Abstract
EMBO J (2012) 31 21, 4124–4139 doi:; DOI: 10.1038/emboj.2012.270; published online October 02 2012
Some 20 years after original work positioned Runx1 as crucial for haematopoiesis and leukaemia formation (Miyoshi et al, 1991; Okuda et al, 1996), a study in this issue of the EMBO Journal (Scheitz et al, 2012) reveals that the RUNX1/Stat3 axis also promotes carcinogenesis in epithelial tissues.
RUNX1, the DNA-binding subunit of heterodimeric transcription factor CBF, was first identified as a fusion protein AML1-MTG8 (RUNX1-ETO) in t(8;21) acute myeloid leukaemia (Miyoshi et al, 1991). Subsequently, Runx1 KO mice were reported to be wholly incapable of generating hematopoietic stem cells (Okuda et al, 1996). These observations prompted extensive studies of RUNX1 in leukaemia and haematopoiesis.
Employing lineage tracing in the DMBA (9,10-dimethyl-1,2-benzanthracene)/TPA (12-O-tetradecanoylphorbol-13-acetate)-carcinogenesis protocol, Scheitz et al, 2012 now report that Runx1-expressing hair follicle stem cells (HFSC) are the source of squamous cell carcinomas (SSCs) in mice. While Runx1 is critical for tumour initiation and long-term maintenance, it was, paradoxically, not required for tumour promotion. Runx1 also appears to serve similar tumorigenic functions in oral SCC, as demonstrated in mice that conditionally express oncogencic KrasG12D. Interestingly, Runx1 expression was also observed at the crypt base of the intestinal epithelium, where it overlaps with Lgr5, an established gastrointestinal stem cell marker (Schuijers and Clevers, 2012). Consistent with these in vivo observations, RNAi-mediated depletion of RUNX1 in human skin and head and neck SCC cells resulted in growth arrest. Thus, although earlier work by the same group indicated that Runx1 is dispensable for differentiation of HFSC, they now establish that RUNX1 drives cancer stem cell proliferation.
In RUNX1-related leukaemia, perturbation of the RUNX1 function either by fusion to other gene products in chromosome translocations or mutations of the RUNX1 gene is a major causative factor leading to human leukaemia. Thus, RUNX1 has been widely recognized as suppressor for leukemogenesis. Therefore, the new evidence for an oncogenic function of RUNX1 raises the fundamental question whether Runx1 prevents or accelerates carcinogenesis.
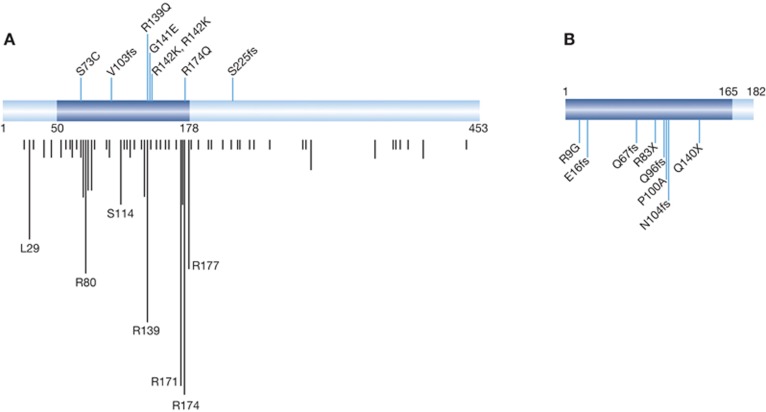
In addition to leukaemia, the involvement of RUNX1 in other cancer types recently emerged; genome-wide analyses such as next-generation sequencing and SNP array exhibited that not only RUNX1 but also the gene encoding the heterodimeric partner of all three RUNX proteins, CBFB, were mutated and of clinical significance in breast cancer (Ellis et al, 2012; Banerji et al, 2012). Deletions of RUNX1 in oesophagus cancer were also found (Dulak et al, 2012). Importantly, in the vast majority of cases, the genetic changes lead to loss of function, suggesting that RUNX1 and CBFB act as tumour suppressors rather than oncogenes (Figure 1, see references and the nature of mutations in legend). In addition, transposon insertion mutagenesis found that Runx1-coding regions are frequently disrupted in an animal model of intestinal tumorigenesis (H Takeda, N Jenkins and N Copeland, personal communication). Given that loss of function of CBFB would inactivate all three RUNX genes, the natural assumption would be that RUNX/CBFB complexes act as a tumour suppressor. However, the current paper reminds us that RUNX proteins can also have a ‘dark side’ as initiator of cancer formation.
Figure 1.
Mutations in the RUNX1 (A) and CBFB (B) genes. Distribution of eight RUNX1 mutations within RUNX1-coding region as reported in breast cancer. The evolutionally conserved and functionally important Runt domain is highlighted (in blue); all six missense mutations are located within Runt domain, which is responsible for both DNA binding and heterodimerization. Four of the mutations fell within two mutation hot spots (amino-acid position 174 and 139/141/142). Vertical black lines indicate respective mutations reported previously ( http://www.sanger.ac.uk/genetics/CGP/). The length of lines represents cumulative number of mutations. Two frameshift (fs) mutations that result in truncated proteins are as indicated. Notably, all breast cancer-related RUNX1 alterations appear to generate loss-of-function mutants. (B) Distribution of eight CBFB mutations along CBFB/PEBP2β-coding region as reported in breast cancer. RUNX-binding domain is indicated in blue. Six of the mutations are either fs or nonsense, both of which result in non-functional proteins. The remaining two missense mutants (R9G and P100A) are also expected to produce non-functional proteins; R9 and P100 are located within α1 helix and β5 sheet, respectively, both of which form the Runt–CBFβ interface. As a consequence, all of these CBFB genetic changes are likely to result in loss-of-function mutants.
Retrospectively, the early retrovirus insertion mutagenesis studies to screen for genes collaborating with c-Myc during T-cell lymphoma development implicate all three Runx as putative oncogenes (Blyth et al, 2005). Curiously, there are also reports describing RUNX3 as an oncogene in skin cancer, head and neck SSC and ovarian cancer, although RUNX3 is considered a tumour suppressor in most tissues (Chuang and Ito, 2010). It is noteworthy that the above tissues in which oncogenic RUNX3 activity was detected were studied in the current paper. Except for ovarian cancer, the common denominator seems to be SSC. This suggests the attractive possibility of a tissue-specific conversion of Runx function in carcinogenesis. It would be worth exploring whether the oncogenic role of RUNX proteins is unique to squamous cells.
The dual and opposite functions of Runx genes have also been extensively studied: RUNX proteins can activate or repress target gene expression depending on whether it interacts with co-activator or corepressor (Blyth et al, 2005). Therefore, as a consequence of context-dependent regulation of target genes expression, RUNX proteins may exert—relative to cell context—tumour suppressive or oncogenic activity. The current paper supports a concept of dual functionality of Runx in carcinogenesis in the field. Given that all three RUNX proteins recognize common DNA sequence motifs, it would be extremely informative to ascertain whether a similar scenario applies to RUNX2 and RUNX3 in exerting dual functions during cancer formation of different tissues.
Antagonistic interplay between RUNX family proteins might also influence cell proliferation. When B cells are immortalized by EBNA-2 of Epstein Barr virus, cells show increased RUNX3 and decreased RUNX1 expression. Following the depletion of RUNX3, cells stop growing; the concomitant increase of RUNX1 suggests an important biological role for RUNX3 to keep B cells immortal, presumably through the regulation of RUNX1 (Brady et al, 2009). Cross regulation among RUNX genes (e.g., RUNX2 and RUNX3) has also been observed in breast cancer cell lines (Chuang and Ito, 2010).
RUNX genes regulate cell specification in development and RUNX1 has been shown to interact with chromatin remodelling proteins (Yu et al, 2012). If we consider anomalous cell differentiation as a cause of cancer, it is likely that we will, in the near future, hear more about the respective roles of RUNX genes in human solid tumours, and thus clarify the ‘bright’ and ‘dark’ sides of RUNX in tumour formation.
References
- Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L, Cortes ML, Fernandez-Lopez JC, Peng S, Ardlie KG, Auclair D, Bautista-Piña V, Duke F, Francis J, Jung J, Maffuz-Aziz A et al. (2012) Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 486: 405–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth K, Cameron ER, Neil JC (2005) The RUNX genes: gain or loss of function in cancer. Nat Rev Cancer 5: 376–387 [DOI] [PubMed] [Google Scholar]
- Brady G, Whiteman HJ, Spender LC, Farrell PJ (2009) Down-regulation of RUNX1 by RUNX3 requires the RUNX3 VWRPY sequence and is essential for Epstein-Barr virus-driven B-cell proliferation. J Virol 83: 6909–6916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang LS, Ito Y (2010) RUNX3 is multifunctional in carcinogenesis of multiple solid tumors. Oncogene 29: 2605–2615 [DOI] [PubMed] [Google Scholar]
- Dulak AM, Schumacher SE, van Lieshout J, Imamura Y, Fox C, Shim B, Ramos AH, Saksena G, Baca SC, Baselga J, Tabernero J, Barretina J, Enzinger PC, Corso G, Roviello F, Lin L, Bandla S, Luketich JD, Pennathur A, Meyerson M et al. (2012) Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res 72: 4383–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, Van Tine BA, Hoog J, Goiffon RJ, Goldstein TC, Ng S, Lin L, Crowder R, Snider J, Ballman K, Weber J, Chen K, Koboldt DC, Kandoth C, Schierding WS et al. (2012) Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486: 353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M (1991) t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci USA 88: 10431–10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR (1996) AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84: 321–330 [DOI] [PubMed] [Google Scholar]
- Scheitz CJF, Lee TS, McDermitt DJ, Tumbar T (2012) Defining a tissue stem cell-driven Runx1/Stat3 signalling axis in epithelial cancer. EMBO J 31: 4124–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijers J, Clevers H (2012) Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. Embo J 31: 2685–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Mazor T, Huang H, Huang HT, Kathrein KL, Woo AJ, Chouinard CR, Labadorf A, Akie TE, Moran TB, Xie H, Zacharek S, Taniuchi I, Roeder RG, Kim CF, Zon LI, Fraenkel E, Cantor AB (2012) Direct recruitment of polycomb repressive complex 1 to chromatin by core binding transcription factors. Mol Cell 45: 330–343 [DOI] [PMC free article] [PubMed] [Google Scholar]