Abstract
Nature advance online publication September 26 2012; doi:; DOI: 10.1038/nature11438
Age associated changes in stem cell function are widely implicated as having a causal role to the declines in tissue function, homeostasis and regenerative ability that accompany aging. However, the signals and mechanisms that lead to altered stem cell functionality in aging are unclear. A recent study published in Nature (Chakkalakal et al, 2012) proposes a unique mechanism whereby a signal from the aged niche causes a cell autonomous and persistent change in the ability of a stem cell to maintain the quiescent state, which, over time, leads into impaired tissue regenerative capacity.
Gradual declines in tissue homeostasis, function, and regenerative ability are hallmarks of the aging process. Tissue-specific adult stem cells are the primary components of tissue regeneration and homeostasis. Therefore, an attractive theory to explain the age-associated decline in these processes centres around the effects of aging on stem cell function (Liu and Rando, 2011). The current debate focuses on the very nature of how stem cells age. Is the decline in stem cell function with age a cell autonomous change that happens due to the cumulative detrimental effects of DNA damage, epigenetic changes, or metabolic and mechanical stresses over time (Klauke and de, 2011)? Or is it an environmentally induced process whereby a perfectly functional stem cell is instructed to behave in a dysfunction manner by an aging niche or systemic milieu (Conboy and Rando, 2012)?
In their recent paper studying the effects of aging on muscle stem cell (satellite cell) function, Brack and colleagues provide a very interesting take on this debate suggesting both a signal from an aging niche induces a persistent and cell autonomous change in stem cell function. They describe important functional differences between satellite cells from young and old mice: aged satellite cells display an increased propensity to enter the cell cycle and to undergo apoptotic cell death. These are interesting observations based on the general acceptance that satellite cells in adult animals are a uniformly quiescent stem cell population. Unlike many other stem cell populations that are continuously cycling or go through phases of cycling and quiescence to maintain tissue homeostasis, muscle tissue exhibits negligible turnover under normal conditions and satellite cells rarely cycle except when called upon to contribute to tissue repair or regeneration in response to injury (Shea et al, 2010). Therefore, any increase in basal cycling and cell death is strongly suggestive of satellite cell functional changes.
In an experiment designed to monitor the cycling of satellite cells, the authors used dilution of a pulse H2B-GFP label as a surrogate marker of satellite cell cycling. What they found is there are two populations of satellite cells based on the ability to retain H2B-GFP: label-retaining (LR) and non-label-retaining (NLR) satellite cells. Furthermore, these two populations had striking functional differences. Compared to LR cells, NLR satellite cells show an increased propensity to exit from the quiescent state (assessed by BrdU incorporation) and, in transplantation experiments, a decreased propensity to self-renew and contribute to muscle regeneration. They also found that a greater proportion of the total satellite cells in aged animals are NLR cells. When the transcriptional changes of LR and NLR cells were compared with bulk populations of adult and aged satellite cells, NLR cells and aged satellite cells shared similar transcriptional changes. Furthermore, both had lower levels of Sprouty (spry1), an inhibitor of FGF signalling, which this group had previously identified as an important factor promoting satellite cell quiescence (Shea et al, 2010). These data suggested to the authors that the increase in NLR cells or a decrease in spry1 expression may explain the functional changes observed in aged satellite cells, but left an important question: what causes a satellite cell to become a NLR cell?
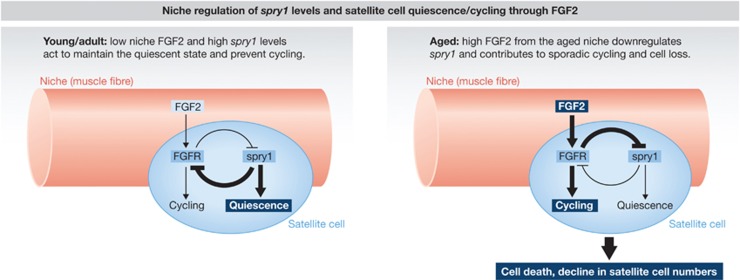
Seeking to identify the root of increased NLR cells in aged animals, the authors made a few very interesting observations that, when combined, lead to a fascinating hypothesis. First, they found that FGF2 signalling is elevated in aged animals and that the stem cell niche, that is, the muscle fibre, is the source of increased FGF2. Exposing satellite cells to FGF2 increases the propensity of cells to break quiescence and cycle, increases cell death, and, importantly, causes downregulation of spry1 expression. They also found that deletion of spry1 in adult satellite cells is sufficient for these satellite cell functional changes. Interestingly, transplantation of satellite cells that have reduced spry1 expression (aged or NLR cells) into control hosts (with presumably low niche FGF2 levels) results in decreased transplant efficiency and reduced contribution of the transplanted cells to muscle regeneration in a cell autonomous manner. Together, these data imply a model wherein a niche-derived signal, FGF2, causes downregulation of spry1, which in turn leads to a cell autonomous alteration in the functionality of satellite cells thereby wedding the niche/environmental and cell autonomous theories of stem cell aging (Figure 1).
Figure 1.
Molecular model integrating both niche/environmental and cell autonomous theories of stem cell aging. Left: low level of niche-derived FGF2 permits spry1-dependent stem cell quiescence. Right: age-induced high FGF2 causes downregulation of spry1, which in turn leads to a cell autonomous alteration in the functionality of satellite cells and ultimately translates into stem cell decline.
What is particularly intriguing about environmental theories of stem cell aging is that they imply that the functional changes that occur in stem cells as they age are potentially reversible when the cells are placed in a ‘young’ environment. In some ways, this study supports that notion showing that suppression of FGF signalling, mimicking a young environment, can rescue the effects of decreased spry1 expression in a population of aged or spry1 null satellite cells. However, it does not address the effect of age-associated, cell autonomous decline in spry1 expression. Does suppression of FGF signalling rescue spry1 expression in aged satellite cells? If not, the only way to protect against increased satellite cell cycling in aged animals is to suppress FGF signalling, which the authors show has potent negative consequences for muscle regeneration. Therefore, it may not be advantageous to protect stem cell quiescence if the effect is decreased regenerative functionality.
One striking piece of data presented in this study is that LR cells in aged animals have apparently been able to retain the H2B-GFP label throughout the life of the animal. An interesting follow-up question emerges regarding these cells: how did they retain label throughout aging? Are they cells that, statistically, have beaten the odds and have just not cycled based on chance. Or is this a unique population of cells that are resistant to FGF2-mediated suppression of spry1? The latter may be consistent with emerging evidence of a more ‘primitive’ population of satellite cells that have enhanced quiescent and self-renewal functional characteristics (Rudnicki et al, 2008; Rocheteau et al, 2012).
This report also touches on another issue that is commonly debated: the role of stem cell number in the effects of aging on tissue function and regeneration. The reported effects of aging on stem cell number vary widely across different stem cell populations but also within the satellite cell literature (Brack and Rando, 2007). Brack and colleagues report reduced satellite cell numbers in aged animals and attribute that decline to the functional changes they describe: decreased spry1 expression leading to increases in cycling and cell death. They propose that functional changes of aged satellite cells, which have previously been shown to impair muscle regeneration (Conboy et al, 2003), are further exacerbated by declining stem cell numbers. It will be interesting to determine if the maintenance of stem cell number can overcome their functional deficits to prevent an age-related decline in regenerative potential.
This study provides a unique model delineating how an environmental signal can translate into a cell autonomous change in functionality. It is also a nice demonstration of how dynamic the quiescent state likely is and that the signals that a stem cell receives in the quiescent state can impact stem cell function and tissue homeostasis/regeneration. Stem cell quiescence has been a poorly characterized cellular state due to the fact that current in vitro models do not satisfactorily recapitulate the in vivo state, making it notoriously difficult to study. Advances in genetic lineage tracing are making it easier to study stem cell quiescence in its physiological state. Such studies, similar to this report, are finding that quiescence state can be dynamic and cells are likely constantly monitoring environmental/niche signals (Cheung et al, 2012). It seems likely that this area of research is only going to expand and we will find that signals the stem cell receives in the quiescent state can programme the stem cell’s future functionality.
Footnotes
The authors declare that they have no conflict of interest.
References
- Brack AS, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3: 226–237 [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Jones KM, Basson MA, Brack AS (2012) The aged niche disrupts muscle stem cell quiescence. Nature (advance online publication 26 September 2012; doi:; DOI: 10.1038/nature11438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA (2012) Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482: 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA (2003) Notch-mediated restoration of regenerative potential to aged muscle. Science 302: 1575–1577 [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA (2012) Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 11: 2260–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauke K, de HG (2011) Polycomb group proteins in hematopoietic stem cell aging and malignancies. Int J Hematol 94: 11–23 [DOI] [PubMed] [Google Scholar]
- Liu L, Rando TA (2011) Manifestations and mechanisms of stem cell aging. J Cell Biol 193: 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S (2012) A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148: 112–125 [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Le GF, McKinnell I, Kuang S (2008) The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73: 323–331 [DOI] [PubMed] [Google Scholar]
- Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS (2010) Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 6: 117–129 [DOI] [PMC free article] [PubMed] [Google Scholar]