Abstract
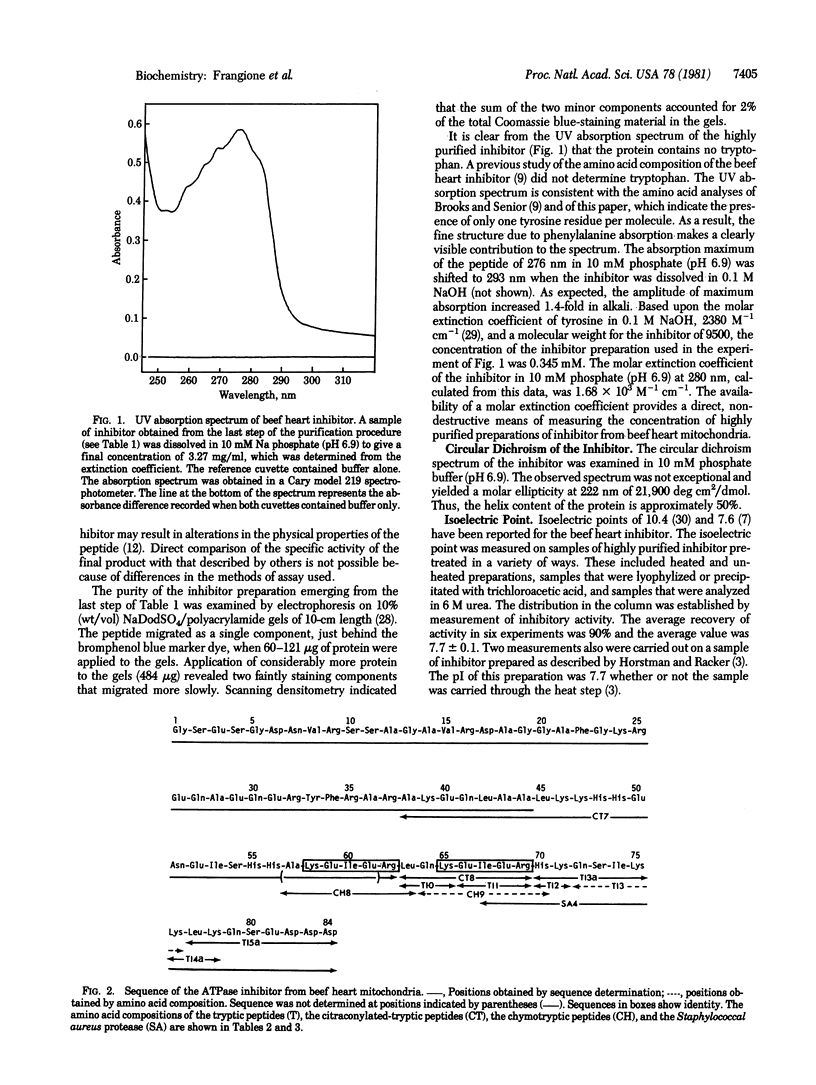
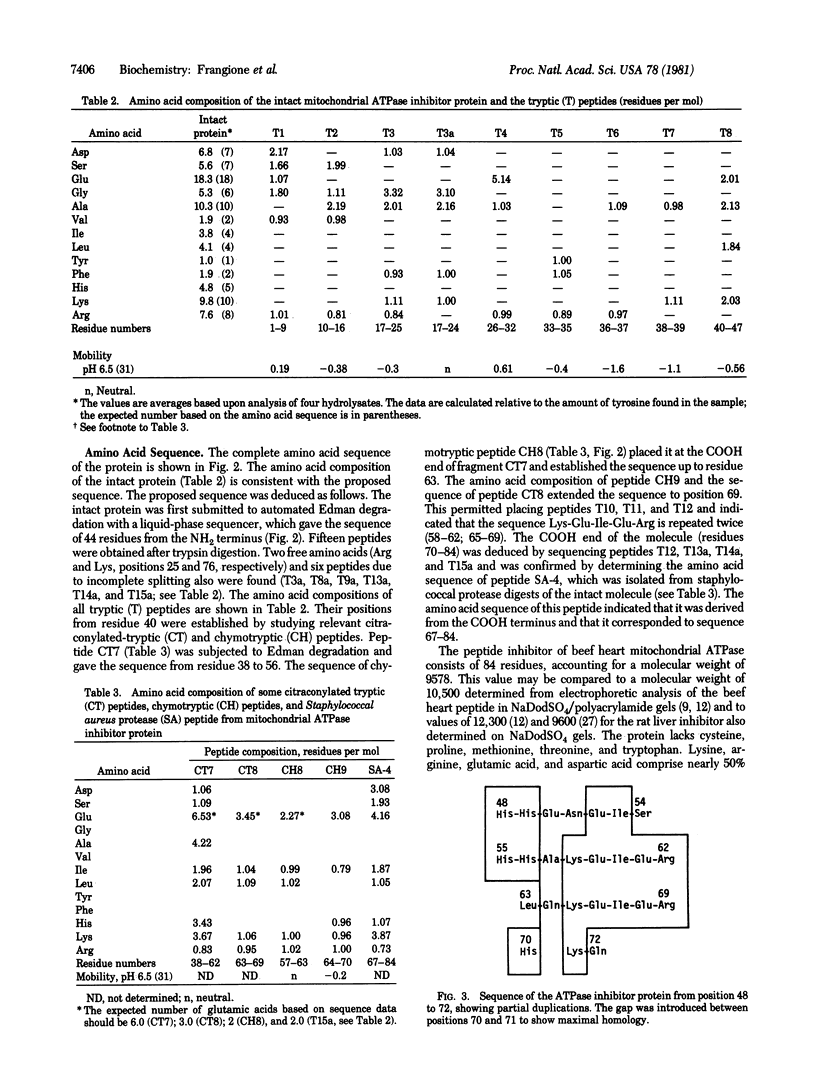
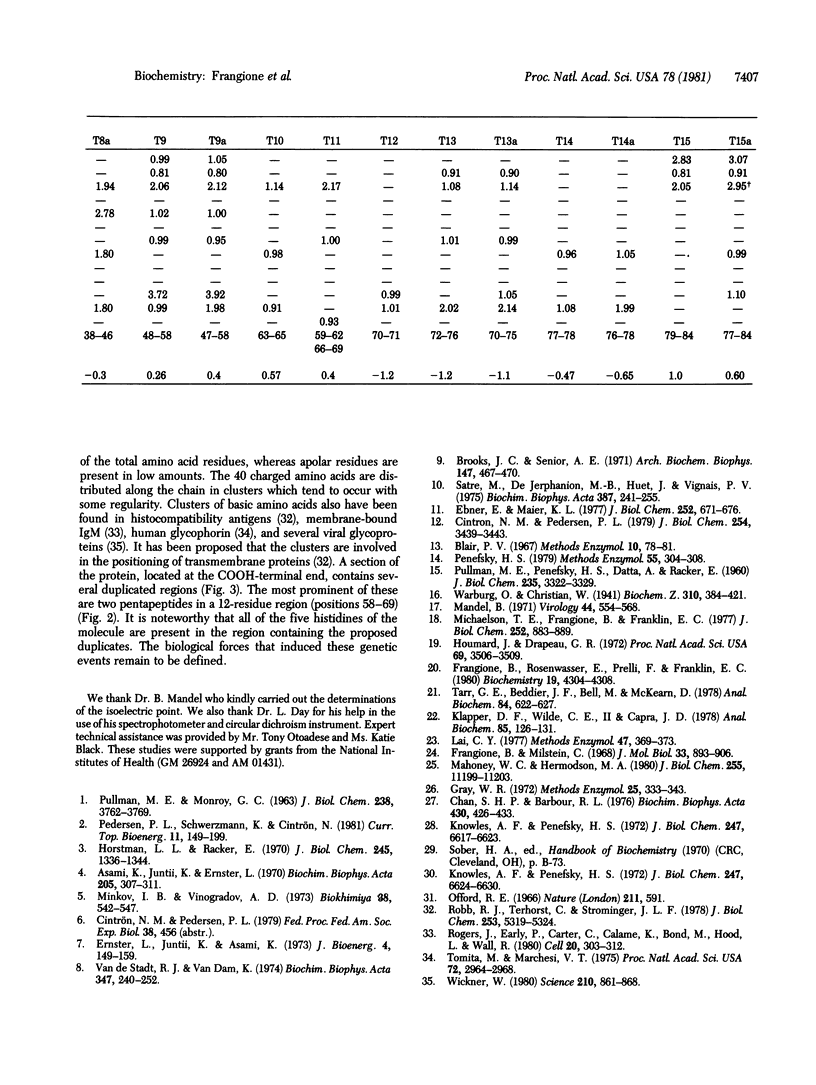
The complete amino acid sequence of the mitochondrial ATPase inhibitor peptide was determined. The molecule contains 84 residues of which 40 are charged amino acids that occur in clusters along the chain. A section of the chain, located at the COOH-terminal end, contains several duplicated regions, the most prominent of which are pentapeptides. This section of the chain also contains all of the five histidines present in the molecule. Some of the physicochemical properties of the protein and an improved purification procedure are described.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asami K., Juniti K., Ernster L. Possible regulatory function of a mitochondrial ATPase inhibitor in respiratory chain-linked energy transfer. Biochim Biophys Acta. 1970;205(2):307–311. doi: 10.1016/0005-2728(70)90261-6. [DOI] [PubMed] [Google Scholar]
- Brooks J. C., Senior A. E. Studies on the mitochondrial oligomycin-insensitive ATPase. II. The relationship of the specific protein inhibitor to the ATPase. Arch Biochem Biophys. 1971 Dec;147(2):467–470. doi: 10.1016/0003-9861(71)90402-4. [DOI] [PubMed] [Google Scholar]
- Chan S. H., Barbour R. L. Purification and properties of ATPase inhibitor from rat liver mitochondria. Biochim Biophys Acta. 1976 Jun 8;430(3):426–433. doi: 10.1016/0005-2728(76)90018-9. [DOI] [PubMed] [Google Scholar]
- Cintrón N. M., Pedersen P. L. A protein inhibitor of the mitochondrial adenosine triphosphatase complex of rat liver. Purification and characterization. J Biol Chem. 1979 May 10;254(9):3439–3443. [PubMed] [Google Scholar]
- Ebner E., Maier K. L. A protein inhibitor of mitochondrial adenosine triphosphatase (F1) from Saccharomyces cerevisiae. J Biol Chem. 1977 Jan 25;252(2):671–676. [PubMed] [Google Scholar]
- Ernster L., Juntti K., Asami K. Mechanisms of energy conservation in the mitochondrial membrane. J Bioenerg. 1973 Jan;4(1):149–159. doi: 10.1007/BF01516053. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Variations in the S-S bridges of immunoglobins G: interchain disulfide bridges of gamma G3 myeloma proteins. J Mol Biol. 1968 May 14;33(3):893–906. doi: 10.1016/0022-2836(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Frangione B., Rosenwasser E., Prelli F., Franklin E. C. Primary structure of human gamma 3 immunoglobulin deletion mutant: gamma 3 heavy-chain disease protein Wis. Biochemistry. 1980 Sep 2;19(18):4304–4308. doi: 10.1021/bi00559a024. [DOI] [PubMed] [Google Scholar]
- Horstman L. L., Racker E. Partial resolution of the enzyme catalyzing oxidative phosphorylation. XXII. Interaction between mitochondrial adenosine triphosphatase inhibitor and mitochondrial adenosine triphosphatase. J Biol Chem. 1970 Mar 25;245(6):1336–1344. [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Isolation procedures. J Biol Chem. 1972 Oct 25;247(20):6617–6623. [PubMed] [Google Scholar]
- Knowles A. F., Penefsky H. S. The subunit structure of beef heart mitochondrial adenosine triphosphatase. Physical and chemical properties of isolated subunits. J Biol Chem. 1972 Oct 25;247(20):6624–6630. [PubMed] [Google Scholar]
- Lai C. Y. Regeneration of amino acids from anilinothiazolinones. Methods Enzymol. 1977;47:369–373. doi: 10.1016/0076-6879(77)47038-1. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Michaelsen T. E., Frangione B., Franklin E. C. Primary structure of the "hinge" region of human IgG3. Probable quadruplication of a 15-amino acid residue basic unit. J Biol Chem. 1977 Feb 10;252(3):883–889. [PubMed] [Google Scholar]
- Minkov I. B., Vinogradov A. D. Vydelenie i svoistva belka, ingibiruiushchego adenozintrifosfatazu mitokhondrii. Biokhimiia. 1973 May-Jun;38(3):542–547. [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Penefsky H. S. Preparation of beef heart mitochondrial ATPase. Methods Enzymol. 1979;55:304–308. doi: 10.1016/0076-6879(79)55035-6. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Terhorst C., Strominger J. L. Sequence of the COOH-terminal hydrophilic region of histocompatibility antigens HLA-A2 and HLA-B7. J Biol Chem. 1978 Aug 10;253(15):5319–5324. [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Satre M., de Jerphanion M. B., Huet J., Vignais P. V. ATPase inhibitor from yeast mitochondria. Purification and properties. Biochim Biophys Acta. 1975 May 15;387(2):241–255. doi: 10.1016/0005-2728(75)90107-3. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. Assembly of proteins into membranes. Science. 1980 Nov 21;210(4472):861–868. doi: 10.1126/science.7001628. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., van Dam K. The equilibrium between the mitochondrial ATPase (F1) and its natural inhibitor in submitochondrial particles. Biochim Biophys Acta. 1974 May 22;347(2):240–252. doi: 10.1016/0005-2728(74)90048-6. [DOI] [PubMed] [Google Scholar]


