Abstract
The first human transfusion was performed by the pioneer Dr Jean-Baptiste Denis in France in 1667 and now, three centuries later, around 50 millions blood units are transfused every year, saving millions of lives. Today, there is a new application for red blood cells (RBCs) in cellular therapy: the effective use of erythrocytes as vehicles for chemical or biological drugs. Using this approach, the therapeutic index of RBC-entrapped molecules can be significantly improved with increased efficacy and reduced side effects. This cell-based medicinal product can be manufactured at an industrial scale and is now used in the clinic for different therapeutic applications.
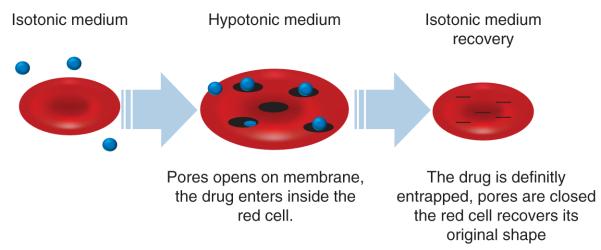
A seminar dedicated to this field of research, debating on this inventive formulation for drugs, was held in Lyon (France) on 28 January 2011. Drs KC Gunter and Y Godfrin co-chaired the meeting and international experts working on the encapsulation of drugs within erythrocytes met to exchange knowledge on the topic ‘The Red Blood Cells as Vehicles for Drugs’. The meeting was composed of oral presentations providing the latest knowledge and experience on the preclinical and clinical applications of this technology. This Meeting Highlights article presents the most relevant messages given by the speakers and is a joint effort by international experts who share an interest in studying erythrocyte as a drug delivery vehicle. The aim is to provide an overview of the applications, particularly for clinical use, of this innovative formulation. Indeed, due to the intrinsic properties of erythrocytes, their use as a drug carrier is one of the most promising drug delivery systems investigated in recent decades. Of the different methods developed to encapsulate therapeutic agents into RBCs [1,2,] the most widely used method is the lysis of the RBCs under tightly controlled hypotonic conditions in the presence of the drug to be encapsulated, followed by resealing and annealing under normotonic conditions (Figure 1). This results in uniform encapsulation of the material into the cells and a final product with good stability, reproducibility and viability. This process, which has now been developed to an industrial scale, is the technique chosen by the majority of the experts presenting their work in this seminar (by R Franco).
Keywords: carriers, drug delivery, erythrocytes, red blood cells, targeting
1. Therapeutic enzyme-loaded RBCs
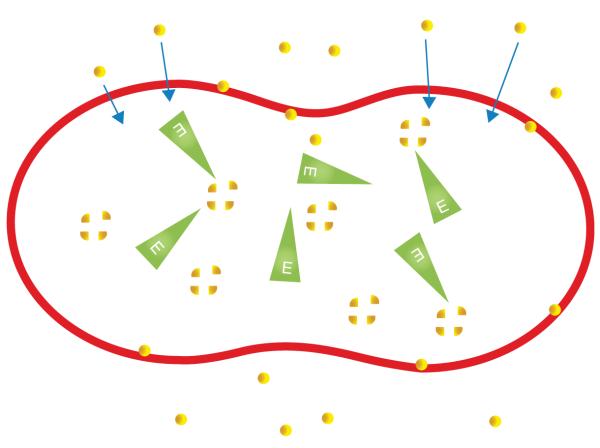
Therapy using RBC encapsulated enzymes has the advantage of prolonging the half-life of the enzyme and maintaining therapeutic blood levels, reducing the dosage and frequency of therapeutic interventions, and preventing the need for expensive chemical modification [3]. The therapeutic index can be strongly improved, especially by reducing immunogenic reactions, which are often observed in enzyme replacement (Figure 2).
Figure 2. The red blood cell as a bioreactor: the substrate (yellow) contained in the plasma permeates the erythrocyte membrane, with the entrapped enzyme (green) catalyzing the metabolism of the substrate to its normal product inside the red cell.
The membrane also protects the enzyme against antibody binding, thus avoiding the hypersensitivity reactions and clearance from the vascular compartment.
1.1 L-asparaginase-loaded RBCs for ASNS-deficient tumor (by E Dufour)
L-asparaginase has been used in the treatment of acute lymphoblastic leukemia for > 40 years. This enzyme converts plasmatic L-asparagine (L-Asn) into L-aspartate plus ammonia. Its use is motivated by the fact that malignant cells (especially leukemic) are deficient in asparagine synthetase (ASNS). Because these cells are unable to synthesize L-Asn to meet metabolic demands, L-Asn deprivation, due to L-asparaginase activity, kills the cancerous cells. However, L-asparaginase can also be responsible for adverse events such as hypersensitivity reactions or blood coagulations disorders, in addition to L-Asn depletion. An approach to decreasing side effects of free L-asparaginase in vivo is to entrap the enzyme in RBCs. Reversible hypotonic dialysis remains the most controlled and reproducible method. Indeed, with this process, human RBCs can be loaded with 116 ± 15 IU of L-asparaginase per milliliter of red cells. The resulting product acts as a bioreactor allowing transport of L-Asn through the RBC membrane where L-asparaginase hydrolyzes it. Due to the RBC membrane, the enzyme is protected from rapid catabolism as well as from potential neutralizing antibodies, resulting in an increased half-life and a reduction in hypersensitivity reactions. A Phase I–II trial testing GRASPA® (ERYTECH Pharma, France) on 24 patients in relapsed acute lymphoblastic leukemia showed a strong reduction in hypersensitive reactions, coagulation disorders and hepatic dysfunctions [4]. The L-asparaginase half-life is enhanced (40 days vs 1 day with the free form) and the mean duration of L-Asn depletion is 18.57 days at a dose of 150 IU/kg in a single injection that corresponds to eight injections of Escherichia coli native L-asparaginase.
This improvement in tolerance allows the introduction of L-asparaginase treatment to other hematological malignancies, such as acute myeloid leukemia, and also in solid tumors. Indeed, the level of expression of L-ASNS, the enzyme responsible for the synthesis of L-Asn in mammalian cells, provides a rationale for testing L-asparaginase in several cancers. For example, about 30 and 40% of pancreatic ductal adenocarcinoma patients (85 – 90% of all pancreatic cancer subjects) have no or low level of expression of ASNS, respectively. A Phase I clinical study is ongoing with pancreatic adenocarcinoma patients.
1.2 Thymidine phosphorylase-loaded RBCs for MNGIE (by BE Bax)
Mitochondrial neurogastroinstestinal encephalomyopathy (MNGIE) is caused by mutations in the gene encoding for the enzyme thymidine phosphorylase, resulting in a complete or partial absence of enzyme activity, and leading to a plasma and tissue accumulation of thymidine and deoxyuridine. This is thought to generate imbalances in the mitochondrial nucleotide pools, leading to damage to mitochondrial DNA and ultimately mitochondrial failure. The consequent failure of cellular energy production directly causes the central clinical manifestation, degeneration of the peripheral nervous system, including the innervation of the alimentary tract, which causes severe gastrointestinal dysmotility, and peripheral polyneuropathy. MNGIE is relentlessly progressive and patients have a shortened life span, with death occurring during early to middle adulthood. There is no recognized specific treatment for MNGIE, and clinical management is based on symptom relief and palliation. In a compassionate patient evaluation, in a named patient treatment, it was observed in a first patient after 210 days of treatment with thymidine phosphorylase-loaded erythrocyte, the daily excretion of thymidine (150 μmol before treatment) and deoxyuridine (260 μmol before treatment) decreased below 63 μmol. This proof of concept was confirmed in a second patient in whom plasma concentrations of thymidine and deoxyuridine were reduced to zero (pretreatment concentrations were 20.5 and 30.6 μmol/l, respectively). Erythrocyte encapsulated thymidine phosphorylase is currently being developed as a therapy for MNGIE, and it was recently awarded the Orphan Medicinal Product Designation by the US FDA and European Medicine Agency [5].
1.3 ADA-loaded erythrocytes for immunodeficiency (by BE Bax)
A deficiency of adenosine deaminase (ADA) leads to elevated cellular levels of deoxyadenosine triphosphate (dATP) and systemic accumulation of its precursor, 2-deoxyadenosine. These metabolites impair lymphocyte differentiation, proliferation and function, and inactivate S-adenosylhomocysteine hydrolase (SAHH), respectively, leading to severe immunodeficiency. One adult-type ADA deficient patient has been treated for nearly 14 years with erythrocyte encapsulated native ADA [6]. The patient is without a matched bone marrow donor and developed anti-ADA neutralizing antibodies to PEG-conjugated ADA. Therapeutic efficacy has been determined by monitoring immunological and metabolic parameters. For the past 12 months, erythrocyte dATP concentrations ranged between 0 and 36 μmol/l (diagnosis, 234; normal value, 0) and SAHH activity between 2.55 and 3.63 nmol/h/mg hemoglobin (diagnosis, 0.34; normal range, 3.6 – 9). Erythrocyte ADA activities have been above the reference range of 40 – 100 nmol/h/mg hemoglobin (0 at diagnosis). The percentages of CD3+, CD4+ and CD8+ T cells are 74% (reference range 58 – 91%), 28% (reference range 27 – 61%) and 61% (reference range 14 – 46), respectively. CD16+ NK cells have increased gradually from a pretreatment value of 0 to 31% (reference range 9 – 16%), and CD20+ B cells from 2 to 9% (reference range 12 – 22%). The patient tolerates the regime of 2-weekly treatment cycles well and has remained clinically stable.
1.4 GS-loaded erythrocytes for ammonia detoxification (by E Kosenko)
High ammonia concentration causes functional disorders in the CNS, which can lead to coma and death. Glutamine synthetase (GS) offers a potential enzymatic detoxification pathway, but has not been previously studied and thus Kosenko et al. investigated the capacity of erythrocytes loaded with GS (GS ammocytes) to remove ammonia from the blood of hyperammonemic mice [7]. The procedure allowed the encapsulation of 3 IU of GS per milliliter of erythrocytes with a 70% cell recovery. Most metabolites and metabolic parameters, including the ATP level and cytosolic NAD:NADH ratio, endogenous phosphofructokinase, glucose-6-phosphate dehydrogenase, hexokinase, lactate dehydrogenase, pyruvate kinase and Na+, K+-ATPase activities, remained unaltered in GS ammocytes compared with native erythrocytes. The mouse GS ammocytes injected into mice survived and retained essentially all of their GS activity for at least 48 h in vivo. Injection of mouse GS ammocytes into hyperammonemic mice reduced blood ammonia levels by about 50%. These are functionally active cells and can be used as a protective system in pathological hyperammonemia, while the method can be regarded as a new technology for medical and veterinary practice.
1.5 Blood coagulation factor IX-loaded erythrocytes to improve pharmacokinetics in hemophilia B (by E Sinauridze)
Hemophilia B is a disorder of blood coagulation caused by an absence or decreased activity of coagulation factor IX. The utilization of erythrocytes as carriers for factor IX has the potential to increase intervals between infusions. In addition, the foreign protein is concealed within an organism’s own cells thereby escaping immune recognition.
Sinauridze et al. studied the use of erythrocytes as carriers for antihemophilic factor IX. To be functional, factor IX is required in the plasma compartment, outside the carrier cells [8], and as RBC carriers are naturally destroyed within the vessels at a low rate, encapsulated drug is released gradually into the circulation [9].
The lifetime of encapsulated factor in the circulation was shown to be 5 – 10 times longer compared to its free form (t1/2 were equal to ~ 73.9 ± 16 and 8.9 ± 5.6 h, respectively). The natural destruction of loaded RBC carriers follows first-order kinetics, and provides a sufficiently high plasma concentration of factor IX over 15 days. Thus, factor IX-loaded RBC offers a promising approach in the clinical setting.
2. Anti-infective drug-loaded RBCs
2.1 IFN-α and RIBA-loaded erythrocytes for treatment of hepatitis C (by OA Skorokhod)
IFN-α and ribavirin (RIBA) are established treatments for hepatitis C. Therapeutic success is moderate, but can be significantly improved by raising IFN-α dosage and associating antivirals such as RIBA. High toxicity limits increase of dosage of both drugs [10]. The aim of Skorokhod et al. was to find innovative ways of enhancing delivery of IFN-α and RIBA to the liver. Homologous or donor erythrocytes loaded with both drugs (IFN-α–ribavirin-loaded red blood cells; RBC-IFN-α-RIBA) and made susceptible to the action of phagocytes by opsonization induce their selective removal by targeting them to the liver macrophages. Encapsulation of IFN-α and RIBA into RBC and targeting to the liver allows: i) organ-specific liberation of high amounts of IFN-α and attainment of higher therapeutically efficient concentrations in the liver; ii) autocrine stimulation of liver (and spleen) macrophages by IFN-α to enhance cell-mediated antiviral defense; and iii) control of viral proliferation within the macrophage. A simplified procedure has been developed to encapsulate IFN-α and RIBA in small volumes of RBCs based on the reversible osmotic lysis method. Results showed the efficiency of IFN-α and RIBA encapsulation was 40% and RBC recovery 60%. RBC-IFN-α-RIBA could be stored up to 3 days at +4°C with retention of IFN-α-RIBA antiviral activity. In addition, it has been shown that human monocytes were activated after ingestion of RBC-IFN-α-RIBA. The induction of surface MHC class II and FC receptors was observed using flow cytometry, and the expression of IFN-α-induced genes, OAS, IRF1 and myxovirus resistance protein A, was demonstrated using quantitative RT-PCR.
IFN-α and RIBA encapsulation in RBCs and targeting of these cells to liver macrophages is a novel approach for the treatment of hepatitis C which deserves further investigation in animal models.
2.2 Anthracycline antibiotics-loaded erythrocytes to reduce side effects (by V Vitvitsky)
Anthracycline antibiotics are among the most utilized antitumor drugs. However, their use is limited by significant cardiotoxicity and other severe side effects. Erythrocytes are among the promising drug carriers to enhance drugs efficacy while reducing their toxicity. Pharmacokinetics of daunorubicin was studied in 14 patients with acute leukemia who received infusions of daunorubicin-loaded erythrocytes [11]. In addition, tolerability of daunorubicin-loaded erythrocytes was evaluated in 26 patients with acute leukemia. After administration of daunorubicin-loaded erythrocytes, daunorubicin peak concentration in blood and plasma reached 20 – 30% of the values obtained with standard (free) daunorubicin form. Daunorubicin clearance from circulation was described using two exponential models with fast (T1) and slow (T2) specific times. In the case of daunorubicin-loaded erythrocytes, administration T1 increased about fourfold and T2 increased about twofold in both blood and plasma, compared to free drug administration. Correspondingly, the AUC increased about 2.5-fold in the case of daunorubicin-loaded erythrocytes administration. Daunorubicin-related toxic effects (particularly nonspecific, non-hematological effects) were significantly reduced in patients receiving daunorubicin-loaded erythrocytes. None of the patients developed clinical or echocardiographic signs of direct cardiotoxicity.
Similarly, pharmacokinetics and tolerability of doxorubicin-loaded erythrocytes were studied in 15 lymphoma patients [12]. Doxorubicin peak concentration decreased by about 60%, T1 and T2 increased two to sevenfold and the AUC increased about five to sixfold in case of doxorubicin-loaded erythrocytes administration, compared with the standard doxorubicin form. No prolonged or severe myelosuppression and cardiotoxicity were observed. Moreover, the doxorubicin-loaded erythrocytes were successfully infused without any negative consequences to one patient who had earlier responded to the standard doxorubicin form with strong paroxysmal tachycardia and cardialgia, and to another patient presenting ciliary arrhythmia.
2.3 Antibiotic-loaded erythrocytes to treat resistant infections due to macrophage retention of the pathogens (by JM Lanao)
Lanao and co-workers are interested in improving the biodistribution of anti-infective drugs in peritoneal macrophages and other tissues using erythrocytes carriers [13]. The aminoglycoside amikacin and the antiretroviral zidovudine were used as model drugs in the rat. The tissue pharmacokinetics of amikacin using erythrocytes in comparison with a control group revealed an accumulation of the antibiotic in specific tissues such as the liver and spleen, a similar pharmacokinetics in the lung, and moderate changes in the pharmacokinetics in the kidney. Studies of tissue concentrations after the injection of glutaraldehyde-treated amikacin-loaded erythrocytes demonstrated important changes in organs of the reticuloendothelial system (RES) in comparison with the results observed for standard erythrocyte carriers, higher levels being observed in the liver whereas spleen levels decreased [14]. When amikacin-loaded erythrocytes were administered by the intraperitoneal (i.p.) route in rats induced with thioglycolate, a higher accumulation in macrophages in vivo was observed. According to the partition coefficients obtained, the relative uptake of amikacin when encapsulated in erythrocytes was spleen > peritoneal macrophages > liver > lung > renal cortex > renal medulla [15]. In the case of zidovudine, the administration of the antiretroviral drug by i.p route using erythrocyte carriers produced significant changes in the plasma and tissue pharmacokinetics with an increase in the zidovudine plasma levels and in the tissue levels and terminal half-life in specific tissues such as spleen, lung, kidney, peritoneal macrophages and monocytes from bone marrow. This shows that loaded erythrocytes are potentially useful for the delivery of anti-infective drugs in specific tissues and in phagocytic cells located in the RES.
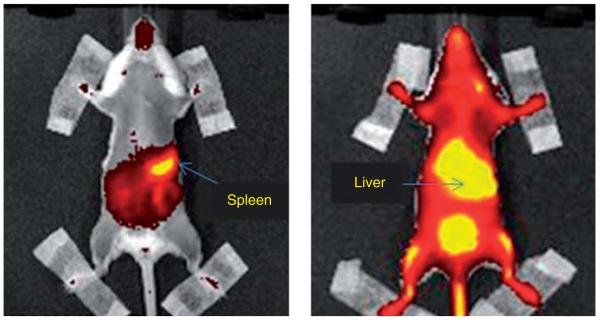
3. Immunomodulation with antigens-loaded RBCs (by A Banz)
The adaptive immune system relies on the capacity of immune cells to distinguish between self and non-self antigens. Dysfunction in this system, such as inappropriate antigen recognition, can result in autoimmune diseases and cancers characterized by inadequate antigen-specific immune responses. The erythrocyte is a convenient system to deliver in vivo antigens to antigen-presenting cells (APCs) leading to the induction or inhibition of antigen-specific immune responses. Using ovalbumin (OVA) as an antigen, we have shown that depending on the treatment performed on the RBC membrane, heat or chemical (BS3-crosslinker), the antigen is delivered to APCs either in the spleen or liver, respectively (Figure 3). The delivery in the spleen in the presence of an immune stimulator, Poly (I:C), induced an increase of OVA-specific CD8 T cells at a higher rate than those from mice injected with free OVA (12% for OVA-entrapped into RBC vs 2% for free OVA). Furthermore, these cells are able to induce OVA-specific cellular lysis (83 vs 53% for free OVA) [16]. Inversely, the antigen delivery in the liver in the absence of an immune stimulator was able to reduce OVA-specific CD8 T-and B-cell responses induced by OVA immunization. In both cases, the responses were dependent on the dose of antigen entrapped and the quantity of RBC injected per mice. These results support the use of RBC as an approach for immunotherapy, cancer and inducing tolerance in autoimmune diseases, or in patients who developed hypersensitivity reactions following repeated drug administration.
Figure 3. RBC targeting to spleen or liver by heat or chemical treatment of the RBC membrane, respectively.
RBC: Red blood cell.
4. Hb allosteric effector-loaded erythrocytes for oxygen release improvement in sickle cell anemia (by V Bourgeaux)
Sickle cell disease (SCD) is a genetic disorder characterized by abnormal hemoglobin (Hb) S that polymerizes under hypoxic conditions leading to sickle-shape RBCs (SS-RBCs). For patients who already experienced vaso-occlusive crisis or stroke, chronic RBCs exchanges are commonly used to prevent further pain episodes or a second stroke [17]; the aim is to dilute patients’ SS-RBCs with normal RBCs to lower the probability of recurrence. The effectiveness of the RBC exchanges can potentially be improved by the entrapment of an allosteric effector of Hb into normal donor RBCs. Indeed, these ‘modified’ RBCs are able to release oxygen at a higher oxygen pressure than SS-RBCs, leading to a higher venous oxygen partial pressure that may significantly reduce sickling. Among available allosteric effectors, inositol hexaphosphate (IHP) which contains six phosphate groups is the most suitable molecule for both efficient Hb binding and RBC entrapment (no possible leakage with negative charges). An in vitro study performed with blood samples from 20 homozygous patients showed that the use of IHP-loaded RBCs (IHP-RBCs) is seven times more effective in reducing sickling than normal RBCs [18]. In vivo proof of concept was performed in the BERK transgenic mouse model that mimics human SCD in childhood with specific features of splenomegaly and reticulocytosis. IHP-RBCs were prepared by loading IHP into murine C57BL6J RBCs using the reversible hypotonic lysis method. Washed murine RBCs were used as a control suspension. The study included repeated RBC exchanges scheduled every 2 weeks using either IHP-RBCs or control suspension. Preliminary results are very encouraging showing increased overall survival (87% for IHP-RBCs vs 67% for control suspension over a period of 7 weeks) and prevention of anemia even after subjecting mice to hypoxic stress (Hb > 7 g/dl for IHP-RBCs vs 5.5 g/dl for control suspension). A decrease in endothelial cell activation and inflammatory processes was also observed in BERK mice treated with IHP-RBCs. In conclusion, both in vitro and in vivo studies bring evidence of the therapeutic potential of IHP-RBCs in SCD. This approach may allow reducing frequency and volumes of RBC exchanges, thus, limiting the emerging problem of blood availability.
5. Conclusion
The work described here illustrates the therapeutic potential for erythrocytes as drug delivery vehicles, which will enable the targeted and less toxic delivery of agents for a variety of disease states. Red cells may be loaded with enzymes to protect against rapid catabolism, diminished toxicity and reduced immunogenicity. The targeted removal of RBCs in the RES may be utilized to enhance the delivery of molecules for modulation of the immune system. Anti-infective agents may also be delivered to the RES system in this manner. The membrane of RBCs may be modified to more effectively target different organs of the RES. In the case of anthracycline-based cytotoxic agents, the cardiotoxic effects may be attenuated with RBC encapsulation. Finally, entrapment of an allosteric effector of Hb may represent a new and promising approach in the treatment of anemic and ischemic diseases. Although the meeting focused mainly on encapsulated drugs alternative strategies such as anchoring therapeutic proteins and fusion to the surface of RBC offer the potential to address additional pathologies, for example, thromboprophylaxis [19]. Based on the variety of approaches, the future of RBCs as drug delivery vehicles is very promising.
6. Expert opinion
The field of cell therapy is rapidly advancing and the exciting work described above illustrates how the intrinsic properties of a simple and specialized cell, the erythrocyte, may be used to deliver drugs and treat a variety of disorders. These advances have been made possible by the profound understanding and considerable experience with RBCs in transfusion medicine settings. In the future, it is possible that other cell types currently in use in cell therapy may be used to delivery therapeutic agents. Such advances will be dependent on a thorough comprehension of the biological properties and in vivo trafficking of these various cell types. In addition, the immunology of RBCs is well understood and matching procedures to enable optimal in vivo half-life of RBCs in allogeneic recipients are currently in place. The work described here illustrates a new paradigm for cell therapy. Currently cell therapies tend to be focused on use of the natural properties of cells for treatment of disease (e.g., hematopoietic stem cell transplantation and mesenchymal stromal cells for immune modulation). However, there are significant opportunities to improve the safety and efficacy of existing treatments if cells can be made to deliver therapeutic agents efficiently. It is possible that this new delivery paradigm will enable the use of therapeutic agents at lower toxicity and increased efficacy. Finally, the manufacturing process can be and must be industrial and reliable to be able to provide such medicinal products for clinical use, meaning to be available on time for the treatment scheduled. This approach to vehicle drugs through the RBCs is very appropriate for life-threatening diseases and where the toxicity of the drug is a limitation. This process is also especially well adapted for rare and/or orphan diseases.
Figure 1. The process of reversible hypotonic lysis of RBCs to entrap molecules.
RBCs are submitted to a hypotonic stress creating pores in the erythrocyte membrane. Drug can pass through the pores and be permanently entrapped after a resealing step using a hypertonic solution.
RBC: Red blood cell.
Footnotes
Declaration of interest Y Godfrin is the co founder and executive vice president of ERYTECH Pharma. F Horand, A Banz, V Bourgeaux, E Dufour are employees of ERYTECH Pharma.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hamidi M, Tajerzadeh H. Carrier erythrocytes: an overview. Drug Deliv. 2003;10:9–20. doi: 10.1080/713840329. [•• This reference has considerable importance because it details the entrapment process.] [DOI] [PubMed] [Google Scholar]
- 2.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv. 2010;7:403–27. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bax BE, Bain MD, Talbot PJ, et al. Survival of human carrier erythrocytes in vivo. Clin Sci (Lond) 1999;96:171–8. [PubMed] [Google Scholar]
- 4.Domenech C, Thomas X, Chabaud S, et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. Br J Haematol. 2011;153:58–65. doi: 10.1111/j.1365-2141.2011.08588.x. [• This reference is important because it details the most recent and important clinical trial using red cells as vehicle for drug.] [DOI] [PubMed] [Google Scholar]
- 5.Moran NF, Bain MD, Muqit MMK, Bax BE. Carrier erythrocyte entrapped thymidine phosphorylasetherapy for MNGIE. Neurology. 2008;71:686–8. doi: 10.1212/01.wnl.0000324602.97205.ab. [DOI] [PubMed] [Google Scholar]
- 6.Bax BE, Bain MD, Fairbanks LD, et al. A 9-yr evaluation of carrier erythrocyte encapsulated adenosine deaminase (ADA) therapy in a patient with adult-type ADA deficiency. Eur J Haematol. 2007;79:338–48. doi: 10.1111/j.1600-0609.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 7.Kosenko EA, Venediktova NI, Kudryavtsev AA, et al. Encapsulation of glutamine synthetase in mouse erythrocytes: a new procedure for ammonia detoxification. Biochem Cell Biol. 2008;86:469–76. doi: 10.1139/O08-134. [DOI] [PubMed] [Google Scholar]
- 8.Sinauridze EI, Vuimo TA, Kulikova EV, et al. A new drug form of blood coagulation factor IX: red blood cell-entrapped factor IX. Med Sci Monit. 2010;16:I19–26. [PubMed] [Google Scholar]
- 9.Biagiotti S, Paoletti MF, Fraternale A, et al. Drug delivery by red blood cells. IUBMB Life. 2011;63:621–31. doi: 10.1002/iub.478. [DOI] [PubMed] [Google Scholar]
- 10.Sulkowski MS. Anemia in the treatment of hepatitis C virus infection. Clin Infect Dis. 2003;37(Suppl 4):S315–22. doi: 10.1086/376911. [DOI] [PubMed] [Google Scholar]
- 11.Skorokhod OA, Garmaeva TT, Vitvitsky VM, et al. Pharmacokinetics of erythrocyte-bound daunorubicin in patients with acute leukemia. Med Sci Monit. 2004;10:I55–64. [PubMed] [Google Scholar]
- 12.Skorokhod O, Kulikova EV, Galkina NM, et al. Doxorubicin pharmacokinetics in lymphoma patients treated with doxorubicin-loaded eythrocytes. Haematologica. 2007;92:570–1. doi: 10.3324/haematol.10770. [DOI] [PubMed] [Google Scholar]
- 13.Briones E, Colino CI, Lanao JM. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J Control Release. 2008;125:210–27. doi: 10.1016/j.jconrel.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez MC, Zarzuelo CA, Gonzalez LF, et al. Pharmacokinetics and biodistribution of amikacin encapsulated in carrier erythrocytes. J Antimicrob Chemother. 2008;61:375–81. doi: 10.1093/jac/dkm477. [DOI] [PubMed] [Google Scholar]
- 15.Briones E, Colino CI, Millan CG, Lanao JM. Increasing the selectivity of amikacin in rat peritoneal macrophages using carrier erythrocytes. Eur J Pharm Sci. 2009;38:320–4. doi: 10.1016/j.ejps.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Banz A, Cremel M, Rembert A, Godfrin Y. In situ targeting of dendritic cells by antigen-loaded red blood cells: a novel approach to cancer immunotherapy. Vaccine. 2010;28:2965–72. doi: 10.1016/j.vaccine.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Wahl S, Quirolo KC. Current issues in blood transfusion for sickle cell disease. Curr Opin Pediatr. 2009;21:15–21. doi: 10.1097/MOP.0b013e328321882e. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeaux V, Hequet O, Campion Y, et al. Inositol hexaphosphate-loaded red blood cells prevent in vitro sickling. Transfusion. 2010;50:2176–84. doi: 10.1111/j.1537-2995.2010.02663.x. [DOI] [PubMed] [Google Scholar]
- 19.Zaitsev S, Spitzer D, Murciano JC, et al. Sustained thromboprophylaxis mediated by an RBC-targeted pro-urokinase zymogen activated at the site of clot formation. Blood. 2010;115:5241–8. doi: 10.1182/blood-2010-01-261610. [DOI] [PMC free article] [PubMed] [Google Scholar]