirement was alleviated when luciferase was thermally aggregated at 100-fold lower concentrations (20 nM) (Figure 2D and E; Supplementary Figure 2C and D). These results suggest that depending on the identity and concentration of the substrate, the presence of an sHsp in preformed heat-induced protein aggregates is essential for disaggregation. Mammalian cells possess a great number of sHsp homologues (11 in human cells) (Kampinga et al, 2009), which can form different hetero-oligomeric complexes (Bukach et al, 2009; Mymrikov et al, 2011; Skouri-Panet et al, 2011). It seems likely therefore that individual sHsps contribute to disaggregation differently. This may involve differential activation of their chaperoning capacity or specific interactions with substrates or other cellular components.
The yeast Hsp70-Hsp40-Hsp110 system (Ssa1-Ydj1-Sse1) is incapable of efficient protein disaggregation without Hsp104, raising the question as to which component(s) of the metazoan disaggregation machinery evolved to support aggregate solubilization. Our finding that yeast Sse1 largely substitutes for Apg2 in the human Hsp70 system indicates that the gain in disaggregation capacity cannot be attributed to the Hsp110 alone, although presence of an Hsp110 is required. Our results contradict recent data suggesting that the yeast and human Hsp70-Hsp40-Hsp110 systems are equally effective for protein disaggregation in vitro, and that yeast Sse1 mutants with impaired substrate binding or ATPase activities are defective in stimulating disaggregation via yeast Hsp70-Hsp40 (Shorter, 2011). Further, reactivation of chemically denatured luciferase reported by the Shorter study is extremely slow and inefficient compared to our data. Our results, however, clearly indicate that chemical aggregation is permissive for disaggregation. Finally, it is not possible to distinguish whether low level reactivation documented in the Shorter study reflects disaggregation or refolding of a sub-population of misfolded substrate, as the fraction of aggregated substrates is unclear. Discrepancies between our data and the Shorter work could arise through use of suboptimal concentrations of Hsp110 variants. We found Hsp110 activity strongly concentration dependent (Figure 4D). Shorter used equimolar concentrations of Hsp110 relative to Hsp70. This ratio reflects a very high Hsp110 concentration that according to our data should inhibit disaggregation. In contrast, in our experiments we titrated all NEFs including Apg2 mutants individually to their optimal concentrations.
Interestingly, we find that the NEFs Bag-1 or Snl1ΔN support in vitro disaggregation of relatively amenable protein aggregates in cooperation with the type I Hsp40 DNAJA2 but fail to do so with the type II Hsp40 Hdj1 (Figure 5), while Hsp110s stimulate disaggregation irrespective of which Hsp40 is employed (Figures 1, 2 and 5). Also, it should be noted that significant reactivation from stringently obtained aggregates requires the presence of Hdj1 (Figures 2B and 5D). Thus, it appears that the combination Hsc70-Hdj1-Hsp110 includes an activity or a level of component integration that other combinations tested are lacking. Whether Hsp40-related differences in structure, substrate interactions or stimulation of the Hsc70 ATPase, differing affinities of the NEFs for Hsc70 or Hsp110–substrate interactions are responsible for the observed differences remains unclear. One explanation may relate to the observation that productive refolding depends on optimal Hsp70 cycling rates (Gassler et al, 2001), because, e.g., premature substrate release from Hsp70 could cause aggregation. Our results are consistent with the interpretation that both NEF and Hsp40 modulate critical parameters of the Hsp70 functional cycle, and in some combinations promote optimal disaggregation in vitro. Alternatively, differences in substrate interaction and holdase activities between Hsp40 proteins may account for the Hsp40-dependent differences we observe: Type I Hsp40 proteins have been reported to be superior to type II Hsp40s in mediating refolding and preventing protein aggregation by acting as holdases. Although both Hsp40 types bind substrates similarly well, the interaction kinetics and substrate specificities differ (Lu and Cyr, 1998; Cintron and Toft, 2006). In addition, substrate binding to Hsp110 may contribute to substrate targeting of the system or to prevention of reaggregation. Conceivably, substrate interactions of Hsp40s and potentially of Hsp110s may affect the efficiency of disaggregation by targeting different substrate populations or by handing over substrate to Hsp70 more or less productively, thus influencing the path of Hsp70 action.
Our work suggests that Hsp70 NEFs have separate functions in vivo, since knock-down of Hsp110, but not of Bag-1, in C. elegans abrogates the solubilization of heat-induced aggregates and causes severe premature ageing. This apparent specialization may derive from differential interaction of Hsp110, Bag-1 and Hsp40 proteins with other cellular factors. Hdj1, which together with an Hsp110 supports efficient disaggregation, is co-induced with Hsp70 after heat shock (Hattori et al, 1993) and is required for survival after heat shock (Uchiyama et al, 2006). In contrast, DNAJA2, which supports less efficient disaggregation with any NEF, is not heat induced and appears to function in mitochondrial protein import (Terada and Mori, 2000; Bhangoo et al, 2007). Therefore, while DNAJA2 serves to dissect mechanistic aspects of disaggregation in metazoans, it is unlikely to be physiologically relevant in co-chaperoning protein disaggregation. Furthermore, Bag-1 is thought to recruit Hsp70 substrates for proteasomal degradation via direct interaction with the proteasome and the E3 ligase CHIP (Alberti et al, 2003). Hsp110 proteins, on the other hand, might recruit Hsp70 to the vicinity of protein aggregates by directly interacting with substrates. Our data do not exclude the possibility that direct substrate interactions of Hsp110 may contribute to aggregate solubilization in the cell where substrate targeting or a high processivity of Hsp70 may be limiting for efficient protein disaggregation. It is worth noting that an NEF-deficient mutant such as Apg2-N619Y/E622A whose interaction with Hsp70 chaperones is compromised (Figure 4B) would consequently also lack a putative Hsp70–substrate-targeting activity, obscuring any contribution of Hsp110 substrate binding.
The large number of Hsp70 chaperones and co-chaperones in metazoans suggests the possibility of a range of functional cooperations for Hsp70 members with NEFs, for instance in protein folding, disaggregation, degradation and translocation across membranes. This could explain the weaker phenotype for single knock-downs of Hsp70 and Hsp110. The stronger effect of Hsp110-Hsp70 double knock-down (Figure 7) suggests that alternative NEFs and Hsp70 cooperate with HSP-1 and C30C11.4, respectively. The C. elegans genome encodes seven cytosolic Hsp70 genes (hsp-1, K09C4, F54C9, C12C8.1, F44E5.4, F44E5.5 and F11F1.1). There are also two cytosolic non-Hsp110 NEFs: F57B10.11 (bag-1) and the largely uncharacterized H14N18.1a (unc-23) (Nikolaidis and Nei, 2004). Our observations in heat-shocked C. elegans clearly implicate Hsp110 in aggregate quality control. However, it remains unclear how localization, availability and specific interactions of components of the cytosolic chaperone network influence the fate of non-native proteins. Studying precisely how metazoan cells call on different components of the cytosolic chaperone network to coordinate substrate flow through degradative or conservative pathways will be highly informative.
Materials and methods
Proteins
Ssa1, Sse1, Hsc70, Hsp70, Hdj1, DNAJA2, Apg1, Apg2 and Apg2 or Sse1 mutants were expressed with an N-terminal His6-Smt3 tag and purified by affinity chromatography using a Ni-IDA matrix as published earlier (Andreasson et al, 2008b). The tag was cleaved with Ulp1. For efficient cleavage, two glycine residues were introduced at the N-terminus of Apg1 and Apg2. Apg2 point mutants were generated by single-stranded mutagenesis. His-Ydj1 was purified by nickel affinity chromatography followed by cation exchange chromatography using an SP sepharose column. Hsp105 was expressed with an N-terminal His6 tag (Dragovic et al, 2006) and purified by Ni-IDA affinity chromatography followed by cleavage of the tag with TEV protease. Hsp104 was purified as described earlier (Tessarz et al, 2008). Firefly luciferase was purified with a C-terminal His-tag employing Ni-NTA chromatography. MDH was purchased from Roche and pyruvate kinase was obtained from Sigma-Aldrich.
Luciferase disaggregation and reactivation
For chemical aggregation, 2 μM luciferase was incubated at 30°C for 30 min in the presence of 4 M urea (Figure 1; data in Figure 1B are an average of data sets using either 4 M or 6 M urea), followed by 100-fold dilution into refolding buffer (40 mM Hepes-KOH pH 7.5, 50 mM KCl, 5 mM MgCl2, 2 mM DTT, 2 mM ATP, 3 mM PEP, 20 ng/μl pyruvate kinase, 10 μM BSA). For stringent thermal aggregation (Figure 2A–C), 2 μM luciferase with 10 μM Hsp26 was incubated at 45°C for 15 min, followed by 100-fold dilution into refolding buffer. In order to obtain luciferase aggregates under less severe conditions of thermal aggregation (Figure 2D and E), 20 nM luciferase with 100 nM Hsp26 was incubated at 45°C for 15 min and the dilution step was omitted. Subsequently, chaperones were added at the following concentrations: Hsp70 2 μM, Hsp40 1 μM, Apg2 0.2 or 0.4 μM, Apg1 0.3 μM, Hsp105 0.4 μM, Bag-1 0.4 or 0.8 μM, Sse1 0.1 or 0.2 μM, Snl1ΔN 1 or 2 μM, Hsp104 1 μM. For disaggregation of aggregates from cell lysates, chaperones were added at the following concentrations: Hsc70, Ssa1 or DnaK 2 μM, Hdj1 or Ydj1 1 μM, DnaJ 0.4 μM, Apg2 0.2 μM, Sse1 0.1 μM, GrpE 0.2 μM, Hsp104 or ClpB 1 μM. Reactivation of luciferase was monitored as published (Gassler et al, 2001).
Assessment of luciferase aggregates
Samples for supernatant-pellet assays were prepared in a similar fashion as for activity assays, however, final concentrations of luciferase were 50 nM. Native or aggregated luciferase was ultracentrifuged at 315 000 g for 30 min. Distribution of luciferase in supernatants and pellets was determined by SDS–PAGE and α-luciferase western blotting using a polyclonal antibody from rabbit that was generated against purified luciferase. Luciferase amounts were quantitated by densitometry using the software ImageJ. For assessment of aggregates by gradient centrifugation, native or thermally aggregated luciferase (20 nM final concentration) was overlaid on a glycerol step gradient (3–23% glycerol in 40 mM Hepes-KOH pH 7.5, 50 mM KCl, 5 mM MgCl2, 2 mM DTT, 1 μM BSA) and ultracentrifuged in an SW60 rotor at 35 000 r.p.m. for 2.5 h. Distribution of luciferase and Hsp26 in the various fractions was determined after acetone or TCA precipitation by SDS–PAGE and α-luciferase and α-Hsp26 western blotting.
MDH disaggregation and reactivation
MDH at a concentration of 0.5 μM was aggregated thermally by incubation at 47°C for 30 min. Subsequently, it was diluted 4 × into refolding buffer (25 mM Hepes-KOH pH 7.5, 50 mM KOAc, 5 mM Mg(OAc)2, 2.5 mM DTT, 10 mM ATP). Chaperones were added at the following concentrations: Hsp70 4 μM, Hdj1 1 μM, Apg2 0.4 μM, Bag1 0.4 μM, Ssa1 2 μM, Ydj1 1 μM, Sse1 0.2 μM, Hsp104 1 μM. MDH reactivation was monitored as published (Mogk et al, 2003b) except that the protocol was adapted to 96-well microtiter plate format. Supernatant-pellet assays were performed as for luciferase, MDH distribution was determined by SDS–PAGE.
Prevention of aggregation
Luciferase at a concentration of 0.2 μM in HKM buffer (25 mM Hepes-KOH pH 7.5, 50 mM KCl, 5 mM MgCl2) with 2 mM DTT, 2 mM ATP, ±0.8 or 1.6 μM Apg2 and BSA to a total protein concentration of 2 μM was shifted from 25°C to 42°C and light scattering was monitored at 600 nm.
Biochemical characterization experiments
Release of the fluorescently labelled ADP-analogue MABA-ADP from Hsp70 or Hsc70 was performed as described earlier for Ssa1 (Andreasson et al, 2008b).
Steady-state ATPase rates were obtained by a coupled enzymatic test as described earlier (Ali et al, 1993; Rampelt et al, 2011).
To assess complex formation between Apg2 variants and Hsc70, pulldowns were performed as published earlier (Andreasson et al, 2010) using 17 μM His6-Smt3-Hsc70, 7 μM Apg2 variants and 50 μM ATP. The proteins were incubated with Co-IDA resin.
Cell culture and immunofluorescence of mammalian cells
The plasmid pCV05 for the creation of stably transfected U2OS-TREX cell line expressing EGFP-labelled luciferase fusion was obtained by subcloning firefly luciferase with the N-terminal EGFP tag into the TOpuro vector (Sandler et al, 2011) using KpnI and BamHI restriction sites, resulting in pCV05.
U2OS-TREX cells kindly obtained from Georg Stoecklin were transfected with pCV05 using polyethylenimine (PEI). Twenty-four hours post transfection, 2 μg/ml puromycin (Sigma) was added to the medium and the selection of stable clones was performed for 2 weeks. Subsequently, cells were harvested and diluted into 96-well plate in a way that only one cell was present per well. After 1 more week under selective pressure, puromycin was removed and cells were expanded to a cell line.
Cells were cultivated in DMEM supplemented with 10% FCS and penicillin/streptomycin. EGFP-luciferase was induced by growth for 1 day in the presence of 1 μg/μl doxycycline. For immunofluorescence, cells were grown on coverslips, fixed with 4% paraformaldehyde for 3–10 min and permeabilized with 0.1% Triton X-100 for 10 min, followed by blocking with 5% BSA for 1 h. Staining for endogenous Apg2 was performed by incubation with α-Apg2 antibody produced in rabbit and subsequent incubation with Cy3-labelled α-rabbit antibody. In addition, nuclei were stained with DAPI. Imaging of Cy3, GFP and DAPI was performed on a Perkin-Elmer ERS-6 spinning disk confocal microscope using the software Volocity by PerkinElmer as well as ImageJ.
Cell lysis, heat shock and light scattering measurements
Lysates of U2OS cells expressing EGFP-luciferase were obtained by scraping PBS-washed cells with lysis buffer (25 mM Hepes-KOH pH 7.5, 10 mM MgCl2, 1 mM EDTA, 1 mM ATP, 1 mM PMSF). Crude lysates were cleared by centrifugation at 1400, r.p.m. for 10 min and ultracentrifugation at 315 000 g for 30 min. Lysates were heat shocked at 45°C for 10 min and the buffer was adjusted to equal the composition of the refolding buffer (see section Luciferase disaggregation). Reactivation of luciferase and supernatant-pellet assays were performed as detailed in the corresponding sections. Light scattering was measured at 600 nm with a Perkin-Elmer LS50B spectrofluorimeter.
Maintenance of C. elegans
Nematodes were grown on NGM plates seeded with E. coli OP50 strain at 20°C. For synchronization, gravid adults from one 10 cm NGM plate were collected in a canonical tube and treated with 20% alkaline hypochlorite solution under vigorous agitation for 4 min. The eggs were then washed three times with cold 0.1 M NaCl solution. The eggs were allowed to hatch in M9 medium at 20°C for 22 h. The arrested L1 larvae were subsequently used for RNAi or lifespan experiments. Strains in this study were as follows:
Bristol strain N2 (wild type) and Punc54::luciferase::yfp (J Kirstein-Miles, unpublished data), which was created by injection of 100 ng/μl of a plasmid encoding unc54::luciferase-yfp::unc54-3’UTR as simple array into the germline of young wild-type (N2) nematodes. The transgenic progeny was then subjected to γ-irradiation to integrate the extrachromosomal arrays and subsequently backcrossed three times.
Lifespan assay
Nematodes were transferred as L1s to IPTG (1 mM) containing NGM plates seeded with the respective dsRNA expressing E. coli strains (J Ahringer, University of Cambridge, UK). Twenty nematodes were used per 3 cm plate and five replica were used for each condition totalling 100 nematodes altogether. Nematodes were passaged regularly to fresh RNAi plates to separate the animals from their progeny. One parallel was heat shocked on day 1 (12 h post hatching) for 1 h at 35°C and subsequently shifted back to 20°C for the remaining time course of the experiment. The number of living animals was scored each day. Animals were scored as dead if they did not respond to gentle prodding on the head and subsequently removed from the plate.
C. elegans aggregation analysis and imaging
Day-one-old Punc54::luciferase::yfp nematodes were heat shocked as L1 (day-1-old animals; 12 h post hatching) for 1 h at 35°C and immediately returned to 20°C and transferred onto C30C11.4 (HSP110), bag-1 or control RNAi plates. The aggregation propensity of Luciferase-YFP was monitored 12 and 24 h post heat shock. For imaging, nematodes were mounted on 2% agarose (Sigma) pads on glass slides and immobilized with 2 mM Levamisole (Sigma). Images were taken on a Zeiss LSM 510 Meta confocal microscope. FRAP analysis was carried out as previously described (Garcia et al, 2007).
Supplementary Material
Acknowledgments
This work received support from grants of the Deutsche Forschungsgemeinschaft and the Network Aging Research (NAR) of the University of Heidelberg to BB and the National Institutes of Health to RIM. HR was supported by a scholarship from the Boehringer Ingelheim Fonds and JK-M was supported by a long-term postdoctoral fellowship of the Human Frontier Science Program. We thank F Seyffer, S Specht, X Wei and H Andreas for contributions in protein purification and cloning, H Raviol for production of the α-Apg2 antibody, V Cherkasova for providing the U2OS cell line expressing EGFP-luciferase, G Stöcklin for providing the U2OS-TREX cell line, J Buchner for providing the α-Hsp26 antibody and A Bracher for providing the pProEx-Hsp110 plasmid. Fluorescence microscopy for mammalian cells was performed at the Nikon Imaging Center at the University of Heidelberg. We thank L Guilbride for critical editing of the manuscript.
Author contributions: HR designed and performed experiments, analysed data and wrote the paper; JK-M, NBN, KC and SRS designed and performed experiments and analysed data; RIM and BB designed experiments, analysed data and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alberti S, Esser C, Hohfeld J (2003) BAG-1--a nucleotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones 8: 225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali JA, Jackson AP, Howells AJ, Maxwell A (1993) The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry 32: 2717–2724 [DOI] [PubMed] [Google Scholar]
- Andreasson C, Fiaux J, Rampelt H, Druffel-Augustin S, Bukau B (2008a) Insights into the structural dynamics of the Hsp110-Hsp70 interaction reveal the mechanism for nucleotide exchange activity. Proc Natl Acad Sci USA 105: 16519–16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson C, Fiaux J, Rampelt H, Mayer MP, Bukau B (2008b) Hsp110 is a nucleotide-activated exchange factor for Hsp70. J Biol Chem 283: 8877–8884 [DOI] [PubMed] [Google Scholar]
- Andreasson C, Rampelt H, Fiaux J, Druffel-Augustin S, Bukau B (2010) The endoplasmic reticulum Grp170 acts as a nucleotide exchange factor of Hsp70 via a mechanism similar to that of the cytosolic Hsp110. J Biol Chem 285: 12445–12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barends TR, Werbeck ND, Reinstein J (2010) Disaggregases in 4 dimensions. Curr Opin Struct Biol 20: 46–53 [DOI] [PubMed] [Google Scholar]
- Bhangoo MK, Tzankov S, Fan AC, Dejgaard K, Thomas DY, Young JC (2007) Multiple 40-kDa heat-shock protein chaperones function in Tom70-dependent mitochondrial import. Mol Biol Cell 18: 3414–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieschke J, Cohen E, Murray A, Dillin A, Kelly JW (2009) A kinetic assessment of the C. elegans amyloid disaggregation activity enables uncoupling of disassembly and proteolysis. Protein Sci 18: 2231–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimston D, Song J, Winchester D, Takayama S, Reed JC, Morimoto RI (1998) BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J 17: 6871–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukach OV, Glukhova AE, Seit-Nebi AS, Gusev NB (2009) Heterooligomeric complexes formed by human small heat shock proteins HspB1 (Hsp27) and HspB6 (Hsp20). Biochim Biophys Acta 1794: 486–495 [DOI] [PubMed] [Google Scholar]
- Cashikar AG, Duennwald M, Lindquist SL (2005) A chaperone pathway in protein disaggregation. Hsp26 alters the nature of protein aggregates to facilitate reactivation by Hsp104. J Biol Chem 280: 23869–23875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron NS, Toft D (2006) Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J Biol Chem 281: 26235–26244 [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A (2006) Opposing activities protect against age-onset proteotoxicity. Science 313: 1604–1610 [DOI] [PubMed] [Google Scholar]
- Diamant S, Ben-Zvi AP, Bukau B, Goloubinoff P (2000) Size-dependent disaggregation of stable protein aggregates by the DnaK chaperone machinery. J Biol Chem 275: 21107–21113 [DOI] [PubMed] [Google Scholar]
- Dragovic Z, Broadley SA, Shomura Y, Bracher A, Hartl FU (2006) Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J 25: 2519–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton DP, Kaneko Y, Subjeck JR (2000) The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones 5: 276–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Graber S, Gaestel M, Buchner J (1997) Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J 16: 221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu B, Moskophidis D, Mivechi NF (2010) Loss of Hsp110 leads to age-dependent tau hyperphosphorylation and early accumulation of insoluble amyloid beta. Mol Cell Biol 30: 4626–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CY, Lee S, Ren HY, Cyr DM (2004) Exchangeable chaperone modules contribute to specification of type I and type II Hsp40 cellular function. Mol Biol Cell 15: 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Mata R, Gao YS, Sztul E (2002) Hassles with taking out the garbage: aggravating aggresomes. Traffic 3: 388–396 [DOI] [PubMed] [Google Scholar]
- Garcia DI, Lanigan P, Webb M, West TG, Requejo-Isidro J, Auksorius E, Dunsby C, Neil M, French P, Ferenczi MA (2007) Fluorescence lifetime imaging to detect actomyosin states in mammalian muscle sarcomeres. Biophys J 93: 2091–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassler CS, Wiederkehr T, Brehmer D, Bukau B, Mayer MP (2001) Bag-1M accelerates nucleotide release for human Hsc70 and Hsp70 and can act concentration-dependent as positive and negative cofactor. J Biol Chem 276: 32538–32544 [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI (2006) Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311: 1471–1474 [DOI] [PubMed] [Google Scholar]
- Gidalevitz T, Krupinski T, Garcia S, Morimoto RI (2009) Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet 5: e1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, Lindquist S (1998) Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94: 73–82 [DOI] [PubMed] [Google Scholar]
- Goloubinoff P, Mogk A, Zvi AP, Tomoyasu T, Bukau B (1999) Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc Natl Acad Sci USA 96: 13732–13737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh K, Nonoguchi K, Higashitsuji H, Kaneko Y, Sakurai T, Sumitomo Y, Itoh K, Subjeck JR, Fujita J (2004) Apg-2 has a chaperone-like activity similar to Hsp110 and is overexpressed in hepatocellular carcinomas. FEBS Lett 560: 19–24 [DOI] [PubMed] [Google Scholar]
- Hageman J, van Waarde MA, Zylicz A, Walerych D, Kampinga HH (2011) The diverse members of the mammalian HSP70 machine show distinct chaperone-like activities. Biochem J 435: 127–142 [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475: 324–332 [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Miess A, Stromer T, Walter S, Buchner J (2005) Disassembling protein aggregates in the yeast cytosol. The cooperation of Hsp26 with Ssa1 and Hsp104. J Biol Chem 280: 23861–23868 [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J (1999) Hsp26: a temperature-regulated chaperone. EMBO J 18: 6744–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslberger T, Bukau B, Mogk A (2010) Towards a unifying mechanism for ClpB/Hsp104-mediated protein disaggregation and prion propagation. Biochem Cell Biol 88: 63–75 [DOI] [PubMed] [Google Scholar]
- Haslberger T, Zdanowicz A, Brand I, Kirstein J, Turgay K, Mogk A, Bukau B (2008) Protein disaggregation by the AAA+ chaperone ClpB involves partial threading of looped polypeptide segments. Nat Struct Mol Biol 15: 641–650 [DOI] [PubMed] [Google Scholar]
- Hattori H, Kaneda T, Lokeshwar B, Laszlo A, Ohtsuka K (1993) A stress-inducible 40 kDa protein (hsp40): purification by modified two-dimensional gel electrophoresis and co-localization with hsc70(p73) in heat-shocked HeLa cells. J Cell Sci 104: Pt 3629–638 [DOI] [PubMed] [Google Scholar]
- Ishihara K, Yamagishi N, Saito Y, Adachi H, Kobayashi Y, Sobue G, Ohtsuka K, Hatayama T (2003) Hsp105alpha suppresses the aggregation of truncated androgen receptor with expanded CAG repeats and cell toxicity. J Biol Chem 278: 25143–25150 [DOI] [PubMed] [Google Scholar]
- Kabani M (2009) Structural and functional diversity among eukaryotic Hsp70 nucleotide exchange factors. Protein Pept Lett 16: 623–660 [DOI] [PubMed] [Google Scholar]
- Kabani M, Beckerich JM, Brodsky JL (2002) Nucleotide exchange factor for the yeast Hsp70 molecular chaperone Ssa1p. Mol Cell Biol 22: 4677–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Craig EA (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 11: 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14: 105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Nollen EA, Kitagawa K, Bindokas VP, Morimoto RI (2002) Polyglutamine protein aggregates are dynamic. Nat Cell Biol 4: 826–831 [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E (1997) A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J 16: 659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowska A, Matuszewska M, Liberek K (2007) Conformational properties of aggregated polypeptides determine ClpB-dependence in the disaggregation process. J Mol Biol 371: 800–811 [DOI] [PubMed] [Google Scholar]
- Lu Z, Cyr DM (1998) Protein folding activity of Hsp70 is modified differentially by the hsp40 co-chaperones Sis1 and Ydj1. J Biol Chem 273: 27824–27830 [DOI] [PubMed] [Google Scholar]
- Miot M, Reidy M, Doyle SM, Hoskins JR, Johnston DM, Genest O, Vitery MC, Masison DC, Wickner S (2011) Species-specific collaboration of heat shock proteins (Hsp) 70 and 100 in thermotolerance and protein disaggregation. Proc Natl Acad Sci USA 108: 6915–6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogk A, Deuerling E, Vorderwulbecke S, Vierling E, Bukau B (2003a) Small heat shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol Microbiol 50: 585–595 [DOI] [PubMed] [Google Scholar]
- Mogk A, Schlieker C, Friedrich KL, Schonfeld HJ, Vierling E, Bukau B (2003b) Refolding of substrates bound to small Hsps relies on a disaggregation reaction mediated most efficiently by ClpB/DnaK. J Biol Chem 278: 31033–31042 [DOI] [PubMed] [Google Scholar]
- Murray AN, Solomon JP, Wang YJ, Balch WE, Kelly JW (2010) Discovery and characterization of a mammalian amyloid disaggregation activity. Protein Sci 19: 836–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymrikov EV, Seit-Nebi AS, Gusev NB (2011) Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 17: 157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis N, Nei M (2004) Concerted and nonconcerted evolution of the Hsp70 gene superfamily in two sibling species of nematodes. Mol Biol Evol 21: 498–505 [DOI] [PubMed] [Google Scholar]
- Oh HJ, Easton D, Murawski M, Kaneko Y, Subjeck JR (1999) The chaperoning activity of hsp110. Identification of functional domains by use of targeted deletions. J Biol Chem 274: 15712–15718 [DOI] [PubMed] [Google Scholar]
- Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM (2011) Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144: 67–78 [DOI] [PubMed] [Google Scholar]
- Packschies L, Theyssen H, Buchberger A, Bukau B, Goody RS, Reinstein J (1997) GrpE accelerates nucleotide exchange of the molecular chaperone DnaK with an associative displacement mechanism. Biochemistry 36: 3417–3422 [DOI] [PubMed] [Google Scholar]
- Parsell DA, Kowal AS, Singer MA, Lindquist S (1994) Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372: 475–478 [DOI] [PubMed] [Google Scholar]
- Polier S, Dragovic Z, Hartl FU, Bracher A (2008) Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell 133: 1068–1079 [DOI] [PubMed] [Google Scholar]
- Polier S, Hartl FU, Bracher A (2010) Interaction of the Hsp110 molecular chaperones from S. cerevisiae with substrate protein. J Mol Biol 401: 696–707 [DOI] [PubMed] [Google Scholar]
- Queitsch C, Hong SW, Vierling E, Lindquist S (2000) Heat shock protein 101 plays a crucial role in thermotolerance in Arabidopsis. Plant Cell 12: 479–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampelt H, Mayer MP, Bukau B (2011) Nucleotide exchange factors for hsp70 chaperones. Methods Mol Biol 787: 83–91 [DOI] [PubMed] [Google Scholar]
- Ratajczak E, Zietkiewicz S, Liberek K (2009) Distinct activities of Escherichia coli small heat shock proteins IbpA and IbpB promote efficient protein disaggregation. J Mol Biol 386: 178–189 [DOI] [PubMed] [Google Scholar]
- Raviol H, Bukau B, Mayer MP (2006a) Human and yeast Hsp110 chaperones exhibit functional differences. FEBS Lett 580: 168–174 [DOI] [PubMed] [Google Scholar]
- Raviol H, Sadlish H, Rodriguez F, Mayer MP, Bukau B (2006b) Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. EMBO J 25: 2510–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler H, Kreth J, Timmers HT, Stoecklin G (2011) Not1 mediates recruitment of the deadenylase Caf1 to mRNAs targeted for degradation by tristetraprolin. Nucleic Acids Res 39: 4373–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H, Langer T, Hartl FU, Bukau B (1993) DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J 12: 4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner L, Trott A, Goeckeler JL, Brodsky JL, Morano KA (2004) The function of the yeast molecular chaperone Sse1 is mechanistically distinct from the closely related hsp70 family. J Biol Chem 279: 21992–22001 [DOI] [PubMed] [Google Scholar]
- Shorter J (2011) The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS ONE 6: e26319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skouri-Panet F, Michiel M, Ferard C, Duprat E, Finet S (2011) Structural and functional specificity of small heat shock protein HspB1 and HspB4, two cellular partners of HspB5: Role of the in vitro hetero-complex formation in chaperone activity. Biochimie 94: 975–984 [DOI] [PubMed] [Google Scholar]
- Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I (2001) Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291: 1553–1557 [DOI] [PubMed] [Google Scholar]
- Terada K, Mori M (2000) Human DnaJ homologs dj2 and dj3, and bag-1 are positive cochaperones of hsc70. J Biol Chem 275: 24728–24734 [DOI] [PubMed] [Google Scholar]
- Tessarz P, Mogk A, Bukau B (2008) Substrate threading through the central pore of the Hsp104 chaperone as a common mechanism for protein disaggregation and prion propagation. Mol Microbiol 68: 87–97 [DOI] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, Bukau B (2010) Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol 11: 777–788 [DOI] [PubMed] [Google Scholar]
- Tzankov S, Wong MJ, Shi K, Nassif C, Young JC (2008) Functional divergence between co-chaperones of Hsc70. J Biol Chem 283: 27100–27109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama Y, Takeda N, Mori M, Terada K (2006) Heat shock protein 40/DjB1 is required for thermotolerance in early phase. J Biochem 140: 805–812 [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU (2010) Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb Perspect Biol 2: a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos MJ, Hageman J, Carra S, Kampinga HH (2008) Structural and functional diversities between members of the human HSPB, HSPH, HSPA, and DNAJ chaperone families. Biochemistry 47: 7001–7011 [DOI] [PubMed] [Google Scholar]
- Wang J, Farr GW, Zeiss CJ, Rodriguez-Gil DJ, Wilson JH, Furtak K, Rutkowski DT, Kaufman RJ, Ruse CI, Yates JR 3rd, Perrin S, Feany MB, Horwich AL (2009) Progressive aggregation despite chaperone associations of a mutant SOD1-YFP in transgenic mice that develop ALS. Proc Natl Acad Sci USA 106: 1392–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibezahn J, Tessarz P, Schlieker C, Zahn R, Maglica Z, Lee S, Zentgraf H, Weber-Ban EU, Dougan DA, Tsai FT, Mogk A, Bukau B (2004) Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell 119: 653–665 [DOI] [PubMed] [Google Scholar]
- Wilbanks SM, DeLuca-Flaherty C, McKay DB (1994) Structural basis of the 70-kilodalton heat shock cognate protein ATP hydrolytic activity. I. Kinetic analyses of active site mutants. J Biol Chem 269: 12893–12898 [PubMed] [Google Scholar]
- Winkler J, Tyedmers J, Bukau B, Mogk A (2012) Hsp70 target Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J Cell Biol 198: 387–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Sarbeng EB, Vorvis C, Kumar DP, Zhou L, Liu Q (2012) Unique peptide substrate binding properties of 110-kDa heat-shock protein (Hsp110) determine its distinct chaperone activity. J Biol Chem 287: 5661–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Ishihara K, Hatayama T (2004) Hsp105alpha suppresses Hsc70 chaperone activity by inhibiting Hsc70 ATPase activity. J Biol Chem 279: 41727–41733 [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Yokota M, Yasuda K, Saito Y, Nagata K, Hatayama T (2011) Characterization of stress sensitivity and chaperone activity of Hsp105 in mammalian cells. Biochem Biophys Res Commun 409: 90–95 [DOI] [PubMed] [Google Scholar]
- Yamashita H, Kawamata J, Okawa K, Kanki R, Nakamizo T, Hatayama T, Yamanaka K, Takahashi R, Shimohama S (2007) Heat-shock protein 105 interacts with and suppresses aggregation of mutant Cu/Zn superoxide dismutase: clues to a possible strategy for treating ALS. J Neurochem 102: 1497–1505 [DOI] [PubMed] [Google Scholar]
- Yasuda K, Nakai A, Hatayama T, Nagata K (1995) Cloning and expression of murine high molecular mass heat shock proteins, HSP105. J Biol Chem 270: 29718–29723 [DOI] [PubMed] [Google Scholar]
- Young JC (2010) Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol 88: 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietkiewicz S, Krzewska J, Liberek K (2004) Successive and synergistic action of the Hsp70 and Hsp100 chaperones in protein disaggregation. J Biol Chem 279: 44376–44383 [DOI] [PubMed] [Google Scholar]
- Zietkiewicz S, Lewandowska A, Stocki P, Liberek K (2006) Hsp70 chaperone machine remodels protein aggregates at the initial step of Hsp70-Hsp100-dependent disaggregation. J Biol Chem 281: 7022–7029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.