Abstract
Directed plant cell growth is governed by deposition and alterations of cell wall components under turgor pressure. A key regulatory element of anisotropic growth, and hence cell shape, is the directional deposition of cellulose microfibrils. The microfibrils are synthesized by plasma membrane-located cellulose synthase complexes that co-align with and move along cortical microtubules. That the parallel relation between cortical microtubules and extracellular microfibrils is causal has been named the alignment hypothesis. Three recent studies revealed that the previously identified pom2 mutant codes for a large cellulose synthases interacting (CSI1) protein which also binds cortical microtubules. This review summarizes these findings, provides structure–function models and discusses the inferred mechanisms in the context of plant growth.
The synthesis and composition of cell walls
The cell wall provides fundamental morphological control for the plant body, and fulfills a range of biological functions, including defense against biotic and abiotic stresses, cell–cell communication and apoplastic water and nutrient transport [1,2]. Two types of cell walls are normally distinguished in vascular plants; an agile primary wall and a robust secondary wall [3]. The primary wall is formed during cell division at the cell plate where first callose is synthesized and a class of galacturonic acid-enriched polysaccharides, pectins, are deposited that emanate from Golgi derived vesicles [4]. This early cell wall provides the basis for the middle lamella, the intercellular glue that maintains cell–cell adhesion. At the maturing cell plate, cellulose is synthesized by the cellulose synthase (CESA) complex (Box 1) and larger proportions of Golgi-produced hemicelluloses are incorporated [2]. Cellulose represents the main load-bearing element in the cell wall, and forms a strong fibrilar mesh that molds the cell shape under turgor pressure. The cellulose microfibrils are inter linked by hemicelluloses, pectins and extracellular glycoproteins that regulate cell wall expansion. The influence of the wall on cell shape may readily be observed in, for example, elongating hypocotyl and root cells.
Box 1. The cellulose synthase complex and associated proteins.
Cellulose is synthesized at the plasma membrane by heteromeric cellulose synthase (CESA) complexes [3] that hold up to 36 CESA proteins. The genome of higher plants codes typically for nine or more CESA genes [5–9]. In Arabidopsis, CESA1, CESA3 and CESA6, are involved in primary wall cellulose synthesis, and CESA4, CESA7 and CESA8 in secondary wall synthesis [10,11]. The position occupied by CESA6 may be substituted by a clade of CESA6-like CESAs; CESA2, CESA5 and CESA9 [11,12]. The CESA proteins can be reversibly phosphorylated. In particular, the bidirectional motility of the CESA complex depends on CESA1 phosphorylation [13]. The changes in motility appear to be microtubule-dependent, as removal of the microtubules restored the bi-directional movement [13]. Migration of the CESA6-related CESA5 depends on its phosphorylation, which is coordinated by phytochrome activation [14]. Lastly, many proteins regulate the synthesis of cellulose, either via a direct interaction with the CESA complex, e.g. the endo-glucanase KORRIGAN [15,16], and POM2/CSI1 (CELLULOSE SYNTHASE INTERACTING 1), or indirectly, e.g. POM1/CTL1 (CHITINASE-LIKE1), COBRA and KOBITO/ELD1 (ELONGATION DEFECTIVE1). Mutations in any of these genes also lead to reduced cellulose content or crystallinity and cell expansion defects of roots and hypocotyls [17–25].
The alignment hypothesis
In cylindrical, anisotropic cells, cellulose microfibrils are typically oriented perpendicular, or obliquely, with respect to the growth axis [26]. Already in the early 1960s, it was noted that the orientation of the microfibrils was sensitive to the destabilizing spindle fiber drug colchicine [27]. Later, these spindle fibers were renamed microtubules and were visualized as highly ordered arrays at the cell cortex during interphase (Box 2) [26,28]. These discoveries led to the hypothesis that in many cell types cortical microtubules guide the synthesis and orientation of cellulose microfibrils [26,43,44]. The motility of the CESA complexes would be due to their catalytic activity, i.e. the emerging cellulose microfibril becomes immobilized in the wall and further synthesis would simply push the complexes forward [45,46]. Consistent with this hypothesis, small rosette-like structures at the plasma membrane that contain CESAs [47,48] co-align with, and move along, cortical microtubules [49]. The co-localization between microtubules and CESA trajectories was also observed when dynamic reorientations of the microtubules occur. In particular, the concomitant rotation of both microtubules and CESA trajectories has been proposed to promote the polylamellate texture of the outer epidermal cell walls in growing hypocotyls [50]. Different models for how the CESAs may be guided by the microtubules have been put forward. It was envisioned that the close interaction of cortical microtubules and the membrane could form barriers that constrained moving CESA complexes to remain between neighboring, parallel microtubules [51]. In another suggestion, coined the ‘templated-incorporation’ model, cellulose microfibrils would adhere to scaffolds arranged by microtubules. The microfibril deposition would subsequently be directed by the scaffolds rather than the microtubules [26,52]. The ‘template-incorporation’ model is in agreement with the observation that CESAs continue their linear movements also in the absence of the microtubules [49], although in an apparently less ordered fashion. The recent discovery that the gene affected in the previously identified cell expansion defective, and cellulose deficient, mutant pom2 is synonymous with CSI1 shed new light on the alignment hypothesis and provides a mechanism for the principle of microtubule guidance of the CESA complexes.
Box 2. Organization of the cortical microtubule arrays.
Microtubules are ‘hollow cables’ with a diameter of 25 nm and are polarized with one end comprising an α-subunit (– end) and the opposite end a β-subunit (+ end) [29]. Both plus and minus ends can grow, shrink and pause. In plants, growth is typically seen only for plus ends. Nevertheless, minus ends are generally observed to pause or shrink [30]. Dynamic instability involves stochastic addition and removal of tubulin subunits leading to switches between growth, shrinkage and pausing [31]. The initiation of catastrophe (transition from growth to shrinkage) and rescue (transition from shrinkage to growth) is controlled by the guanosine triphosphate (GTP) occupancy of tubulins. Once the rate of GTP hydrolysis is faster than the growth rate of the microtubule, GTP is depleted from the plus end, which leads to catastrophe [32]. Dynamic instability at the plus end and slow depolymerization at the minus end lead to hybrid treadmilling, which allows for polymerization-based migration of microtubules [33]. In contrast to animal cells, nucleation of microtubules occurs at multiple sites throughout the whole cell cortex and the nuclear envelope in plant cells [34,35]. After generation of new microtubules, they are transported to sites of assembly by treadmilling. Building on their dynamic instability and on their geometrical and biochemical interactions (cross-over, zippering and ‘touch and go’), microtubule encounters lead to their self-organization in cortical arrays or bundles with defined orientation and anisotropy at the cell cortex [35–42].
The structural characteristics of the CSI1 protein
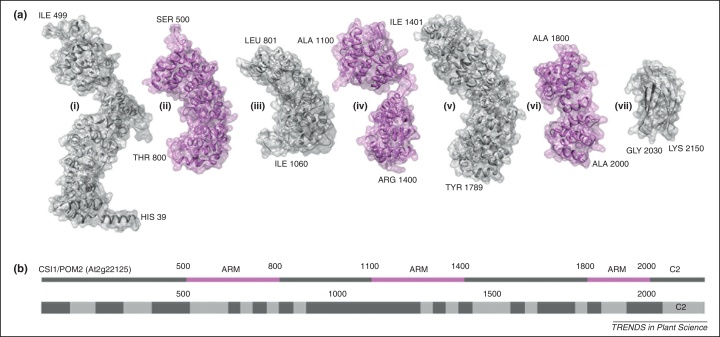
CSI1 is a protein of 2150 amino acids with up to 21 armadillo (ARM)/β-catenin-like repeats [53,54]. ARM repeats consist of about 40 amino acids that fold into three α helices. Often they occur in repetitive clusters on proteins forming a right-handed superhelix that serves as platform for protein–protein interactions [55]. Homology modeling of the CSI1 sequence revealed a repeated pattern of α-helices arranged in bundles typical for ARM, or ARM-like, domains (Figure 1a and b). CSI binds microtubules with an affinity in the range of the microtubule associated protein 2 (MAP2) [56,57]. Furthermore, the conserved 3D structure of ARM repeats is related to the structure of HEAT repeats, which have already been shown to interact with microtubules. For example, the N-terminal HEAT repeat of the MOR1 (MICROTUBULE ORGANIZATION1)/GEM1 (GEMENI1)/MAP125 protein and its homologs in other organisms is essential for microtubule binding [58–60]. Some of the best characterized ARM repeat proteins are the importin alpha-subunit/karyopherin with eight, the fungal VAC8 with 11, or the β-catenins with 12 ARM repeats. Given the number of potential ARM repeats in CSI1, the protein is probably forming a superhelix with more than twice the length of β-catenins. Interestingly, the strictly metazoan β-catenins serve some related functions with CSI1, because they link a transmembrane protein, cadherin, to the cytoskeleton and thus function in cell–cell adhesion [61] and mechano-transduction [62]. Similar functions are known for the VAC8 proteins where the ARM repeats are important for protein–protein interaction, including interaction with actin, and the remaining domains mediate interaction with membranes [63].
Figure 1.
3D structure models for the Arabidopsis CSI1 protein domains. (a) Molecular ribbon/surface depictions of model 3D structures for seven regions (i–vii) of the CSI1 proteins that were generated via homology modeling using the software HHPred [98]. The pink structures (ii), (iv) and (vi) correspond to regions that were found to match 3D structures containing Armadillo(-like) repeats in a BLAST-query against the Protein Data Bank [99]. The gray structures (i), (iii) and (v) correspond to regions that were not covered by said BLAST query, whereas the structure (vii) contains a C2 domain. Note, however, that regions (i), (iii) and (vi) appear to contain Armadillo(-like) repeats as well. Sequence indices and amino acid codes for the respective N- and C-terminals are specified. (b) Top: Sequence of CSI1 (At2g22125) annotated with the positions of the modeled regions and the domain types (ARM: Armadillo(-like) domain, C2: C2 domain). Bottom: Sequence of CSI1 (At2g22125) annotated with pfam/smarts domain annotation obtained via TAIR. Light gray regions correspond to Armadillo domains or to the C-terminal C2 domain (labeled ‘C2’). Molecular graphics were produced with the UCSF Chimera package [100].
Similar to the β-catenins, CSI1 has a number of putative sites for Glycogen synthase kinase 3 phosphorylation. Phosphorylation, and thus changing the surface charge of proteins, has been shown to regulate interactions of MAP65s and microtubules [64,65]. In addition, it has been reported that the microtubule-binding ARMADILLO REPEAT KINESIN1 (ARK1) interacts with the NEVER IN MITOSIS A (NIMA)-related kinase 6 (NEK6) [66]. This interaction influence cell morphogenesis in Arabidopsis epidermal cells by promotion of microtubule depolymerization at the cell cortex. Similar to csi1, ark1 mutants show root twisting that in ark1 could be suppressed by microtubule stabilizing drugs, such as taxol or propyzamide.
Apart from the many ARM repeats, CSI1 also harbors a C-terminal C2 domain (Figure 1). C2 domains form layers of β-sheets, which can bind to phospholipids in membranes. This association can be stimulated by binding of calcium, which enhances the interaction through changes in electrostatic potential of the C2 domain. Once the membrane association becomes stronger, lipophilic loops can interlace to the membrane, leading to membrane buckling [67]. Therefore, C2 domains, e.g. in SYNAPTOTAGMIN1, are considered electrostatic switches with great importance for vesicle shuttling, affecting both exocytosis and endocytosis in animal neurons [68]. Hence the C-terminal C2 domain could target CSI1 to the plasma membrane. Interestingly, removal of the C2 domain resulted in the localization of the CSI1 in the cytoplasm [54], supporting the membrane interaction function of the C2 domain.
Integrating CSI into a refined alignment hypothesis model
The discovery that CSI1 binds directly to both CESA subunits and microtubules, associate with the plasma membrane and is important for the co-alignment of CESA complexes [54,56,57] allows for refined models of the alignment hypothesis. For example, how does CSI1 allow for smooth movement of CESA complexes along microtubules considering its tight binding to microtubules in vitro? One possibility is that some form of gliding/sliding mechanism occurs between the two components in vivo that is not captured during the in vitro experiments (Figure 2) [56]. Alternatively, frequent switches between a binding and releasing state of CSI1 on microtubules might be possible, perhaps similar to the ATP-dependent movement of kinesins, i.e. via some form of energy-dependent process. This could for example be envisioned if CSI1 forms an extended inverted V-shaped structure, in which the mid-part binds the CESA complexes (as shown by [53]), and the ‘legs’ associate with microtubules. Migration could be fueled via phosphorylation/de-phosphorylation events, similar to the MAP65 proteins (Figure 2a) [64,65]. Another tentative model for the guidance could include a balance between the two forces that control CESA complex movement. These forces are the propulsion of the CESA complexes by the nascent microfibril, and the binding to microtubules through CSI1. In this model, multiple CSI1s would attach to CESA complexes and one or a couple of CSI1 protein(s) would anchor the complexes to microtubules (Figure 2b). The synthesis of the cellulose microfibril would push the complex forward, which would break the association of CSI1 and microtubules and instead allow for a neighboring CSI1 to take over the association (Figure 2b) leading to a rotary movement of the CESA complexes. The microfibrils form right-handed twisted structures [3], therefore a rotary movement of the CESA complexes around its own axis during cellulose synthesis seems, not only plausible, but necessary to avoid torsional stress. Photobleaching experiments indicated that multiple CSI1s were associated with each CESA complex (estimated from increasing POM2-CFP fluorescence intensities at distinct CESA foci over time (Figure 2b) [54]). Thus, under the assumption that each complex holds 36 CESAs, an equivalent number of CSI1s might also be associated. This seems to be consistent with a rotary model; however, X-ray or NMR structures of the CESAs and CSI1 will be necessary to confirm or revise this hypothesis.
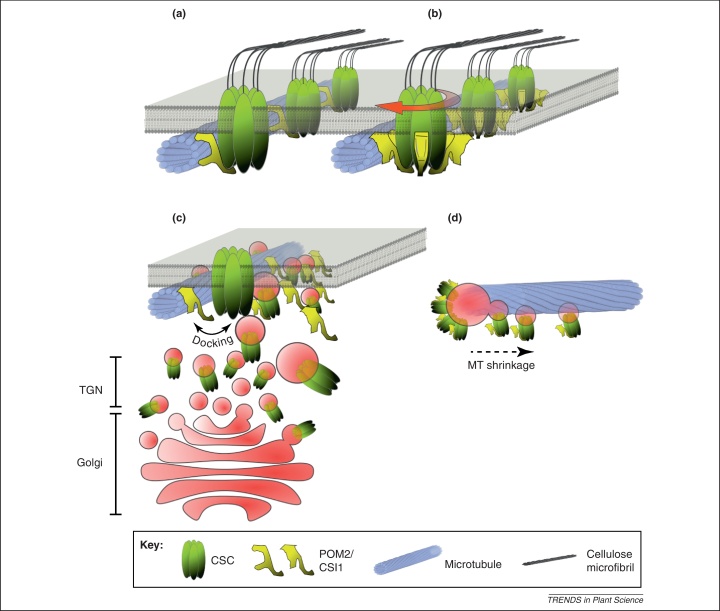
Figure 2.
Schematic model for potential functions of the CSI1 protein in cellulose synthesis. (a) and (b) CSI1 is involved in the guidance of the CESA complexes along microtubules at the cell cortex. One tentative model for this is that the CSI1 slide, or actively migrates in an energy-dependent fashion, along the microtubules while the complexes synthesize cellulose (a). Another scenario could include a rotary model, in which multiple CSI1s are associated with the CESA complexes. In this case their catalytic activity would force the complex forward which would cause neighboring CSI1 proteins to loosen and engage at the microtubule, respectively (b). (c) CSI1 can interact with the CESAs either at the plasma membrane or at sites of smaCCs/MASCs in the cell cortex. Although unclear at this point, the CSI1 could work as docking sites for the CESA complexes at the microtubules, probably in conjunction with other proteins. (d) CSI1 proteins associate with and behave as smaCCs/MASCs at the cell cortex. Proteins and structures are not to scale.
It is important to note that removal of microtubules does not appear to affect the overall motility of CESA complexes [13,14], although this is controversial [56]. If CSI1 only guides CESA complexes along microtubules it would be expected that the velocity of the complexes would not change when CSI1s were removed. However, in the csi1 mutants the motility of the complexes is reduced to a third of what is observed in the controls [53]. If the driving force behind the movement is generated through the activity of the complexes, i.e. the propulsion of the extruded cellulose microfibril, it seems improbable that the lack of attachment to microtubules would have an impact on their velocity. However, changes in spatial organization, and deviations from straight linear trajectories of CESA complexes were observed upon microtubules or CSI1 impairment [49,54]. Therefore, CSI1 may be important for both the activity and guidance of CESA complexes along the microtubules.
Uncoupling the role of microtubules in CESA movement from CESA delivery
Although synthesis of cellulose occurs at the plasma membrane, it is hypothesized that the assembly of the CESA complexes takes place in the Golgi and the complex is then exocytosed to the plasma membrane [15,69]. The Golgi derived vesicles, which probably partake in the exocytosis of the complexes, have been referred to as small CESA compartments (smaCCs) [70], or microtubule-associated cellulose synthase compartments (MASCs) [71]. However, it has also been suggested that this pool of vesicles may represent internalized CESAs [71]. Nevertheless, it is clear that the complexes are preferably delivered to sites at the plasma membrane that coincide with cortical microtubules [70]. The delivered complexes then remain immobile for some seconds, and subsequently start to move at constant speed in a bidirectional pattern along underlying microtubules [70].
Interestingly smaCCs/MASCs can also move along microtubules, [70]; however, the motility of the smaCCs/MASCs is distinct from that of the CESA complexes, and occurs in bursts of rapid movement interspersed with infrequent stops [70,71]. While CSI1 is present at sites of smaCC/MASC populations (Figure 2c and d), the insertion of the complexes do not appear to depend on CSI1 [54]. It therefore seems that different mechanisms govern the insertion of CESA complexes adjacent to microtubules, and their tracking along the microtubules.
Other molecular implications of CSI1
The characterization of CSI1 consolidates the alignment hypothesis, but it might also help unravel other related issues.
First, while disruption of the microtubule array does influence the behavior of the CESA complexes [49,54] and cell growth [27], some form of self-organized cellulose synthesis appears to occur [26,52,72]. Although it is not clear how this type of self-assembly is mediated, it is possible that the already established cell wall structure inherently aligns the newly formed cellulose microfibrils [26,73,74]. Because in csi1 mutants cellulose organization is uncoupled from microtubule-oriented cellulose deposition, the mutants will certainly be a key tool to address this issue.
Second, CSI1 may help in understanding how CESA activity is regulated. In particular, CSI1 binds to the putative catalytic domain of CESAs [53]. It is hence possible that CSI1, apart from its role in guiding CESAs along microtubules, may also play a role in the regulation of CESA activity (Box 1). This could be mediated by a direct influence of CSI1 on the conformation of the catalytic domain of CESAs, or perhaps via recruitment of proteins necessary for CESA activity. For example, it was suggested that sucrose synthase, together with a catalytic component, can dock to CESA complexes [75], a process that could be mediated via CSI1.
Third, cellulose synthesis, via CSI1, might be involved in the control of microtubule behavior in a feedback loop. In particular, impairment of the CESA complex motility may lead to microtubule disorganization [76]. However, no impact on microtubule organization was observed after partial digestion of the cellulose microfibrils by cellulases [76]. These data suggest the existence of crosstalks between the tracking behavior of CESA complexes and the organization of microtubules. A direct influence of CSI1 on microtubule organization was proposed, as csi1 mutants showed typical microtubule-related phenotypes, e.g. twisted growth and disturbed parallel microtubule arrays in elongating seedlings [54,57].
Putting CSI1 in context to cell growth and shape
Given its role in microtubule-driven cellulose deposition, CSI1 also provides an exciting avenue to investigate the cellular mechanisms behind cell and plant shape changes.
Compared to starch, cellulose can reach a much higher degree of structural order, or crystallinity. This has a strong impact on the mechanical properties of cell walls, as wall stiffness depends on the ratio between crystalline and amorphous cellulose. Decreased cellulose crystallinity is typically associated with an increased elongation rate. For example, microtubule fragmentation in the thermo-sensitive mutant mor1 maintains high levels of crystalline cellulose, supporting a role of microtubules in promoting the formation of amorphous cellulose in the wall, and thus softer cell walls [77]. Considering that the CSI1 protein affects both microtubule organization, and interaction between the microtubules and CESA complexes, csi1 mutants may then contain higher levels of crystalline cellulose. Hence, the CSI1 protein could potentially help to explain how microtubules regulate the organization of cellulose microfibrils in the wall, and by extension how this relates to cell and plant growth.
The relation between cellulose content and growth rate is not only restricted to the function of CESA complexes, but also involves additional regulators, such as accessory proteins, various signal transduction elements and hormones. Generally, growth rates decrease when cellulose content is reduced, which may lead to a compensatory increase in other cell wall components, such as pectin and lignin, presumably providing some mechanical strength to the wall [78]. As in yeast, and other walled organisms, a wall integrity mechanism can activate a complex signal transduction scheme that counteracts the wall rupture, thus leading to decreased growth rates [79–82]. This signaling scheme appears to involve various classical cell signals, such as reactive oxygen species, Rho GTPase and calcium [82–84], and also hormonal changes that may regulate cellular growth [79,82,84]. Considering that defects in CESA complex motility affect microtubule organization, it appears possible that some form of signaling process communicates the cellulose synthesis impairment to microtubules. This could be facilitated via the integrity signaling framework described above in which the impaired CESA complex activity would be sensed, leading to alterations in hormone levels that affect microtubule organization. Alternatively, a direct feedback loop between CESA complexes and microtubules could be involved, perhaps mediated by CSI1 or associated proteins.
Analysis of CSI1 may also provide mechanistic insight into how the cytoskeleton relays mechanical anisotropy of the cell wall. As discussed above, the linear structure of the cellulose microfibrils and their parallel alignments is guided by microtubules orientation [44] that regulates directional and anisotropic cell growth (Figure 3b). It should however be noted that growth anisotropy can also result from other factors. For instance, the boundary between two groups of cells growing isotropically, albeit at different growth rates, will be forced to undergo anisotropic growth, as a result of elastic deformation between contiguous cells, e.g. [85,86] (Figure 3a). In tissues, it is likely that cortical microtubules provide the most direct way to modulate anisotropic cell growth locally [87]. This seems to be true at both supracellular and subcellular scales. For example, the puzzle shape of pavement cells has been correlated with microtubules aiding in the reinforcement of the wall necks between lobes [88] (Figure 3c). Furthermore, tissue folding in the shoot apical meristem (SAM) has been correlated with supracellular microtubule alignment in the boundary domain between the meristem and the emerging organ [89] (Figure 3d). These models are based on the assumption that microtubule arrays reflect cellulose deposition, a hypothesis that can be tested by uncoupling the guiding principle, e.g. in csi1 mutants or through depolymerization of microtubules. However, the presence of relatively weak phenotypic defects observed in the csi1 mutants suggests that other mechanisms are also involved, such as a self-assembly of microfibrils from CESA complexes. This further supports the idea that the main role of the microtubule-dependent CESA complex behavior is to provide a fast way to reorient growth in response to signals, rather than the one and only way to control cellulose orientation in the wall [52].
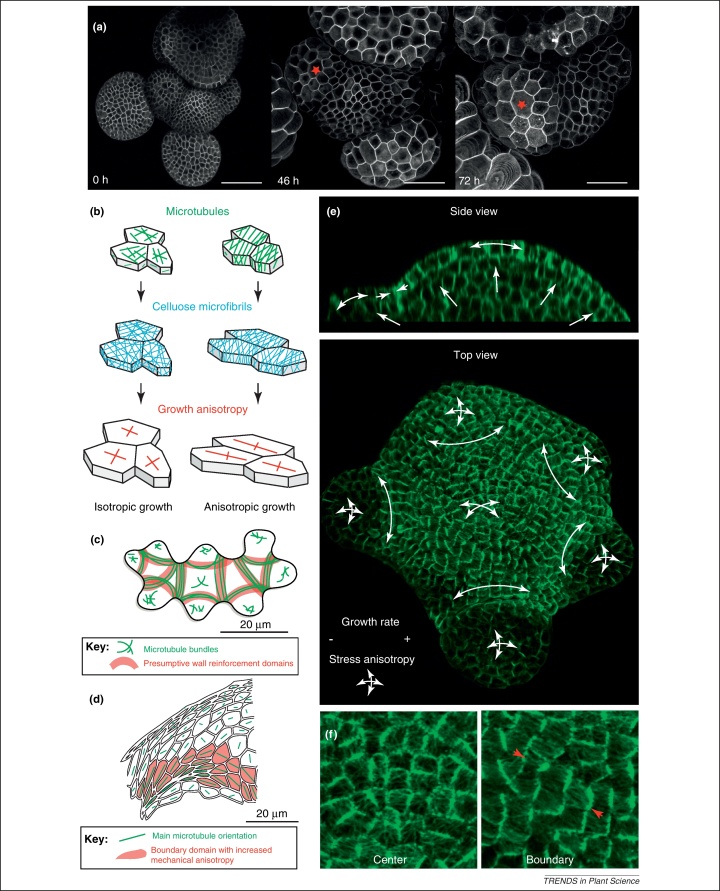
Figure 3.
Shape changes rely on the coupling between microtubule and CESA. (a) Cell and organ shape changes in the shoot apical meristem (SAM) expressing the GFP-LTI6b marker after microtubule depolymerization: cell growth becomes isotropic and tissue folding is reduced. Differential growth between the organ (fast growing) and the center of the meristem (growing more slowly) is maintained. A red star points at the same cell in two successive time points. Scale bar: 50 μm. (b) Microtubule orientation generally defines the main direction of cell growth anisotropy through the guided deposition of cellulose microfibrils in the cell wall. (c) The subcellular heterogeneity in microtubule bundling is correlated with the presence of neck and lobes in pavement cells. (d) The presence of coherent microtubule alignments in files of cells at the boundary domain of the SAM is correlated with tissue folding. (e) Side view and top view of a SAM expressing a GFP-MBD marker. Assuming that the epidermis is stiffer than the internal tissues, the meristem can be represented as an elastic shell inflated by turgor pressure and the principal direction of stress can be calculated (white arrows). In this scenario, microtubules align along the direction of maximal stress. Organ initiation is associated with a softening of the cell walls that is circumscribed by boundary domains exhibiting reduced growth rates and coherent microtubules alignments. (f) Close-ups showing microtubule orientation in the center and the boundary domains of the SAM revealed with the GFP-MBD marker. Red arrows point at the main microtubule orientation in the boundary.
Among the various cues involved in controlling microtubule behavior and cellulose deposition, the contribution of cell shape itself is attracting more and more attention, notably thanks to new developments in 4D live imaging. For instance, the CLASP protein has been proposed to help microtubules bend at cell corners, providing a scenario in which microtubule orientation depends on the cell geometry by default [90]. Intriguingly, cortical microtubules and CESA complexes also exhibit distinct behaviors depending on their proximity to the outer or inner wall in epidermal cells [91,92]. In addition to cell geometry, many signals, such as light, hormones or mechanical stress have been shown to promote the reorientation of cortical microtubules, thus impacting growth direction and morphogenesis [93–95]. For instance, the preferential alignment of cortical microtubules along the direction of maximal stress can in principle explain how a stem remains cylindrical during growth: assuming that the epidermis of the stem is under tension, the maximal direction of stress is circumferential and thus maintains the microtubules, and arguably the deposition of cellulose, in a transverse orientation. This could help shaping the stem as a cylindrical object, and as a consequence is able to maintain the stress pattern in a feedback loop [89] (Figure 3e and f).
Could CSI1 be a target of these signals? Inhibition of cellulose deposition leads to weaker walls [96] and thus to increased mechanical stress levels. Strikingly, the microtubule response to the predicted stress pattern is enhanced in these conditions [97]. Therefore, CSI1 and the cellulose synthesis machinery are probably not required for the transduction of mechanical stress to microtubules. However, this does not exclude the possibility that CSI1 modulates the microtubule response to these signals [54,57]. More generally, the CSI1 protein is a perfect candidate to decipher the cause and effect of signals on microtubule behavior and growth, as the corresponding mutant provides the first instance in which the physical coupling between microtubules and CESA complexes is largely reduced. In particular, in csi1 mutants, cellulose deposition becomes independent from microtubule behavior. One would predict that cortical microtubules in the csi1 mutants still respond to biochemical and mechanical stimuli, while cellulose deposition does not. The role of these signals in controlling anisotropic growth could be investigated in this context. Other players are likely to be involved, but a careful analysis of the csi1 phenotypes could already shed some light on the role of the coupling between microtubule and CESA complexes in plant morphogenesis and architecture.
Acknowledgments
We would like to thank Dr Dirk Walther for useful comments on the structural aspects of the CSI1. M.B. and S.P. were funded in part by the Max-Planck Gesellschaft, and through the DFG grant PE1642/5-1. M.T.H. was funded by the Austrian Science Fund (FWF, and the following grants; SFB F37/F03707, P14477-GEN, P11001-GEN). B.L. and O.H. were funded in part by a grant from Agence Nationale de la Recherche ANR-10-BLAN-1516 « Mechastem ». C.S. was supported by the TROST grant (22011208) from the BMELV (German Ministry for Food, Agriculture and Consumer Protection).
References
- 1.Carpita N., McCann M. The plant cell wall. In: Buchanan B., editor. Biochemistry and Molecular Biology of Plants. American Society of Plant Biologists; 2000. pp. 52–109. [Google Scholar]
- 2.Sánchez-Rodríguez C. Phytohormones and the cell wall in Arabidopsis during seedling growth. Trends Plant Sci. 2010;15:291–301. doi: 10.1016/j.tplants.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Somerville C. Toward a systems approach to understanding plant cell walls. Science. 2004;306:2206–2211. doi: 10.1126/science.1102765. [DOI] [PubMed] [Google Scholar]
- 4.Mohnen D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008;11:266–277. doi: 10.1016/j.pbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Richmond T. Higher plant cellulose synthases. Genome Biol. 2000;1 doi: 10.1186/gb-2000-1-4-reviews3001. Reviews 3001.1–3001.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland N. A comparative analysis of the plant cellulose synthase (CesA) gene family. Plant Physiol. 2000;123:1313–1324. doi: 10.1104/pp.123.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar M. An update on the nomenclature for the cellulose synthase genes in Populus. Trends Plant Sci. 2009;14:248–254. doi: 10.1016/j.tplants.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang L. Expression profiling and integrative analysis of the CESA/CSL superfamily in rice. BMC Plant Biol. 2010;10:282. doi: 10.1186/1471-2229-10-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts A.W., Bushoven J.T. The cellulose synthase (CESA) gene superfamily of the moss Physcomitrella patens. Plant Mol. Biol. 2007;63:207–219. doi: 10.1007/s11103-006-9083-1. [DOI] [PubMed] [Google Scholar]
- 10.Taylor N.G. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1450–1455. doi: 10.1073/pnas.0337628100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Persson S. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15566–15571. doi: 10.1073/pnas.0706592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desprez T. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15572–15577. doi: 10.1073/pnas.0706569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S. Mutations of cellulose synthase (CESA1) phosphorylation sites modulate anisotropic cell expansion and bidirectional mobility of cellulose synthase. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17188–17193. doi: 10.1073/pnas.1012348107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff V. Phytochrome regulation of cellulose synthesis in Arabidopsis. Curr. Biol. 2011;21:1822–1827. doi: 10.1016/j.cub.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Crowell E.F. Regulated trafficking of cellulose synthases. Curr. Opin. Plant Biol. 2010;13:700–705. doi: 10.1016/j.pbi.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Song D. Characterization of cellulose synthase complexes in Populus xylem differentiation. New Phytol. 2010;187:777–790. doi: 10.1111/j.1469-8137.2010.03315.x. [DOI] [PubMed] [Google Scholar]
- 17.Hauser M.T. Conditional root expansion mutants of Arabidopsis. Development. 1995;121:1237–1252. doi: 10.1242/dev.121.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicol F. A plasma membrane-bound putative endo-1,4-β-d-glucanase is required for normal wall assembly and elongation in Arabidopsis. EMBO J. 1998;17:5563–5576. doi: 10.1093/emboj/17.19.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arioli T. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science. 1998;279:717–720. doi: 10.1126/science.279.5351.717. [DOI] [PubMed] [Google Scholar]
- 20.Fagard M. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell. 2000;12:2409–2424. doi: 10.1105/tpc.12.12.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schindelman G. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001;15:1115–1127. doi: 10.1101/gad.879101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane D.R. Temperature-sensitive alleles of RSW2 link the KORRIGAN endo-1,4-beta-glucanase to cellulose synthesis and cytokinesis in Arabidopsis. Plant Physiol. 2001;126:278–288. doi: 10.1104/pp.126.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagant S. KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. Plant Cell. 2002;14:2001–2013. doi: 10.1105/tpc.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J. Chimeric proteins suggest that the catalytic and/or C-terminal domains give CesA1 and CesA3 access to their specific sites in the cellulose synthase of primary walls. Plant Physiol. 2006;142:685–695. doi: 10.1104/pp.106.084004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez-Rodríguez C. CHITINASE-LIKE1/POM-POM1 and its homolog CTL2 are glucan-interacting proteins important for cellulose biosynthesis in Arabidopsis. Plant Cell. 2012;24:589–607. doi: 10.1105/tpc.111.094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baskin T.I. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma. 2001;215:150–171. doi: 10.1007/BF01280311. [DOI] [PubMed] [Google Scholar]
- 27.Green P.B. Mechanism for plant cellular morphogenesis. Science. 1962;138:1404–1405. doi: 10.1126/science.138.3548.1404. [DOI] [PubMed] [Google Scholar]
- 28.Ledbetter M.C., Porter K.R. A “microtubule” in plant cell fine structure. J. Cell Biol. 1963;19:239–250. doi: 10.1083/jcb.19.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waterman-Storer C.M., Salmon E.D. Microtubule dynamics: treadmilling comes around again. Curr. Biol. 1997;7:R369–R372. doi: 10.1016/s0960-9822(06)00177-1. [DOI] [PubMed] [Google Scholar]
- 30.Ehrhardt D.W., Shaw S.L. Microtubule dynamics and organization in the plant cortical array. Annu. Rev. Plant Biol. 2006;57:859–875. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- 31.Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 32.Caplow M., Fee L. Concerning the chemical nature of tubulin subunits that cap and stabilize microtubules. Biochemistry. 2003;42:2122–2126. doi: 10.1021/bi027010s. [DOI] [PubMed] [Google Scholar]
- 33.Shaw S.L. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science. 2003;300:1715–1718. doi: 10.1126/science.1083529. [DOI] [PubMed] [Google Scholar]
- 34.Vaughn K.C., Harper J.D. Microtubule-organizing centers and nucleating sites in land plants. Int. Rev. Cytol. 1998;181:75–149. doi: 10.1016/s0074-7696(08)60417-9. [DOI] [PubMed] [Google Scholar]
- 35.Ehrhardt D.W. Straighten up and fly right: microtubule dynamics and organization of non-centrosomal arrays in higher plants. Curr. Opin. Cell Biol. 2008;20:107–116. doi: 10.1016/j.ceb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Bartolini F., Gundersen G.G. Generation of noncentrosomal microtubule arrays. J. Cell Sci. 2006;119:4155–4163. doi: 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- 37.Pastuglia M. Gamma-tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell. 2006;18:1412–1425. doi: 10.1105/tpc.105.039644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burk D.H. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell. 2001;13:807–827. [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura M. Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat. Cell Biol. 2010;12:1064–1070. doi: 10.1038/ncb2110. [DOI] [PubMed] [Google Scholar]
- 40.Dixit R., Cyr R. The cortical microtubule array: from dynamics to organization. Plant Cell. 2004;16:2546–2552. doi: 10.1105/tpc.104.161030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoppin-Mellet V. Katanin's severing activity favors bundling of cortical microtubules in plants. Plant J. 2006;46:1009–1017. doi: 10.1111/j.1365-313X.2006.02761.x. [DOI] [PubMed] [Google Scholar]
- 42.Wasteneys G.O., Ambrose J.C. Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol. 2009;19:62–71. doi: 10.1016/j.tcb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Heath I.B. A unified hypothesis for the role of membrane bound enzyme complexes and microtubules in plant cell wall synthesis. J. Theor. Biol. 1974;48:445–449. doi: 10.1016/s0022-5193(74)80011-1. [DOI] [PubMed] [Google Scholar]
- 44.Lloyd C. Dynamic microtubules and the texture of plant cell walls. Int. Rev. Cell Mol. Biol. 2011;287:287–329. doi: 10.1016/B978-0-12-386043-9.00007-4. [DOI] [PubMed] [Google Scholar]
- 45.Emons A.M. Microtubules and cellulose microfibrils: how intimate is their relationship? Trends Plant Sci. 2007;12:279–281. doi: 10.1016/j.tplants.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Guerriero G. What do we really know about cellulose biosynthesis in higher plants? J. Integr. Plant Biol. 2010;52:161–175. doi: 10.1111/j.1744-7909.2010.00935.x. [DOI] [PubMed] [Google Scholar]
- 47.Kimura S. Immunogold labeling of rosette terminal cellulose-synthesizing complexes in the vascular plant Vigna angularis. Plant Cell. 1999;11:2075–2085. doi: 10.1105/tpc.11.11.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller S.C., Brown R.M., Jr. Evidence for an intramembrane component associated with a cellulose microfibril-synthesizing complex in higher plants. J. Cell Biol. 1980;84:315–326. doi: 10.1083/jcb.84.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paredez A.R. Visualization of cellulose synthase demonstrates functional association with microtubules. Science. 2006;312:1491–1495. doi: 10.1126/science.1126551. [DOI] [PubMed] [Google Scholar]
- 50.Chan J. The rotation of cellulose synthase trajectories is microtubule dependent and influences the texture of epidermal cell walls in Arabidopsis hypocotyls. J. Cell Sci. 2010;123:3490–3495. doi: 10.1242/jcs.074641. [DOI] [PubMed] [Google Scholar]
- 51.Giddings T.H., Staehelin L.A. Microtubule-mediated control of microfibril deposition; a re-examination of the hypothesis. In: Lloyd C.W., editor. The Cytoskeletal Basis of Plant Growth and Form. Academic Press; 1991. pp. 85–100. [Google Scholar]
- 52.Wasteneys G.O. Progress in understanding the role of microtubules in plant cells. Curr. Opin. Plant Biol. 2004;7:651–660. doi: 10.1016/j.pbi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Gu Y. Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:12866–12871. doi: 10.1073/pnas.1007092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bringmann M. POM-POM2/CELLULOSE SYNTHASE INTERACTING1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell. 2012;24:163–177. doi: 10.1105/tpc.111.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conti E. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell. 1998;94:193–204. doi: 10.1016/s0092-8674(00)81419-1. [DOI] [PubMed] [Google Scholar]
- 56.Li S. Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc. Natl. Acad. Sci. U.S.A. 2012;109:185–190. doi: 10.1073/pnas.1118560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mei Y. The Arabidopsis ARCP protein, CSI1, which is required for microtubule stability, is necessary for root and anther development. Plant Cell. 2012;24:1066–1080. doi: 10.1105/tpc.111.095059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whittington A.T. MOR1 is essential for organizing cortical microtubules in plants. Nature. 2001;411:610–613. doi: 10.1038/35079128. [DOI] [PubMed] [Google Scholar]
- 59.Twell D. MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat. Cell Biol. 2002;4:711–714. doi: 10.1038/ncb844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spittle C. The interaction of TOGp with microtubules and tubulin. J. Biol. Chem. 2000;275:20748–52073. doi: 10.1074/jbc.M002597200. [DOI] [PubMed] [Google Scholar]
- 61.McCrea P.D., Gu D. The catenin family at a glance. J. Cell Sci. 2010;123:637–642. doi: 10.1242/jcs.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Desprat N. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 63.Coates J.C. Armadillo repeat proteins: beyond the animal kingdom. Trends Cell Biol. 2003;13:463–471. doi: 10.1016/s0962-8924(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 64.Smertenko A.P. Control of the AtMAP65-1 interaction with microtubules through the cell cycle. J. Cell Sci. 2006;2119:3227–3237. doi: 10.1242/jcs.03051. [DOI] [PubMed] [Google Scholar]
- 65.Smertenko A.P. The C-terminal variable region specifies the dynamic properties of Arabidopsis microtubule-associated protein MAP65 isotypes. Plant Cell. 2008;20:3346–3358. doi: 10.1105/tpc.108.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sakai T. Armadillo repeat-containing kinesins and a NIMA-related kinase are required for epidermal-cell morphogenesis in Arabidopsis. Plant J. 2008;53:157–171. doi: 10.1111/j.1365-313X.2007.03327.x. [DOI] [PubMed] [Google Scholar]
- 67.Martens S., McMahon H.T. Mechanisms of membrane fusion: disparate players and common principles. Nat. Rev. Mol. Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 68.Yao J. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat. Neurosci. 2011;15:243–249. doi: 10.1038/nn.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geisler D.A. Laying down the bricks: logistic aspects of cell wall biosynthesis. Curr. Opin. Plant Biol. 2008;11:647–652. doi: 10.1016/j.pbi.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 70.Gutierrez R. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 2009;11:797–806. doi: 10.1038/ncb1886. [DOI] [PubMed] [Google Scholar]
- 71.Crowell E.F. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell. 2009;21:1141–1154. doi: 10.1105/tpc.108.065334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Himmelspach R. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J. 2003;36:565–575. doi: 10.1046/j.1365-313x.2003.01906.x. [DOI] [PubMed] [Google Scholar]
- 73.Neville A.C. New model for cellulose architecture in some plant-cell walls. Protoplasma. 1976;90:307–317. [Google Scholar]
- 74.Vian B., Roland J.C. Tansley Review. 9. The helicoidal cell-wall as a time register. New Phytol. 1987;105:345–357. doi: 10.1111/j.1469-8137.1987.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 75.Fujii S. Sucrose synthase is an integral component of the cellulose synthesis machinery. Plant Cell Physiol. 2010;51:294–301. doi: 10.1093/pcp/pcp190. [DOI] [PubMed] [Google Scholar]
- 76.Paredez A.R. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol. 2008;147:1723–1734. doi: 10.1104/pp.108.120196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujita M. Cortical microtubules optimize cell-wall crystallinity to drive unidirectional growth in Arabidopsis. Plant J. 2011;66:915–928. doi: 10.1111/j.1365-313X.2011.04552.x. [DOI] [PubMed] [Google Scholar]
- 78.Burton R.A. Virus-induced silencing of a plant cellulose synthase gene. Plant Cell. 2000;12:691–706. doi: 10.1105/tpc.12.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hematy K. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 80.Levin D.E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005;69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seifert G.J., Blaukopf C. Irritable walls: the plant extracellular matrix and signaling. Plant Physiol. 2010;153:467–478. doi: 10.1104/pp.110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf S. Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 2012;63:381–407. doi: 10.1146/annurev-arplant-042811-105449. [DOI] [PubMed] [Google Scholar]
- 83.Hamann T. Identification of cell-wall stress as a hexose-dependent and osmosensitive regulator of plant responses. Plant J. 2009;57:1015–1026. doi: 10.1111/j.1365-313X.2008.03744.x. [DOI] [PubMed] [Google Scholar]
- 84.Denness L. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coen E. The genetics of geometry. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4728–4735. doi: 10.1073/pnas.0306308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwiatkowska D., Dumais J. Growth and morphogenesis at the vegetative shoot apex of Anagallis arvensis L. J. Exp. Bot. 2003;54:1585–1595. doi: 10.1093/jxb/erg166. [DOI] [PubMed] [Google Scholar]
- 87.Williamson R.E. Alignment of cortical microtubules by anisotropic wall stresses. Aust. J. Plant Physiol. 1990;17:601–613. [Google Scholar]
- 88.Fu Y. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 89.Hamant O. Developmental patterning by mechanical signals in Arabidopsis. Science. 2008;322:1650–1655. doi: 10.1126/science.1165594. [DOI] [PubMed] [Google Scholar]
- 90.Ambrose C. A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat. Commun. 2011;2:430. doi: 10.1038/ncomms1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan J. Microtubules and CESA tracks at the inner epidermal wall align independently of those on the outer wall of light-grown Arabidopsis hypocotyls. J. Cell Sci. 2011;124:1088–1094. doi: 10.1242/jcs.086702. [DOI] [PubMed] [Google Scholar]
- 92.Crowell E.F. Differential regulation of cellulose orientation at the inner and outer face of epidermal cells in the Arabidopsis hypocotyl. Plant Cell. 2011;23:2592–2605. doi: 10.1105/tpc.111.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamant O., Traas J. The mechanics behind plant development. New Phytol. 2010;185:369–385. doi: 10.1111/j.1469-8137.2009.03100.x. [DOI] [PubMed] [Google Scholar]
- 94.Lucas J., Shaw S.L. Cortical microtubule arrays in the Arabidopsis seedling. Curr. Opin. Plant Biol. 2008;11:94–98. doi: 10.1016/j.pbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 95.Sambade A. The influence of light on microtubule dynamics and alignment in the Arabidopsis hypocotyl. Plant Cell. 2012;24:192–201. doi: 10.1105/tpc.111.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ryden P. Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiol. 2003;132:1033–1040. doi: 10.1104/pp.103.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heisler M.G. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 2010;8:e1000516. doi: 10.1371/journal.pbio.1000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Söding J. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33:W244–W248. doi: 10.1093/nar/gki408. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berman H.M. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pettersen E.F. UCSF Chimera – a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]