Abstract
EMBO J (2012) 31 21, 4106–4123. doi:; DOI: 10.1038/emboj.2012.202; published online August 14 2012
Alzheimer’s disease (AD) is characterized by the loss of hippocampal and cortical neurons as a consequence of the accumulation of amyloid-β (Aβ). Aβ is produced from the amyloid precursor protein (APP) by the γ-secretase complex components presenilin-1 (PS1) and -2 (PS2), which are mutated in genetic forms of AD. In this issue, Schon and coworkers show that PS1 and PS2 are located at the interface between mitochondria and endoplasmic reticulum (ER). In models of familial and sporadic AD, these two organelles are juxtaposed closely, affecting shared lipid metabolic pathways. The interface between mitochondria and ER emerges as a new potential determinant of AD pathogenesis.
Mitochondria participate in the pathogenesis of several neurodegenerative diseases, including Parkinson’s, Huntington’s and the heterogeneous group of mitochondrial diseases of mitochondrial and nuclear origin. Indeed, most of the metabolic processes that are performed in, associated with, or controlled by mitochondria, are known to be perturbed in neurodegeneration (Schon and Przedborski, 2011). However, mitochondria seem to have a less defined role in the pathogenesis of AD, one of the most devastating neurodegenerative conditions (Morais and De Strooper, 2010). In particular, while mitochondrial dysfunction has been extensively reported in models of AD, as well as in cells from AD patients, the prevailing consensus is that this represents an epiphenomenon of a dysfunctional neuron, secondary to well-characterized pathogenetic pathways at other cellular sites. Indeed, according to the currently prevalent pathogenetic theory, AD results from a cascade of proteolytic events including PS1 and PS2, the catalytic subunits of the γ-secretases, a collection of protease complexes involved in the proteolysis of integral membrane proteins including the APP (De Strooper, 2010). Cleavage of APP yields the well-known Aβ peptides of amyloid plaques, which provide, together with the neuronal tangles, the characteristic pathological signature of AD. Aβ is upstream in a pathological cascade that induces plaques, tangles, and finally leads to neurodegeneration and dementia (De Strooper, 2010). In this hypothesis, PS1 and PS2 are linked to AD via their well-established roles in Aβ generation and mitochondria may play a role downstream of Aβ, providing for instance the signals that trigger caspase activation to cause apoptosis of the neuronal spines (D’Amelio et al, 2011) or degeneration of the whole neuron (Hardy and Selkoe, 2002). However, the picture is complicated by a spatial paradox: how can this organelle be a primary target of Aβ produced by the γ-secretase at the plasma membrane and in intracellular vesicles? In this issue, Schon and colleagues try to solve this conundrum, showing that the γ-secretase complex is enriched at the interface between mitochondria and the ER and that the communication between the two organelles becomes dysfunctional in models of AD (Figure 1).
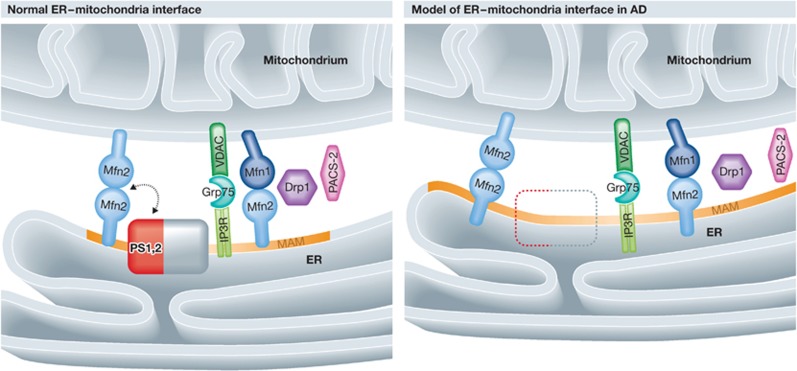
Figure 1.
Schematic representation of the changes in the ER–mitochondria interface in models of AD. Left: the normal ER–mitochondria interface; the known molecular tethers and PS1 and PS2 are shown. Right: the closer juxtaposition observed in models of AD, where PS is lacking. Note that the MAM has increased in size. Mfn, mitofusin; Drp1, dynamin-related protein 1; IP3R, inositol triphosphate receptor; VDAC, voltage-dependent anion channel; GRP75, heat-shock protein 75; PS, presenilin; the orange region on the ER illustrates the MAM.
Area-Gomez et al (2009) build on their previous findings that PS might be localized in mitochondria-associated membranes (MAMs). MAMs are specialized subdomains of the ER, originally identified by Vance (1990) as regions of the ER that are in close contact with mitochondria. Mitochondria and ER are known to engage in close connections that are essential for several functions shared by the two organelles: transfer of Ca2+, lipid metabolism, morphology of mitochondria and the control of apoptosis and autophagy (de Brito and Scorrano, 2010). The functional importance of the ER–mitochondria interface was originally recognized by laboratories studying the biosynthesis of lipids, often performed by pathways that are shared by the two organelles and that require the transfer of intermediates between the two (Vance, 1990). Subsequently, the interface was shown to be a crucial site for Ca2+ signalling, where microdomains of high concentration of this ion are generated upon release by the ER and taken up by the neighbouring mitochondria (Rizzuto et al, 1993). Despite the increasing awareness of the importance of this region in physiology, its structural composition remains largely uncharacterized. Physical bridges between mitochondria and ER have been imaged by electron tomography (Csordas et al, 2006) and several proteins are known to be enriched in this interface. So far the only structural component identified in mammalian cells to physically link ER and mitochondria is the mitochondria-shaping protein mitofusin 2 (Mfn2), which is essential for the establishment of a close interface that functions in Ca2+ and lipid transfer (de Brito and Scorrano, 2008).
While MAMs are operationally defined as a membrane fraction that can be isolated following specific differential centrifugation procedures, their nature remains largely elusive. Schon and colleagues provide evidence that these regions float in sucrose gradients when extracted in specific detergents, which characterizes them as raft-like domains. Lipid rafts are specialized domains enriched in cholesterol and sphingolipids (Lingwood and Simons, 2010). Lipid rafts have been characterized extensively in the plasma membrane, where they are crucial platforms for the assembly of signalling complexes. Interestingly, proteins are targeted to MAMs by certain lipidation modifications that are also known to facilitate targeting of proteins to plasma membrane lipid rafts (Lynes et al, 2012). Notably, the components of the γ-secretase complex are known to be enriched in lipid rafts (Vetrivel et al, 2004); however, the activity of this complex at the plasma membrane is low, suggesting that other intracellular membranes with similar biophysical properties could be the major physiologically relevant location for γ-secretase. Area-Gomez et al found that the active complex can be retrieved with MAMs, along with the structural components of the γ-secretase, including PS.
Since mutations in PS are the major cause of inherited AD, this raises the interesting questions whether PS is involved in MAM function and whether this function is disturbed in familial AD. One function of MAM is intermediary lipid metabolism and a series of remarkable observations were made in PS knockout fibroblasts in this regard. The cells accumulate lipid droplets, display increased cholesterol and cholesterylester levels, and synthesize phospholipids at increased rates (Area-Gomez et al, 2012). At the morphological level, ER–mitochondrial contacts were two- to three-fold larger, showing that genetic deletion of PS results in dramatic effects on MAM organization. Interestingly, in a set of genetic experiments, Schon and colleagues found that PS and Mfn2, the master regulator of ER–mitochondria juxtaposition, work in the same pathway. Upon ablation of Mfn2, the activity of the γ-secretase complex was reduced, despite PS still being localized in MAMs. Downregulation of Mfn2 in PS-deficient cells corrected the increased juxtaposition between ER and mitochondria and complemented all the lipid metabolism defects that characterize cells lacking PS. Conversely, ablation of PS in an Mfn2-deficient background restored the juxtaposition between the two organelles, indicating that in the absence of PS, mitochondria and ER can come in close contact even when Mfn2 is lacking. These results are important for the elucidation of the cell biology of the ER–mitochondrial interface, as they suggest that the presence of PS itself is a repulsive factor. Likely, other tethers are unveiled upon PS ablation: maybe at steady state the products of γ-secretase activity at the MAMs somehow interfere with the function of these uncharacterized tethers, in a mechanism that uses for instance rhomboid proteases to control this crucial cellular process.
The importance of the observations of Area-Gomez et al for AD was accentuated by demonstrating that fibroblasts derived from patients with mutations in PS genes display similar phenotypes. To a certain extent, it is puzzling that similar phenotypes were also retrieved in fibroblasts derived from patients suffering from sporadic AD. These patients have no known mutations in PS, and the MAM dysfunction must therefore be caused by other molecular pathways. How the different causes of AD (PS mutations, APP mutations, APOE4, deficient clearance of Aβ and other unknown factors) all converge on MAM is indeed an intriguing and important question to be addressed in the future.
When Schon and colleagues investigate to what extent the proteolytic function of PS is involved in MAM functions, the answers remain unclear. On the one hand, overexpression of catalytically inactive PS mutants could not rescue the morphological and lipid metabolism defects of the knockout cells, which suggests the involvement of the enzymatic activity of the complex. However, the authors point out this correctly, experiments with such dominant-negative forms of PS can be difficult to interpret. High overexpression will not only lead to the generation of catalytically inactive enzyme but also to an altered stoichiometry of complex subunits and thus potentially to the generation of partially assembled complexes. Thus, such dominant-negative mutants can affect the function of γ-secretase at several levels and the interpretation of the results can become very complicated. The problem can be controlled by performing native gel electrophoresis and other experiments to monitor the effects of overexpression, but the authors have chosen an alternative approach using a γ-secretase inhibitor to evaluate the role of proteolytic function in MAM. The inhibitor affects lipid metabolism in wild-type cells in a similar way as that observed in the knockout cell line, suggesting that the proteolytic activity of γ-secretase is involved in that aspect of MAM function. However, morphological alterations of the ER–mitochondria contacts (MAM) were not observed in the treated cells, showing separation of effects on morphology and on lipid metabolism. Thus, how precisely PS is involved in MAM integrity, and how it genetically interacts with Mfn2 remains unclear.
The observations of Schon et al are for the time being difficult to integrate with the canonical knowledge in the AD field. At the cell biological level for instance it is quite difficult to understand how fully glycosylated γ-secretase complex would become integrated in the ER–mitochondrial interface. The nicastrin subunit of the active γ-secretase in particular is heavily glycosylated in the trans-Golgi. How the mature complex returns from there to the ER–mitochondrial interface cannot be explained at the moment. The same holds true for highly glycosylated APP, which appears to be at least partially processed by the γ-secretase at the MAMs (Area-Gomez et al, 2009). However, in yeast, an ER component of the ERMES complex that tethers ER and mitochondria is also glycosylated, suggesting that unknown pathways exist to allow MAM localization of glycosylated proteins (Kornmann et al, 2009).
Since alterations in the ER–mitochondrial interface are also observed in fibroblasts from sporadic AD patients, they might have a broad relevance in AD pathogenesis. Further work is however necessary to consolidate some of the conclusions proposed in the paper. For example, what happens in neurons and in the brain of AD patients at the level of the MAM? Can the alterations in MAM functions be subject to drug targetting? For example, selective inhibitors of Mfn2 could revert the increased ER–mitochondria juxtaposition and ameliorate AD if the observations in the current manuscript hold true in the intact organism. At the end, finding effective drugs is the ultimate goal of AD research and the paper of Schon and coworkers might indicates a possible new horizon in the biology and pharmacology of AD.
Footnotes
The authors declare that they have no conflict of interest.
References
- Area-Gomez E, de Groof AJ, Boldogh I, Bird TD, Gibson GE, Koehler CM, Yu WH, Duff KE, Yaffe MP, Pon LA, Schon EA (2009) Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am J Pathol 175: 1810–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA (2012) Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J 31: 4106–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174: 915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, Diamantini A, De ZD, Carrara P, Battistini L, Moreno S, Bacci A, Ammassari-Teule M, Marie H, Cecconi F (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat Neurosci 14: 69–76 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2010) An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J 29: 2715–2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B (2010) Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 90: 465–494 [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297: 353–356 [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327: 46–50 [DOI] [PubMed] [Google Scholar]
- Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T (2012) Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J 31: 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais VA, De Strooper B (2010) Mitochondria dysfunction and neurodegenerative disorders: cause or consequence. J Alzheimers Dis 20: Suppl 2S255–S263 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T (1993) Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262: 744–747 [DOI] [PubMed] [Google Scholar]
- Schon EA, Przedborski S (2011) Mitochondria: the next (neurode)generation. Neuron 70: 1033–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265: 7248–7256 [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G (2004) Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 279: 44945–44954 [DOI] [PMC free article] [PubMed] [Google Scholar]