Abstract
The microtubule motor protein kinesin-5 (Eg5) provides an outward force on centrosomes, which drives bipolar spindle assembly. Acute inhibition of Eg5 blocks centrosome separation and causes mitotic arrest in human cells, making Eg5 an attractive target for anti-cancer therapy. Using in vitro directed evolution, we show that human cells treated with Eg5 inhibitors can rapidly acquire the ability to divide in the complete absence of Eg5 activity. We have used these Eg5-independent cells to study alternative mechanisms of centrosome separation. We uncovered a pathway involving nuclear envelope (NE)-associated dynein that drives centrosome separation in prophase. This NE-dynein pathway is essential for bipolar spindle assembly in the absence of Eg5, but also functions in the presence of full Eg5 activity, where it pulls individual centrosomes along the NE and acts in concert with Eg5-dependent outward pushing forces to coordinate prophase centrosome separation. Together, these results reveal how the forces are produced to drive prophase centrosome separation and identify a novel mechanism of resistance to kinesin-5 inhibitors.
Keywords: centrosome separation, dynein, Eg5, resistance, spindle
Introduction
Successful chromosome segregation in mitosis requires the formation of a bipolar spindle. In mammalian cells, spindle organization is to a large extent dominated by the centrosomes. In prophase, centrosomes move to opposite sides of the nucleus along the nuclear envelope (NE) (Tanenbaum and Medema, 2010). After NE breakdown (NEB), microtubules interact with chromosomes and the bipolar spindle is formed (Walczak and Heald, 2008). A key player driving centrosome separation and bipolar spindle assembly is the microtubule motor protein kinesin-5 (Eg5 in humans). Eg5 has a unique tetrameric configuration, which allows it to crosslink and slide microtubules apart (Kashina et al, 1996; Kapitein et al, 2005). In this way, Eg5 is thought to push centrosomes apart, thereby promoting bipolar spindle formation. This function of Eg5 is conserved from yeast to humans (Ferenz et al, 2010; Roostalu et al, 2011), and inhibition of Eg5 activity was shown to inhibit centrosome separation in prophase (Whitehead and Rattner, 1998; Tanenbaum et al, 2008; Woodcock et al, 2010; Bertran et al, 2011; Smith et al, 2011) and block bipolar spindle assembly in prometaphase (Sawin et al, 1992; Blangy et al, 1995; Kashina et al, 1996; Whitehead and Rattner, 1998; Mayer et al, 1999). Consequently, inhibition of Eg5 arrests cells in mitosis with a monopolar spindle (Ferenz et al, 2010) and results in cell death (Kapoor et al, 2000; Sakowicz et al, 2004). Because of this essential role of Eg5 in bipolar spindle assembly, much attention has focussed on Eg5 as a drug target for cancer therapy.
While Eg5 is clearly a key player in bipolar spindle assembly, recent studies identified a second kinesin, kinesin-12 (known as Kif15/Hklp2 in humans), which acts together with Eg5 in bipolar spindle assembly (Tanenbaum et al, 2009; Vanneste et al, 2009). Normally, kinesin-12 activity is not sufficient for bipolar spindle formation, as acute inhibition of Eg5 results in monopolar spindles. Nonetheless, the existence of such redundant pathways for bipolar spindle assembly has major implications, not only for our understanding of the mechanism of spindle assembly, but also for the use of Eg5 inhibitors as anti-cancer agents.
To address whether redundant pathways can take over the functions of Eg5, we asked if human cells could be established that bypass the need for Eg5 in spindle assembly. To this end, we used an in vitro ‘directed evolution’ approach to obtain human cells that can grow in the complete absence of Eg5 activity. Characterization of these Eg5-independent cells (EICs) reveals that centrosome separation occurs relatively normal, both in prophase and in prometaphase. We show that bipolar spindle assembly in EICs depends on kinesin-12 in prometaphase, but that prophase centrosome separation does not. Rather, we show that a pathway involving dynein drives prophase centrosome separation in EICs and find that this pathway is essential for Eg5-independent bipolar spindle assembly. Surprisingly, the NE-associated pool of dynein, rather than the well-studied cortical pool of dynein, is required for Eg5-independent prophase centrosome separation. Finally, we show that in the parental cells, where Eg5 is fully active, NE-associated dynein acts in concert with Eg5 to coordinate prophase centrosome separation. Thus, our data have uncovered a pathway of centrosome separation in human cells that is driven by NE-associated dynein and may play an important role in the resistance to Eg5 inhibitors.
Results
Generation and characterization of cells that can divide independently of Eg5
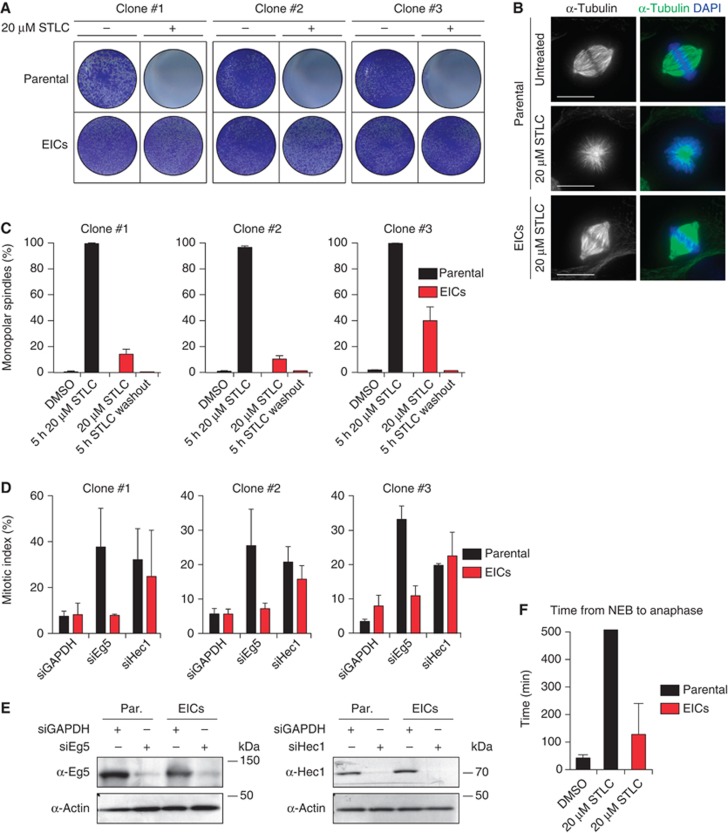
In an attempt to generate human cells that grow independently of Eg5, we treated HeLa cells for several weeks with increasing concentrations of the Eg5 inhibitor S-trityl-L-cysteine (STLC; DeBonis et al, 2004). Using this method, we generated three different EIC clones that can grow in the presence of a high dose (20 μM) of STLC, sufficient to fully inhibit Eg5 activity (Skoufias et al, 2006). Colony formation assays confirmed that proliferation was efficiently blocked upon STLC treatment in parental HeLa cells (hereafter referred to as parental cells), while the newly derived EICs survived in the presence of STLC (Figure 1A). Further analysis of EICs indicated that the majority of cells in all three EIC clones were able to assemble a bipolar spindle (Figure 1B and C) (EICs were always cultured in the presence of 20 μM STLC unless stated otherwise). To confirm that EICs acquired resistance to STLC by bypassing Eg5 function, rather than via mutations in Eg5 or upregulation of multi-drug resistance genes, we depleted Eg5 from both parental and EICs by siRNA. Knockdown of Eg5 in parental cells resulted in a dramatic increase of the mitotic index, while it did not affect EICs (Figure 1D and E), demonstrating that EICs are truly Eg5-independent. As a control, kinetochore disruption by Hec1 depletion increased the mitotic index similarly in both cell lines, indicating that the EICs are not impaired in the ability to maintain a mitotic arrest (Figure 1D). While EICs can form bipolar spindles, mitotic timing was increased and they proliferated slightly slower than parental cells (Figure 1F and data not shown). Together, these results show that cells can be generated that form a bipolar spindle and proliferate in the absence of Eg5 activity, indicating that redundant pathways can take over all essential functions of Eg5.
Figure 1.
Characterization of cells that grow in the absence of kinesin-5 activity. (A) Colony formation assays of three different HeLa clones. Both parental and EICs were left untreated or treated for 5 days with 20 μM STLC, fixed with methanol and stained with crystal violet. (B) Representative images of parental and EICs (clone #1) treated as indicated. Cells were stained for α-tubulin to visualize spindles and DAPI was used to stain DNA. (C) Quantification of the percentage of monopolar spindles from (B) (n=300 per condition). (D) Parental and EIC clones were treated for 48 h with either control (GAPDH), Eg5 or Hec1 siRNA and stained for phospho-H3. Mitotic index was determined as described in the Materials and methods section. (E) Parental and EICs (clone #1) were transfected with the indicated siRNA’s and 48 h after transfection, cells were harvested and protein levels were analysed by western blot. (F) Parental and EICs (clone #1) were treated as indicated and analysed by time-lapse microscopy. Time from NEB to anaphase was determined based on DIC (n=150 per condition). Results in (C, D, F) are averages of at least three independent experiments. Error bars represent s.d.
Kinesin-12 is essential for bipolar spindle assembly in EICs
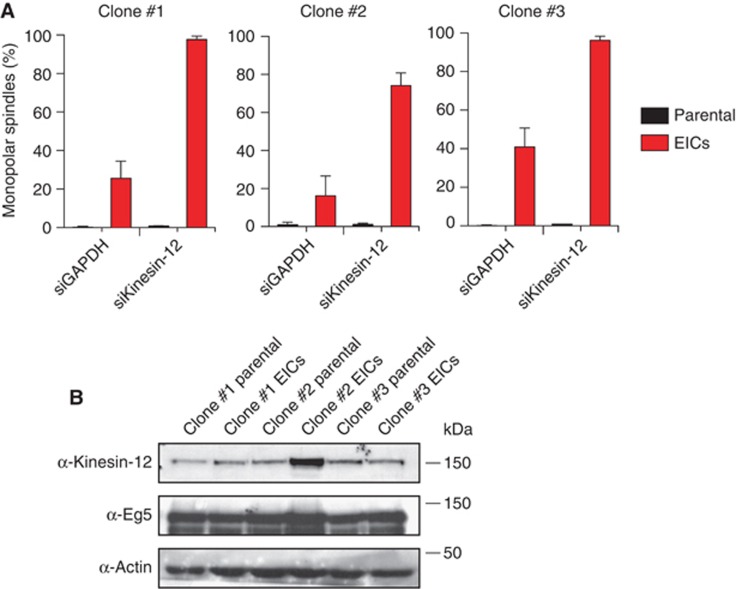
Recently, we and others showed that the plus-end-directed motor kinesin-12 (Kif15/Hklp2 in humans) cooperates with Eg5 in bipolar spindle assembly (Tanenbaum et al, 2009; Vanneste et al, 2009). We therefore tested whether kinesin-12 is required for Eg5-independent bipolar spindle assembly in the EICs. Indeed, depletion of kinesin-12 resulted in a dramatic increase in the percentage of monopolar spindles in all three clones of EICs, while it had no effect on parental cells (Figure 2A). Thus, kinesin-12 becomes essential for bipolar spindle assembly in human cells that divide independent of Eg5. We therefore tested if Eg5-independent growth of EICs is due to kinesin-12 overexpression. Interestingly, although clone #1 and #3 do not upregulate kinesin-12, clone #2 showed a clear upregulation in kinesin-12 protein levels (Figure 2B). Thus, upregulation of kinesin-12 protein levels may contribute to EIC growth, but additional mechanisms must exist.
Figure 2.
Kinesin-12 is essential for bipolar spindle assembly in EICs. (A) Parental and EIC clones were treated for 48 h with control (GAPDH) or kinesin-12 siRNA. Spindles were stained for α-tubulin and DAPI was used to visualize the DNA. The percentage of monopolar spindles was determined (n=300 per condition). Results are averages of at least three independent experiments. Error bars represent s.d. (B) Western blot analysis of protein levels in parental and EIC clones.
The dynein complex drives prophase centrosome separation in EICs
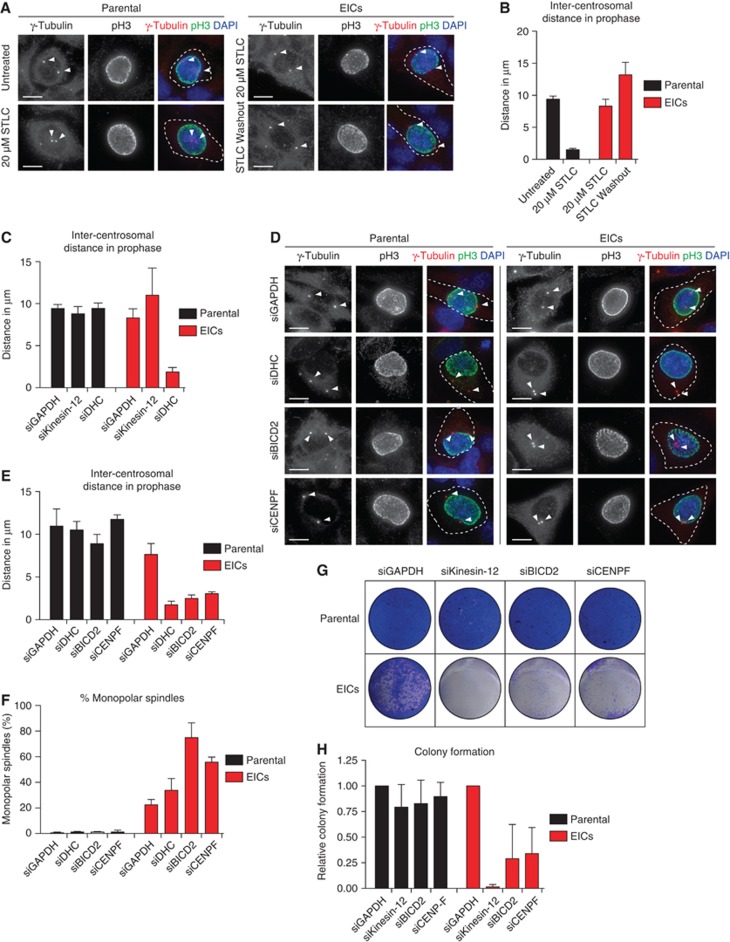
Eg5 was shown to be essential for prophase centrosome separation in human cells (Whitehead and Rattner, 1998; Tanenbaum et al, 2008; Woodcock et al, 2010; Bertran et al, 2011; Smith et al, 2011; Figure 3A and B). Surprisingly, all three clones of EICs separated their centrosomes in prophase in the absence of Eg5 activity to almost the same extent as parental cells (Figure 3A and B; Supplementary Figure S1A–C), indicating that an Eg5-independent pathway takes over prophase centrosome separation in these cells. Interestingly, washout of STLC in EICs resulted in excessive prophase centrosome separation (Figure 3A and B), suggesting that EICs have hyperactivated the Eg5-independent pathway for prophase centrosome separation.
Figure 3.
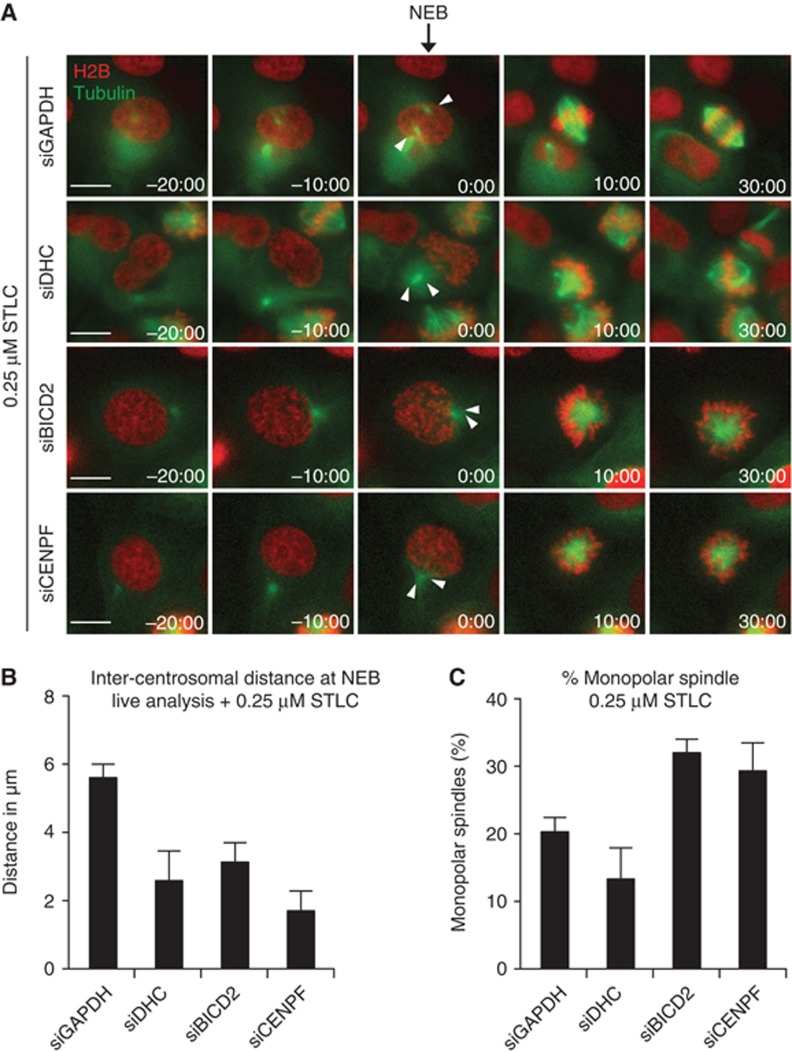
NE-Dynein is required for prophase centrosome separation in EICs. (A) Representative images of parental and EICs (clone #1) treated as indicated. Cells were stained for γ-tubulin to visualize the centrosomes, phospho-H3 (pH3) to mark prophase cells and DAPI to visualize the DNA. Arrowheads mark the centrosomes. (B) Quantification of inter-centrosomal distance in prophase from (A) (n=45 per condition). (C) Parental and EICs (clone #1) were treated for 48 h with either control (GAPDH), kinesin-12 or dynein heavy chain (DHC) siRNA. Inter-centrosomal distance in prophase was calculated as in (B) (n=45 per condition). (D) Parental and EICs (clone #1) were treated for 72 h with either control (GAPDH), DHC, BICD2 or CENPF siRNA. Cells were stained for centrosomes (γ-tubulin), pH3 (prophase cells) and DNA (DAPI). Arrowheads mark the centrosomes. (E) Quantification of inter-centrosomal distance in prophase from (D) (n=45 per condition). (F) Parental and EICs were treated as in (D), stained for α-tubulin and DAPI to visualize the DNA and the percentage of monopolar spindles was determined (n=300 per condition). (G) Colony formation of parental and EICs (clone #1). Cells were treated for 7 days with either control (GAPDH), kinesin-12, BICD2, CENPF siRNA and stained as in Figure 1A. Re-transfection was performed every 3 days. (H) Quantification of the colony formation in (G). Results in (B, C, E, F, H) are averages of at least three independent experiments. Error bars represent s.d. Scale bars represent 10 μm. See also Supplementary Figures S1–S4.
In contrast to bipolar spindle assembly after NEB, we found that prophase centrosome separation is not dependent on kinesin-12 (Figure 3C), consistent with the fact that kinesin-12 does not act before NEB (Boleti et al, 1996; Tanenbaum et al, 2009; Vanneste et al, 2009). Previous studies implicated the minus-end-directed motor dynein in prophase centrosome separation in certain cell types (Vaisberg et al, 1993; Gonczy et al, 1999; Robinson et al, 1999). Furthermore, NE-associated dynein can transport nuclei along microtubules, indicating it is capable of producing significant forces on microtubules (Reinsch and Karsenti, 1997). Therefore, we tested whether dynein was involved in centrosome separation in the EICs. Indeed, depletion of dynein completely blocked prophase centrosome separation in all three clones of EICs (Figure 3C; Supplementary Figure S1A–C). In contrast, robust centrosome separation was observed after dynein depletion in all three parental HeLa clones (Figure 3C; Supplementary Figure S1A and C), although a small decrease in centrosome separation was observed after dynein RNAi in one of the three clones (P<0.001, Supplementary Figure S1B). Similarly, depletion of Lis1 or dynein intermediate chain 2 (DIC), two other proteins essential for dynein function (Kardon and Vale, 2009), completely blocked prophase centrosome separation in EICs, while they did not inhibit centrosome separation in parental cells (Supplementary Figure S1A). Together, these results show that dynein is required for prophase centrosome separation in the absence of Eg5 activity.
The NE-associated pool of dynein drives prophase centrosome separation
How can dynein promote prophase centrosome separation? Dynein localizes to several distinct intracellular compartments (Kardon and Vale, 2009; Tanenbaum and Medema, 2010), including the cortex, intracellular vesicles, microtubules plus-ends and the NE (Kardon and Vale, 2009; Tanenbaum et al, 2010) and could therefore exert force from distinct locations. Recent studies identified BICD2 and CENPF as independent specific recruiters of dynein and the dynein-activating proteins Nde1/L1 at the NE, respectively (Splinter et al, 2010; Bolhy et al, 2011), allowing us to address if the NE-associated pool of dynein is involved in centrosome separation. Indeed, we were able to confirm that depletion of BICD2 and CENPF resulted in loss of dynein and Nde1/L1 from the NE, respectively (Supplementary Figure S2A and B). Depletion of BICD2 and CENPF does not affect localization of dynein to the centrosomes (Supplementary Figure S2C). Furthermore, Nde1/NdeL1 are not found at the centrosomes during prophase, indicating that its dynein-activating function is restricted to the NE during prophase (Supplementary Figure S2C). Nde1/NdeL1 localization is not affected by BICD2 depletion (Supplementary Figure S2A and B), consistent with previous findings (Bolhy et al, 2011). Surprisingly, we did not observe a detectable decrease in DIC or p150glued levels at the NE upon CENPF depletion, while we were able to effectively deplete CENPF, as judged by western blot and by the strongly decreased Nde1/L1 levels at the NE after CENPF depletion (Supplementary Figures S2A and B and S3A). Furthermore, we found that CENPF depletion resulted in an increased distance between centrosomes and the NE, confirming that CENPF is likely required for dynein activity at the NE (Supplementary Figure S3B; Bolhy et al, 2011). It should be noted that, in contrast to Bolhy et al, 2011, our experiments where done in the presence of nocodazole to better visualize NE-dynein, and this may explain the difference between the two studies. In any case, these results validate siRNAs targeting BICD2 and CENPF as good tools to specifically inactivate dynein at the NE, by either preventing dynein recruitment to the NE, preventing NE-dynein activation or both.
Strikingly, depletion of either BICD2 or CENPF resulted in an almost complete block of prophase centrosome separation in EICs, similar to depletion of dynein itself (Figure 3D and E; Supplementary Figure S1B and C). These results indicate that specifically the NE pool of dynein drives prophase centrosome separation. Interestingly, inhibition of NE-dynein by depletion of either BICD2 or CENPF not only blocked prophase centrosome separation, but also robustly inhibited bipolar spindle assembly in EICs (Figure 3F), demonstrating the importance of prophase centrosome separation for Eg5-independent bipolar spindle assembly. Note that dynein depletion itself did not increase the fraction of cells that formed a monopolar spindle, likely because dynein has a second, independent function in prometaphase in pulling centrosomes together (Mitchison et al, 2005; Tanenbaum et al, 2008; Ferenz et al, 2009). This result also confirms that depletion of BICD2 or CENPF does not perturb dynein function in general, but specifically inhibits NE-associated dynein activity.
Since depletion of kinesin-12 or removal of dynein from the NE in EICs results in a dramatic increase in monopolar spindles, these pathways may be key to survival of EICs. Indeed, depletion of kinesin-12 or removal of NE-associated dynein in EICs potently blocked their proliferation, while having no substantial effect on parental cells (Figure 3G and H). These results show that NE-associated dynein-dependent centrosome separation during prophase and subsequent kinesin-12 activity during prometaphase drive bipolar spindle assembly and long-term cellular proliferation in cells lacking Eg5 activity.
The balance of motor activities at the NE is altered in EICs
Previous studies found that during late G2 and prophase, a balance of NE-dynein and kinesin-1 activity controls proper localization of centrosomes relative to the nucleus (Splinter et al, 2010). Depletion of dynein results in detachment of centrosomes from the nucleus in normal cells, which is dependent on kinesin-1 activity (Figure 3D; Supplementary Figure S4A; Splinter et al, 2010). Surprisingly, centrosome detachment from the nucleus was strongly reduced in EICs depleted of dynein compared with parental cells (Supplementary Figure S4A). Importantly, there was no apparent difference in the depletion of dynein when comparing both cell lines (Supplementary Figure S4B) and the differential effect was also not due to STLC treatment of EICs (Supplementary Figure S4A, C and D). These results indicate that the balance of forces that link the centrosomes to the NE is altered in EICs (i.e., either kinesin-1 activity is reduced in EICs or NE-dynein activity is increased) and could explain why dynein is able to drive centrosome separation in the absence of Eg5 in EICs, while it is unable to do so in parental HeLa cells.
To obtain further insights into the altered motor balance in EICs, we first tested the involvement of kinesin-1 in prophase centrosome separation. Depletion of kinesin-1 did not significantly affect prophase centrosome separation in EICs, nor did it affect prophase centrosome separation in U2OS cells treated with a low dose of STLC (Supplementary Figure S5A and B). However, depletion of kinesin-1 in cells treated with a high dose of STLC (20 μM) promoted centrosome separation in U2OS cells (P=0.0024, Supplementary Figure S5C), consistent with the model that dynein promotes centrosome separation and is counteracted by kinesin-1.
We also examined the levels of several components of the dynein complex at the NE, including DIC, p150glued, CENPF and BICD2, to test if the EICs have altered the expression of these dynein components at the NE. However, we found that NE levels of these proteins were unchanged (Supplementary Figure S6A and B), suggesting that the altered motor balance is due to a change in NE-dynein activity rather than an increase in protein levels.
Dynein cooperates with Eg5 to drive prophase centrosome separation
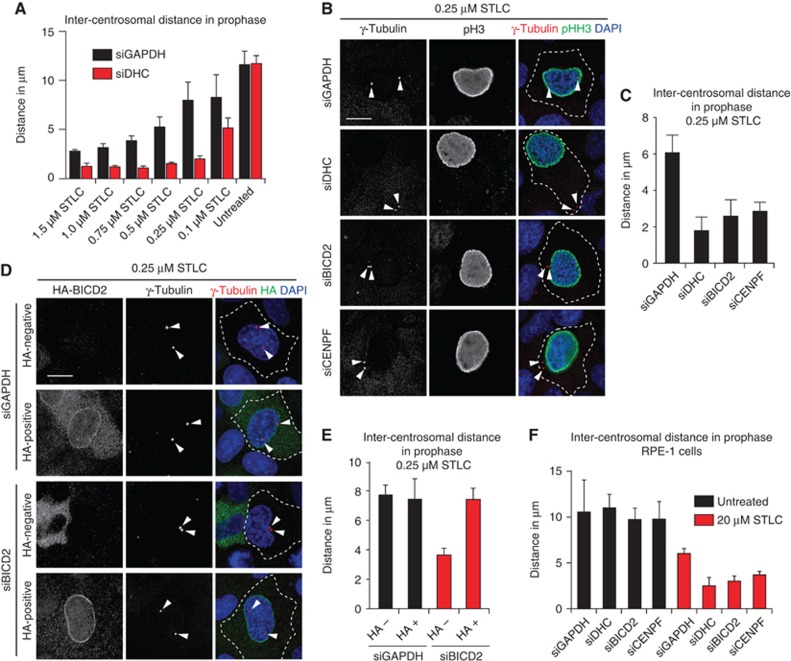
Since NE-associated dynein drives prophase centrosome separation in EICs, we wondered if a similar pathway is active in parental cells. Depletion of dynein does not result in a significant block in centrosome separation in most of the parental cell lines (Figure 3C; Tanenbaum et al, 2008), suggesting that high Eg5 activity may compensate for a lack of dynein activity during prophase centrosome separation. To test this, Eg5 activity was partially inhibited using increasing concentrations of STLC in U2OS and HeLa cells (Figure 4A; Supplementary Figure S7A). Strikingly, concentrations as low as 250 nM of STLC, which barely affected centrosome separation in control cells, almost completely eliminated prophase centrosome separation in cells lacking dynein. Similar results were observed for depletion of Lis1 or DIC (Supplementary Figures S7B and S8), confirming the importance of the dynein complex for prophase centrosome separation. These results indicate that, while dynein is not essential for prophase centrosome separation in cells with full Eg5 activity, it becomes essential when Eg5 activity is slightly compromised.
Figure 4.
NE-Dynein cooperates with Eg5 to drive prophase centrosome separation. (A) STLC titration curve. U2OS cells were transfected with siRNAs targeting either control (GAPDH) or DHC. The indicated concentrations of STLC were added to the cells 16 h before fixation (n=30 per condition). (B) U2OS cells were treated for 72 h with either control (GAPDH), DHC, BICD2 or CENPF siRNA. At 16 h before fixation, cells were treated with 0.25 μM STLC. Centrosomes (γ-tubulin), prophase cells (pH3) and DNA (DAPI) were stained. (C) Quantification of inter-centrosomal distance in prophase from (B) (n=30 per condition). (D) U2OS cells were treated for 72 h with either control (GAPDH) or BICD2 siRNA. At 24 h after siRNA transfection, cells were transfected with HA–BICD2. At 16 h before fixation, cells were treated with 0.25 μM STLC. Cells were stained for centrosomes (γ-tubulin), DNA (DAPI) and HA. Arrowheads mark the centrosomes. (E) Quantification of inter-centrosomal distance in prophase from (D) (n=30 per condition). (F) RPE-1 cells were transfected with indicated siRNA’s. Cells were left untreated or treated with 20 μM STLC 6 h before fixation. Inter-centrosomal distance in prophase was determined (n=45 per condition). Results in (A, C, E, F) are averages of at least three independent experiments. Error bars represent s.d. Scale bars represent 10 μm.
Similar to dynein depletion, BICD2 and CENPF depletion also potently blocked prophase centrosome separation in parental cells when Eg5 activity is reduced (Figure 4B and C; Supplementary Figure S7C), confirming the involvement of the NE pool of dynein. The block in centrosome separation could be reverted by expression of an RNAi-insensitive HA-tagged BICD2 (Figure 4D and E).
To obtain more insights into the kinetics of centrosome separation and bipolar spindle assembly, time-lapse imaging was performed to follow prophase centrosome separation and spindle assembly in living cells. Consistent with fixed cell experiments, live-cell imaging revealed that depletion of dynein, BICD2 or CENPF inhibited prophase centrosome separation when Eg5 was slightly inhibited (Figure 5A). Furthermore, depletion of BICD2 or CENPF also increased the fraction of cells that form a monopolar spindle, consistent with the notion that robust prophase centrosome separation is important for subsequent spindle bipolarity (Figures 3F and 5B and C). Again, dynein depletion did not increase the fraction of cells that formed a monopolar spindle (Figure 5C), likely due to loss of the inward force produced by dynein in the spindle in prometaphase (Mitchison et al, 2005; Tanenbaum et al, 2008; Ferenz et al, 2009).
Figure 5.
Live-cell analysis of prophase centrosome separation in U2OS cells. (A) U2OS cells stably expressing mCherry-α-tubulin and GFP–H2B were transfected with the indicated siRNA’s and imaged using time-lapse microscopy. Images were acquired every 2.5 min. Arrow indicates NEB, arrowheads mark the centrosomes. (B) Quantification of the inter-centrosomal distance from the movies in (A). Inter-centrosomal distance was measured one frame (2.5 min) before NEB (n=30 per condition). (C) Quantification of the number of monopolar spindles from the movie in (A) (n=150 per condition).
To test the contribution of Eg5- and dynein-dependent pathways in non-transformed cells, we inhibited Eg5 and/or dynein activity in RPE-1 cells. Surprisingly, in contrast to HeLa and U2OS cells, full inhibition of Eg5 in RPE-1 cells did not fully block prophase centrosome separation (Figure 4F), whereas it did block bipolar spindle formation after NEB (data not shown). This Eg5-independent centrosome separation depends on NE-associated dynein, as depletion of DHC, BICD2 and CENPF resulted in a substantial decrease in inter-centrosomal distance in cells lacking Eg5 activity (Figure 4F). Taken together, these results show that NE-associated dynein cooperates with Eg5 to drive prophase centrosome separation in human cells, but the relative contribution of each pathway appears to differ per cell type. Consistent with this, depletion of dynein resulted in a decrease in prophase centrosome separation in one of the parental HeLa clones (Supplementary Figure S1B).
Dynein pulls on individual centrosomes, while Eg5 acts on centrosome pairs
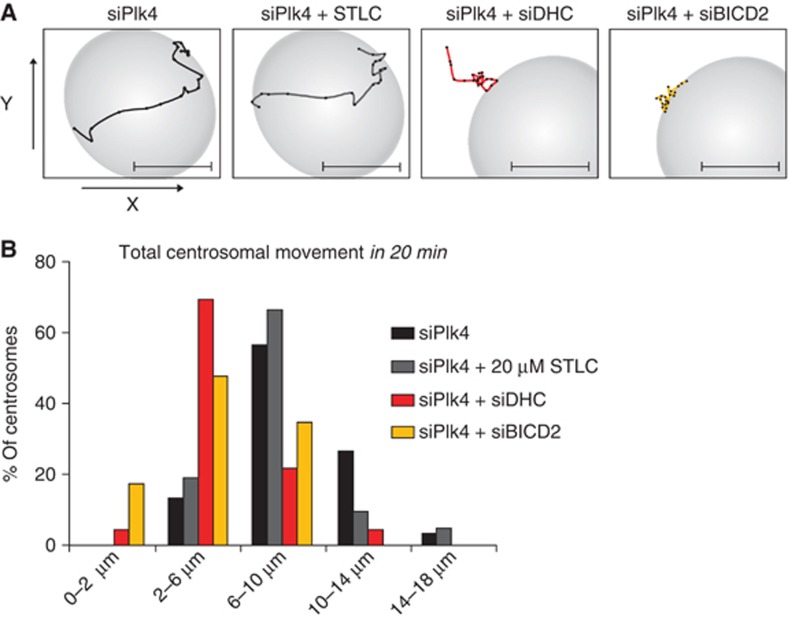
Eg5 is known to slide two anti-parallel microtubules apart, and this activity is thought to allow Eg5 to exert an outward pushing force on both centrosomes simultaneously. In contrast, we hypothesize that NE-associated molecules of dynein, by continuously walking towards the minus ends of microtubules emanating from either the one or the other centrosome, generate a pulling force on individual centrosomes. To test this, we generated cells possessing only one centrosome by depletion of Plk4 to inhibit centriole duplication (Habedanck et al, 2005). At 48 h post-transfection >92% of Plk4-depleted prophase cells contained only one centrosome (Supplementary Figure S9A and B). Time-lapse imaging of prophase centrosome movement in these cells showed that a large fraction of the individual centrosomes moved substantial distances along the NE (Figure 6A and B; Supplementary Movie S1). Importantly, treatment with a high dose of STLC did not significantly affect these movements (P=0.251, Student’s t-test), demonstrating that Eg5 does not act on individual centrosomes (Figure 6A and B). In contrast, depleting DHC or BICD2 in cells with a single centrosome resulted in a substantial reduction of the total observed movement of the centrosome (P<0.0001, Student’s t-test) (Figure 6A and B; Supplementary Movie S2 and S3). Together, these results show that, while NE-associated dynein and Eg5 cooperate in prophase centrosome separation, they act mechanistically different; NE-associated dynein pulls on individual centrosomes, while Eg5 pushes centrosome pairs apart.
Figure 6.
NE-Dynein moves individual centrosomes. (A, B) Cells stably expressing mCherry-α-tubulin were depleted of Plk4 and centrosome movements were analysed by time-lapse microscopy. Time interval for each tracking point is 60 s for the duration of 20 min prior to NEB. Movements were corrected for nuclear movement for the duration of the movie. (A) Representative tracks from individual centrosome movements are shown. (B) Histogram of the total movements of individual centrosomes from (A) (n>20 cells per conditions). See also Supplementary Figure S9 and Supplementary Movies S1–S3.
Discussion
Eg5 is thought to be the key regulator of centrosome separation and bipolar spindle assembly, making it an attractive drug target for cancer therapy. However, relatively little is known about Eg5-independent pathways that contribute to spindle bipolarity. Here, we have generated human cells that divide and proliferate in the absence of Eg5 activity. Using this approach, we show that kinesin-12 is essential for bipolar spindle assembly in the absence of Eg5. Furthermore, we identify a pathway involving NE-associated dynein that can substitute for Eg5 in prophase centrosome separation and find that this pathway is essential for bipolar spindle assembly in the absence of Eg5. Importantly, the action of this NE-dynein pathway is not restricted to EICs, but is also seen in Eg5-dependent cells, where it acts together with Eg5 to coordinate prophase centrosome separation.
Mechanisms of prophase centrosome separation
Initial centrosome separation occurs in late G2/prophase with the migration of the two centrosomes to opposite sides of the nucleus, allowing spindle assembly in prometaphase to initiate with separated centrosomes. However, the mechanism of prophase centrosome separation has remained unclear, in part due to conflicting data from various organisms concerning the involvement of different motors (Vaisberg et al, 1993; Gonczy et al, 1999; Robinson et al, 1999; Sharp et al, 2000; Saunders et al, 2007; Tanenbaum et al, 2008). In this study, we show that in human cells, dynein and Eg5 act together to drive prophase centrosome separation. Interestingly, while both Eg5 and dynein are involved in prophase centrosome separation in all cell types we tested, the relative importance of each pathway differs between cell types. Perhaps, a similar effect might also explain why studies have found contradictory results regarding the involvement of these motor proteins in prophase centrosome separation in different organisms. Future experiments in other systems involving double inhibition of dynein and Eg5 will hopefully address this intriguing notion and lead to a unifying model of prophase centrosome separation.
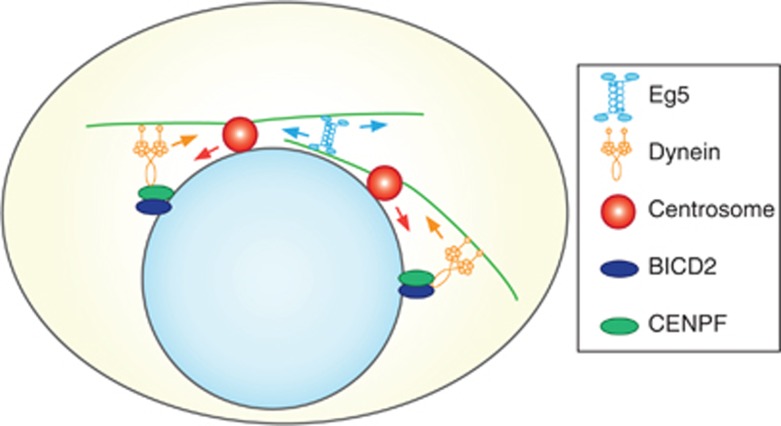
While Eg5 and dynein have redundant functions in prophase centrosome separation, their activities are mechanistically distinct. Our results show that Eg5 specifically generates an outward force on centrosome pairs, likely by crosslinking and sliding antiparallel inter-centrosomal microtubules apart (Figure 7). In contrast, dynein pulls on single microtubules emanating from centrosomes, enabling dynein to generate forces specific to each individual centrosome (Figure 7).
Figure 7.
Model of NE-dynein and Eg5 function in prophase centrosome separation. Both Eg5-dependent antiparallel microtubule sliding, as well as minus-end-directed microtubule pulling forces generated by NE-dynein mediate centrosome separation. During late G2/prophase, CENPF and BICD2 are recruited to the NE and in turn recruit/activate dynein at the NE. NE-dynein can bind centrosomal microtubules and pull centrosomes apart through its minus-end-directed motility. Blue and orange arrows indicate the direction of Eg5 and dynein motility, respectively. Red arrows indicate the movement of the centrosomes.
An interesting question is how NE-dynein can generate asymmetric pulling forces on centrosomes, required for juxtaposed movement of the two centrosomes along the NE. One possibility, albeit speculative, is that productive microtubule interactions with NE-dynein occur stochastically, resulting in random walk of individual centrosomes. In this respect, it is of interest to note that we observed discrete periods of prolonged unidirectional movement of single centrosomes (Figure 6A). In cells with two centrosomes, it is possible that microtubules emanating from one centrosome physically collide with MTs from the other aster, resulting in catastrophes specifically between the two centrosomes (Janson et al, 2003). This would result in biased MT growth away from the opposing aster and could result in an asymmetric distribution of pulling forces and movement of centrosomes away from each other. Addition of the Eg5-dependent outward sliding force between centrosomes will further skew movement of centrosomes in opposite directions.
Mechanisms for prophase and prometaphase centrosome separation are redundant
While most cells separate centrosomes in prophase (Figures 3 and 4; Magidson et al, 2011), in certain HeLa clones a fraction of cells does not undergo prophase centrosome separation and these cells can still form a bipolar spindle (Toso et al, 2009). However, without prophase centrosome separation, subsequent chromosome segregation becomes more error-prone (Kaseda et al, 2012). Furthermore, the prophase centrosome separation becomes essential for spindle bipolarity, when spindle assembly in prometaphase is put under stress (Toso et al, 2009). Consistent with this, we found that inhibition of prophase centrosome separation in cells with reduced Eg5 activity also decreased spindle bipolarity (Figure 5). Thus, it is likely that prophase and prometaphase mechanisms for centrosome separation act redundantly to allow robust bipolar spindle assembly. When such redundancy is removed, as is the case in EICs, grown in the presence of Eg5 inhibitor, bipolar spindle assembly becomes less robust and all remaining pathways become essential.
Mechanism of resistance to Eg5 inhibitors
How can EICs build a bipolar spindle in the absence of Eg5 activity? Previously, we reported that 5- to 10-fold overexpression of kinesin-12 is sufficient to establish spindle bipolarity in the absence of Eg5 (Tanenbaum et al, 2009). However, EICs can form a bipolar spindle without kinesin-12 overexpression, so other mechanisms must exist. Dynein-dependent prophase centrosome separation in EICs allows spindle assembly in prometaphase to initiate with highly separated centrosomes, a situation that likely results in a strong bias towards spindle bipolarity (Ferenz et al, 2009; Tanenbaum et al, 2009) and thus, relatively low kinesin-12 activity might be sufficient to tip the balance towards spindle bipolarity. Consistent with this, endogenous kinesin-12 activity is not sufficient for spindle bipolarity in parental HeLa cells treated with Eg5 inhibitors, as these cells enter prometaphase with unseparated centrosomes.
However, other changes have likely occurred in EICs as well that could enhance spindle bipolarity in prometaphase, and future work will be directed towards identification of these additional changes. Nonetheless, eliminating prophase centrosome separation in EICs by depletion of BICD2 or CENPF, results in a dramatic decrease in spindle bipolarity, clearly pointing at an important role for prophase centrosome separation as a modulator of bipolar spindle assembly in the absence of Eg5 activity. Although the exact mutations/changes that allow dynein to drive prophase centrosome separation in EICs are currently unknown, these might involve increased activation of NE-dynein or weakening of the linkage that holds centrosomes together (Mardin et al, 2010), and could be different for each clone.
Taken together, the development of EICs has allowed us to delineate multiple levels of redundancy in centrosome separation and bipolar spindle assembly. This approach will be useful to identify additional pathways involved in spindle assembly that have previously been overlooked due to redundancy. A similar approach can easily be adopted for other motor and non-motor proteins, allowing widespread identification of redundancy, as well as potential resistance to clinically relevant drugs.
Materials and methods
Cell culture, transfection and drug treatment
HeLa and U2OS cells were cultured in Dulbecco’s modified Eagle’s medium (GIBCO) with 6% fetal calf serum, 100 U/ml penicillin and 100 μg/ml streptomycin. siRNA was transfected with HiPerFect (Qiagen) according to the manufacturer’s guidelines. DNA was transfected using X-tremeGENE (Roche) according to the manufacturer’s guidelines. The following siRNAs were used in this study: GAPDH OTP SMARTpool (Dharmacon), Eg5 OTP SMARTpool (Dharmacon), Hec1 OTP SMARTpool (Dharmacon), dynein HC AAGGAUCAAACAUGACGGAAU (Draviam et al, 2006) (Dharmacon), kinesin-12 OTP SMARTpool (Dharmacon), Lis1 GAGTTGTGCTGATGACAAG (Shu et al, 2004) (Dharmacon), dynein IC OTP GUAAAGCUUUGGACAACUA (Dharmacon), BICD2 SMARTpool and OTP AGACGGAGCGCGAACAGAA (Dharmacon), CENPF SMARTpool and OTP GAAGCUAUGCUAAGAAAUA (Dharmacon), Kinesin-1 OTP CGAGGAACGUCUAAGAGUA (Dharmacon) and Plk4 OTP GGACUUGGUCUUACAACUA (Dharmacon). The following expression construct was used: Mouse HA–BICD2 (Matanis et al, 2002).
Generation of EICs
Different HeLa clones were treated with increasing concentrations of STLC over the course of 5 weeks. Cells were washed three times a week and passaged into increased STLC concentrations twice a week. After reaching the final concentration of 20 μM STLC, Eg5 RNAi was used to test if the cells were truly dividing independently of Eg5.
Immunofluorescence
Cells were grown on 10 mm glass coverslips and fixed with either 4% formaldehyde with 1% triton X-100 in PBS at room temperature or ice-cold methanol for 15 min. α-Tubulin antibody (Sigma) was used at 1:10 000, γ-tubulin (Abcam) was used at 1:500, phospho-H3 (Ser10) (Millipore) was used at 1:1.500, BICD2 antibody (Splinter et al, 2010) was used 1:300, P150glued antibody (BD) was used 1:500, CENPF (Novus) was used 1:500, DIC (70.1, Sigma) was used 1:1.000, Nde1/NdeL1 (Stehman et al, 2007) was used 1:100, HA (HA11, Covance) was used 1:1.000, Lamin B (Santa Cruz) and Lamin A/C (Santa Cruz) antibodies were used 1:200. Primary antibodies were incubated overnight at 4°C and secondary antibodies (Alexa 488 and 561, Molecular Probes) were incubated for 1 h at room temperature. DAPI was added before mounting using Vectashield (Vectorlabs). Prophase cells were selected based on phospho-H3 (Ser10) signal, DNA condensation status and the lack of cytoplasmic proteins in the nucleus and inter-centrosomal distances were measured in 3D images using Zeiss LSM 510 confocal software. Images were acquired on a Zeiss LSM510 META confocal microscope (Carl Zeiss) with a Plan Apochromat × 63 NA 1.4 objective. Brightness and contrast were adjusted with Adobe Photoshop.
Mitotic indexes were determined using automated image acquisition and analysis based on the pHH3 signal using a Cellomics ArrayScan VTI (Thermo Scientific) as described previously (Raaijmakers et al, 2009).
Time-lapse microscopy
U2OS cells stably expressing mCherry-α-tubulin and GFP–H2B were plated on eight-well glass-bottom dishes (Labtek). Cells were imaged in a Zeiss Axiovert 200M microscope equipped with a Plan-Neofluar × 40/1.3 NA oil objective in a permanently heated chamber in Leibovitz L15 CO2-independent medium. Images were acquired every 60 s using a Photometrics Coolsnap HQ charge-coupled device (CCD) camera (Scientific) and GFP/mCherry filter cube (Chroma Technology Corp.). Z-stacks were acquired with 3.33 μm intervals between Z-slices. Images were processed using Metamorph software (Universal Imaging). Centrosome movement was tracked in 3D using ImageJ. Statistical analysis was carried out using Prism 5 (Graphpad Software Inc.).
Colony formation
Parental and STLC-resistant HeLa cells were seeded at a density of 5.000 cells per well in a 96-well plate. Cells were treated with the indicated concentrations of STLC and siRNAs and grown for 7 days. siRNA transfections were repeated every 72 h. Cells were fixed with methanol and stained with crystal violet. Colony density was quantified using ImageJ.
Western blotting
Cells were lysed with Laemmli buffer (120 mM Tris (pH 6.8), 4% SDS, 20% glycerol). Protein levels were analysed by western blot. Kinesin-12 antibody (Tanenbaum et al, 2009) was used 1:500, actin antibody (Santa Cruz) was used 1:1.500, BICD2 antibody (Splinter et al, 2010) was used 1:1.000, Eg5 antibody (Abcam) was used 1:500, Hec1 antibody (Genetex) was used 1:1.000, CENPF antibody (Novus) was used 1:500 and DIC (70.1, Sigma) was used 1:1.000. Actin levels served as a loading control.
Supplementary Material
Acknowledgments
We thank Livio Kleij for maintaining the microscopes, Richard Vallee for the Nde1/NdeL1 antibody, Anna Akhmanova and Casper Hoogenraad for the mouse HA–BICD2 expression construct. We also thank Pierre Gonzcy and members of the Medema, Kops and Lens labs for helpful discussion. We thank Ron Vale, Peter Bieling, Benjamin Rowland and Aniek Janssen for critical reading of the manuscript. This work was supported by the Netherlands Genomic Initiative of NOW and a ZonMW TOP project (40-00812-98-10021) to RHM. MET was supported by fellowships from the European Molecular Biology Organization (EMBO) and the Dutch Cancer Society (KWF).
Author contributions: JAR, RGHPH, RHM and MET designed the experiments. JAR, RGHPH and JLM carried out and analysed the experiments. BFG and EFG provided technical assistance. JAR, RGHPH, RHM and MET wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bertran MT, Sdelci S, Regue L, Avruch J, Caelles C, Roig J (2011) Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J 30: 2634–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d'Herin P, Harper M, Kress M, Nigg EA (1995) Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83: 1159–1169 [DOI] [PubMed] [Google Scholar]
- Boleti H, Karsenti E, Vernos I (1996) Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell 84: 49–59 [DOI] [PubMed] [Google Scholar]
- Bolhy S, Bouhlel I, Dultz E, Nayak T, Zuccolo M, Gatti X, Vallee R, Ellenberg J, Doye V (2011) A Nup133-dependent NPC-anchored network tethers centrosomes to the nuclear envelope in prophase. J Cell Biol 192: 855–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBonis S, Skoufias DA, Lebeau L, Lopez R, Robin G, Margolis RL, Wade RH, Kozielski F (2004) In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol Cancer Ther 3: 1079–1090 [PubMed] [Google Scholar]
- Draviam VM, Shapiro I, Aldridge B, Sorger PK (2006) Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J 25: 2814–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenz NP, Gable A, Wadsworth P (2010) Mitotic functions of kinesin-5. Semin Cell Dev Biol 21: 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenz NP, Paul R, Fagerstrom C, Mogilner A, Wadsworth P (2009) Dynein antagonizes eg5 by crosslinking and sliding antiparallel microtubules. Curr Biol 19: 1833–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P, Pichler S, Kirkham M, Hyman AA (1999) Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol 147: 135–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA (2005) The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol 7: 1140–1146 [DOI] [PubMed] [Google Scholar]
- Janson ME, de Dood ME, Dogterom M (2003) Dynamic instability of microtubules is regulated by force. J Cell Biol 161: 1029–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF (2005) The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435: 114–118 [DOI] [PubMed] [Google Scholar]
- Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ (2000) Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol 150: 975–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon JR, Vale RD (2009) Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol 10: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseda K, McAinsh AD, Cross RA (2012) Dual pathway spindle assembly increases both the speed and the fidelity of mitosis. Biol Open 1: 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM (1996) A bipolar kinesin. Nature 379: 270–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson V, O'Connell CB, Loncarek J, Paul R, Mogilner A, Khodjakov A (2011) The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell 146: 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardin BR, Lange C, Baxter JE, Hardy T, Scholz SR, Fry AM, Schiebel E (2010) Components of the Hippo pathway cooperate with Nek2 kinase to regulate centrosome disjunction. Nat Cell Biol 12: 1166–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matanis T, Akhmanova A, Wulf P, Del NE, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, Barnekow A, Hoogenraad CC (2002) Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol 4: 986–992 [DOI] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286: 971–974 [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Maddox P, Gaetz J, Groen A, Shirasu M, Desai A, Salmon ED, Kapoor TM (2005) Roles of polymerization dynamics, opposed motors, and a tensile element in governing the length of Xenopus extract meiotic spindles. Mol Biol Cell 16: 3064–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers JA, Tanenbaum ME, Maia AF, Medema RH (2009) RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J Cell Sci 122: 2436–2445 [DOI] [PubMed] [Google Scholar]
- Reinsch S, Karsenti E (1997) Movement of nuclei along microtubules in Xenopus egg extracts. Curr Biol 7: 211–214 [DOI] [PubMed] [Google Scholar]
- Robinson JT, Wojcik EJ, Sanders MA, McGrail M, Hays TS (1999) Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J Cell Biol 146: 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostalu J, Hentrich C, Bieling P, Telley IA, Schiebel E, Surrey T (2011) Directional switching of the kinesin Cin8 through motor coupling. Science 332: 94–99 [DOI] [PubMed] [Google Scholar]
- Sakowicz R, Finer JT, Beraud C, Crompton A, Lewis E, Fritsch A, Lee Y, Mak J, Moody R, Turincio R, Chabala JC, Gonzales P, Roth S, Weitman S, Wood KW (2004) Antitumor activity of a kinesin inhibitor. Cancer Res 64: 3276–3280 [DOI] [PubMed] [Google Scholar]
- Saunders AM, Powers J, Strome S, Saxton WM (2007) Kinesin-5 acts as a brake in anaphase spindle elongation. Curr Biol 17: R453–R454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, LeGuellec K, Philippe M, Mitchison TJ (1992) Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359: 540–543 [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Brown HM, Kwon M, Rogers GC, Holland G, Scholey JM (2000) Functional coordination of three mitotic motors in Drosophila embryos. Mol Biol Cell 11: 241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Ayala R, Nguyen MD, Xie Z, Gleeson JG, Tsai LH (2004) Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44: 263–277 [DOI] [PubMed] [Google Scholar]
- Skoufias DA, DeBonis S, Saoudi Y, Lebeau L, Crevel I, Cross R, Wade RH, Hackney D, Kozielski F (2006) S-trityl-L-cysteine is a reversible, tight binding inhibitor of the human kinesin Eg5 that specifically blocks mitotic progression. J Biol Chem 281: 17559–17569 [DOI] [PubMed] [Google Scholar]
- Smith E, Hegarat N, Vesely C, Roseboom I, Larch C, Streicher H, Straatman K, Flynn H, Skehel M, Hirota T, Kuriyama R, Hochegger H (2011) Differential control of Eg5-dependent centrosome separation by Plk1 and Cdk1. EMBO J 30: 2233–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter D, Tanenbaum ME, Lindqvist A, Jaarsma D, Flotho A, Yu KL, Grigoriev I, Engelsma D, Haasdijk ED, Keijzer N, Demmers J, Fornerod M, Melchior F, Hoogenraad CC, Medema RH, Akhmanova A (2010) Bicaudal D2, dynein, and kinesin-1 associate with nuclear pore complexes and regulate centrosome and nuclear positioning during mitotic entry. PLoS Biol 8: e1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehman SA, Chen Y, McKenney RJ, Vallee RB (2007) NudE and NudEL are required for mitotic progression and are involved in dynein recruitment to kinetochores. J Cell Biol 178: 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Akhmanova A, Medema RH (2010) Dynein at the nuclear envelope. EMBO Rep 11: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Macurek L, Galjart N, Medema RH (2008) Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J 27: 3235–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Macurek L, Janssen A, Geers EF, Alvarez-Fernandez M, Medema RH (2009) Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr Biol 19: 1703–1711 [DOI] [PubMed] [Google Scholar]
- Tanenbaum ME, Medema RH (2010) Mechanisms of centrosome separation and bipolar spindle assembly. Dev Cell 19: 797–806 [DOI] [PubMed] [Google Scholar]
- Toso A, Winter JR, Garrod AJ, Amaro AC, Meraldi P, McAinsh AD (2009) Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J Cell Biol 184: 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Koonce MP, McIntosh JR (1993) Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J Cell Biol 123: 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste D, Takagi M, Imamoto N, Vernos I (2009) The role of Hklp2 in the stabilization and maintenance of spindle bipolarity. Curr Biol 19: 1712–1717 [DOI] [PubMed] [Google Scholar]
- Walczak CE, Heald R (2008) Mechanisms of mitotic spindle assembly and function. Int Rev Cytol 265: 111–158 [DOI] [PubMed] [Google Scholar]
- Whitehead CM, Rattner JB (1998) Expanding the role of HsEg5 within the mitotic and post-mitotic phases of the cell cycle. J Cell Sci 111: Part 172551–2561 [DOI] [PubMed] [Google Scholar]
- Woodcock SA, Rushton HJ, Castaneda-Saucedo E, Myant K, White GR, Blyth K, Sansom OJ, Malliri A (2010) Tiam1-Rac signaling counteracts Eg5 during bipolar spindle assembly to facilitate chromosome congression. Curr Biol 20: 669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.