Abstract
Seagrass ecosystems are expected to benefit from the global increase in CO2 in the ocean because the photosynthetic rate of these plants may be Ci-limited at the current CO2 level. As well, it is expected that lower external pH will facilitate the nitrate uptake of seagrasses if nitrate is cotransported with H+ across the membrane as in terrestrial plants. Here, we investigate the effects of CO2 enrichment on both carbon and nitrogen metabolism of the seagrass Zostera noltii in a mesocosm experiment where plants were exposed for 5 months to two experimental CO2 concentrations (360 and 700 ppm). Both the maximum photosynthetic rate (Pm) and photosynthetic efficiency (α) were higher (1.3- and 4.1-fold, respectively) in plants exposed to CO2-enriched conditions. On the other hand, no significant effects of CO2 enrichment on leaf growth rates were observed, probably due to nitrogen limitation as revealed by the low nitrogen content of leaves. The leaf ammonium uptake rate and glutamine synthetase activity were not significantly affected by increased CO2 concentrations. On the other hand, the leaf nitrate uptake rate of plants exposed to CO2-enriched conditions was fourfold lower than the uptake of plants exposed to current CO2 level, suggesting that in the seagrass Z. noltii nitrate is not cotransported with H+ as in terrestrial plants. In contrast, the activity of nitrate reductase was threefold higher in plant leaves grown at high-CO2 concentrations. Our results suggest that the global effects of CO2 on seagrass production may be spatially heterogeneous and depend on the specific nitrogen availability of each system. Under a CO2 increase scenario, the natural levels of nutrients will probably become limiting for Z. noltii. This potential limitation becomes more relevant because the expected positive effect of CO2 increase on nitrate uptake rate was not confirmed.
Keywords: CO 2 enrichment, glutamine synthetase, growth, nitrate reductase, nitrogen uptake, photosynthesis, seagrasses
Introduction
Projections that the current atmospheric CO2 concentration will double by the end of this century and that oceanic CO2 level will rise (IPCC [Intergovernmental Panel on Climate Change] 2007) have caused increasing interest in the research of the direct impacts of elevated CO2 on the marine environment (Gattuso et al. 1998; Feely et al. 2004; Guinotte and Fabry 2008; Hall-Spencer et al. 2008; Pörtner 2008; Porzio et al. 2011). It is expected that the seawater pH will decrease 0.3–0.4 units relative to present values before the year 2100 (Caldeira and Wickett 2003; Feely et al. 2004). The acidification of the seawater will induce changes in the carbonate chemistry, that is, in the relative proportions of the inorganic carbon species, carbon dioxide (CO2), bicarbonate (HCO3−), and carbonate (CO32−), shifting the total dissolved inorganic carbon away from CO32− toward more HCO3− and CO2 (Riebesell et al. 2007). This shift toward more HCO3− is expected to benefit species that use it as a carbon source for photosynthesis in addition to CO2 (Beer et al. 2002; Mercado et al. 2003).
Seagrass-dominated ecosystems play an important role in the carbon cycle of coastal areas (Duarte and Chiscano 1999; Hemminga and Duarte 2000). The responses of seagrasses to elevated CO2 concentrations must be considered for an effective management of coastal regions in the future. Seagrass meadows are reported as one of the few ecosystems that may benefit from rising CO2 levels because their photosynthetic rates have been considered Ci-limited at the current oceanic CO2 concentration (Beer and Koch 1996; Thom 1996; Zimmerman et al. 1997; Invers et al. 2001). Consequently, increases in seagrass production and growth may occur in a future high-CO2 scenario.
CO2 enrichment may also affect nitrogen uptake and the assimilation process, as growth enhancement at high-CO2 concentrations is expected to increase the nitrogen demand of plants (Stitt and Krapp 1999). In addition, the relative uptake rates of ammonium and nitrate may be altered by the acidification of the seawater resulting from CO2 enrichment, due to the involvement of protons (H+) in the nitrogen transport across the plasma membrane. In terrestrial plants, nitrate is cotransported with H+ across the membrane and consequently lower external pH facilitates nitrate uptake because of the higher H+ gradient outside the cell (e.g., Vessey et al. 1990). On the other hand, the lower external pH affects ammonium uptake because the higher content of H+ reduces the activity of H+-ATPase, which is involved in the cation transport into the cells (Marschner 1995). From the ionic balance perspective, lower pH levels in the seawater may reduce the ammonium uptake rates of seagrasses, whereas nitrate uptake rates may be unaffected or even increased.
The effects of CO2 enrichment on seagrasses have focused mainly on how elevated CO2 concentrations will affect seagrass productivity, light requirements, and nutrient content (Beer and Koch 1996; Thom 1996; Zimmerman et al. 1997; Palacios and Zimmerman 2007; Jiang et al. 2010). However, these effects were investigated with short-term (days) laboratory experiments, except the study of Palacios and Zimmerman (2007), in which experiments were run in outdoor aquaria for 1 year. Longer term studies are thus needed to account for the acclimation potential of seagrass species to increasing CO2. Furthermore, the response of seagrass nitrogen metabolism to CO2 enrichment is not known.
Here, we investigate the effects of CO2 enrichment on the carbon and nitrogen metabolism of the seagrass Zostera noltii in a mesocosm experiment where plants were exposed for 5 months to present (360 ppm) and future (700 ppm) seawater CO2 concentrations. We specifically aimed to assess the effects of CO2 enrichment on photosynthesis and growth, on the ammonium and nitrate uptake rates, and on the activity of nitrate reductase and glutamine synthetase, the two key enzymes of nitrogen assimilation. To the best of our knowledge, this is the first report on the effects of the global CO2 increase on the nitrogen metabolism of seagrasses.
Methods
Plant collection and experimental design
Zostera noltii is the most abundant seagrass species in Ria Formosa coastal lagoon, South Portugal (37°00′N, 7°58′W). The species develop along subtidal and intertidal areas and plays a major role in the lagoon's metabolism (Santos et al. 2004). In this system, the nutrient concentration in the water column is typically less than 5 μM due to a high water exchange between the lagoon and the adjacent ocean in each tidal cycle. Ammonium and phosphate concentrations in the sediment porewater are higher (12–38 μM for NH4+ and 2.5–14 μM for PO4−), whereas the concentration of nitrate is almost negligible (<1 μM) (Cabaço et al. 2008).
In order to preserve the integrity of the Z. noltii belowground plant parts and of its associated community, 20-cm diameter cores were carefully collected including plants and sediment, in March 2010. The cores were used to fill plastic boxes of 55 × 35 × 14 cm, which were placed in an outdoor mesocosm system at Centre of Marine Sciences (CCMAR) field station, near the donor meadow. The mesocosm consisted of two flow-through open systems running in parallel, one with seawater at the present CO2 concentration (360 ppm) and the other with twofold the present CO2 concentration (700 ppm), close to the “business as usual” scenario for 2100 of IPCC (Intergovernmental Panel on Climate Change) (2007) projections. Each system consisted of one head tank (1500 L) connected to two independent tanks (660 L each). Each of these tanks included four plastic boxes of Z. noltii and its associated community. Consequently, the experiment consisted of 2 CO2 levels × 2 replicates (660 L tanks), each bearing four plant units. The seawater used in the mesocosm was pumped from the lagoon into the head tanks after passing through a sand filter. The flow rate to each replicate unit was about 210 L/h. CO2 was bubbled into the head tanks from a CO2 tank to achieve the experimental CO2 concentrations (360 and 700 ppm). The rate of CO2 injection into the system was controlled by the pH level of the seawater using pH probes connected to CO2 controllers (EXAtx 450; Yokogawa, Tokyo, Japan). We acknowledge that this is a pseudoreplicated design, but the alternative option to control pCO2 individually in each tank would result in an added degree of error related to the difficulties of maintaining the same pCO2 values between tank replicates. The maintenance and control of elevated pCO2 levels in experimental tanks is not a straightforward process, but rather a difficult task, with countless small problems. Therefore, we considered that it was preferable to supply all the tanks with the same batch of water (and hence the same pCO2), even at the cost of falling into pseudoreplication. We trust that there is a high probability that the observed effects are due to the CO2 variable rather than to some undetected confounding effect between head tanks because the tanks were exactly the same size and type with exactly the same set up except for the CO2 enrichment. We considered that the perils of possible artifacts derived from pseudoreplication are small compared with the probability of Type II error associated with the error introduced when attempting at controlling CO2 independently in each replicated treatment. The plants were exposed to the experimental CO2 levels for 5 months (from March to August).
Seawater chemistry
The daily fluctuations of dissolved inorganic carbon (CO2, HCO3−, and CO32−), pH, and total alkalinity of the seawater in both CO2 treatments were monitored throughout the experiment at different hours during the day. In July, a complete 24 h cycle was made to illustrate the diel variation in seawater carbon chemistry. Triplicate water samples were collected inside the seagrass canopy in each mesocosm replicate every 2 h. For each replicate sample, total alkalinity was determined by measuring pH directly (Multimeter 340; WTW, Weilheim, Germany; accuracy of ±0.004 for the temperature range 15–35°C) in 4 mL of seawater before and after acidification with 1 mL of HCl 0.01 M, according to Parsons et al. (1984) and modified by Semesi et al. (2009). The concentration of dissolved inorganic carbon (CO2, HCO3−, and CO32−) was calculated from total alkalinity, temperature, and salinity of the seawater using the Excel-based program CO2SYS.XLS 1.0 (Pelletier et al. 1997). Salinity was measured using a hand refractometer, whereas temperature was measured using a combined pH + temperature probe (SenTixHWS; WTW). Water samples for nutrient analysis were also collected in triplicate, filtered through cellulose acetate filters, and stored at −20°C. The concentrations of ammonium, nitrate, and phosphate in the seawater were determined in a loop-flow analyzer (μMac-1000; Systea, Anagni, Italy). Ammonium concentration was determined using the hypochlorite method and nitrate concentration was determined using the Cd-Cu column reduction method. Phosphate was determined using the molybdate and ascorbic acid colorimetric method.
Photosynthetic measurements
All photosynthetic measurements were performed on the second youngest leaf of Z. noltii shoots. Electron transport rates (ETR) of Z. noltii plants exposed to both CO2 levels (360 and 700 ppm) were measured in vivo along 1 day in June, using a submersible pulse amplitude modulated (PAM) fluorometer (Diving-PAM; Heinz Walz, Effeltrich, Germany). Ambient light was measured simultaneously with the Diving-PAM external quantum sensor. ETR (μmol e−/m2/s) was calculated using the equation ETR = Y × I × AF × 0.5, where I is irradiance (μmol photon/m2/s), AF is the absorption factor, that is, the fraction of incident photosynthetic photon flux absorbed by the leaves, and 0.5 is the assumed proportion of photons absorbed by pigments associated with each photosystem. We acknowledge that intraspecific AF values can vary with geographic location, time of year, depth, leaf age, and nitrogen status. However, in our study, ETR were accessed at the same time for each point along the day in leaves of the same age with a similar previous light history. Therefore, we used a previously determined absorption factor (0.79 ± 0.02, n = 10; Silva and Santos 2003). The effective quantum yield of photosystem II (Y) was calculated using the equation (F′m−Fs)/F′m, where Fs is the fluorescence in the light when only part of the reaction centers are closed and Fm′ is the maximal fluorescence of a light adapted leaf immediately after closure of all reaction centers obtained through the application of a saturating light pulse (Genty et al. 1989).
Light response curves were determined in the laboratory by following oxygen evolution in square section incubation chambers (15 mL) coupled to a Clark-type oxygen electrode (DW3/CB1; Hansatech, Norfolk, U.K.). Actinic light was provided by a slide projector (Pradovit 150; Leica, Solms, Germany) equipped with a halogen lamp (Xenophot 150W; Osram, MüFCnchen, Germany). Ten light intensities (between 0 and 875 μmol quanta/m2/s) were achieved using a series of neutral density filters. For both CO2 concentrations (360 and 700 ppm), GF/F filtered seawater from the respective treatment was used as incubation medium. For each replicate measurement (n = 4 for the current CO2 and n = 3 for the enriched CO2 concentration), two independent segments (≍2 cm long) of Z. noltii leaves were held vertically inside the chamber. During the measurements, the water in the incubation chamber was continuously stirred and the temperature was kept constant at 20°C. Each light step took approximately 7 min at steady-state photosynthesis, and the water in the reaction chambers was replaced by new water before each light step, to prevent experimental artifacts (Silva and Santos 2003). After each light curve, the area of the leaf segments was measured (122–148 mm2) and leaf tissues were dried at 60°C for 24 h. The adapted hyperbolic tangent model equation of Jassby and Platt (1976) was fitted to the net photosynthesis versus irradiance data plots:
where Pm is the maximum photosynthetic rate (μmol O2/m2/s), I is irradiance (μmol quanta/m2/s), and α is the ascending slope at limiting irradiances (μmol O2/μmol quanta).
Growth measurements
Leaf growth rates were determined in Z. noltii plants exposed to the two CO2 levels (360 and 700 ppm) at the end of the experiment using the classical punching method described for seagrasses by Zieman (1974) and modified by Peralta et al. (2000). For each CO2 level, the leaves of five random shoots in each mesocosm replicate unit were marked with fine plastic fibers immediately above the leaf sheath. The total length of nonmarked leaves (small or new leaves) was also recorded. After 3 days, the length from the leaf base to the punching mark and the total leaf length of nonmarked leaves were recorded. Leaf growth rate (LGR) (cm/d/shoot) was calculated following the equation:
where Gnm is the growth rate of nonmarked leaves, Gm is the growth rate of marked leaves, and t is the time elapsed (days) between the punching and the final measurements (tf–t0). Gnm (cm/d/shoot) = TLLf–TLLi, where TLLi and TLLf are the total leaf length at t0 and tf, respectively. Gm (cm/d/shoot) = MLLf−MLLi, where MLLi and MLLf is the length from the leaf base to the punching mark at t0 and tf, respectively.
Combined effect of nitrogen and CO2 concentration on nitrogen uptake rates
Leaf nitrogen uptake rates were estimated at the end of the experiment using two-compartment cylindrical chambers that physically separated the leaves from the belowground plant parts. Leakage between compartments was avoided using molding clay and sterile vaseline as sealants. The leaves of plants grown at 360 and 700 ppm CO2 were simultaneously incubated for 2 h in seawater enriched with 15NH4Cl or 15KNO3 solutions (atom% = 99; Cambridge Isotope Laboratories, Andover, MA) in a walk-in culture chamber at constant temperature (21°C) and light intensity (200 μmol quanta/m2/s). The seawater used in the incubations was collected from the respective CO2 treatment batch. The uptake rates were determined at two nitrogen concentrations, one concentration that was representative of the typical values in the lagoon (5 μM) and at another which represented a nutrient enriched scenario (30 μM). This concentration is common in Ria Formosa in the vicinity of waste water treatment plants (Cabaço et al. 2008). Incubations at different CO2 levels were performed simultaneously for each nitrogen concentration, and replicate incubations (n = 3) were performed sequentially. One single shoot with the respective rhizome and roots was placed inside each split chamber. In the leaf compartment, an average leaf biomass of 0.04 g dry weight was incubated in 1.5 L of seawater, constantly mixed with a flow rate of ≍250 mL/min by a peristaltic pump. The nitrogen concentration (ammonium or nitrate) in the media did not vary noticeably throughout the incubation period. Root compartments were left without nutrients. Even though in natural conditions the rhizosphere of Z. noltii is mostly anoxic, in these experiments we incubated the whole plants in a nonanoxic medium. Previous experiments reported elsewhere (Alexandre et al. 2010) showed no effects of rhizosphere oxygenation on the ammonium and nitrate uptake rates of leaves.
At the end of incubations, the plants were removed from the chambers, the leaves were immediately separated from the rhizomes and roots and were briefly rinsed with deionized water to remove adherent label. Leaf tissues were dried at 60°C for 48 h and reduced to a fine powder. Total nitrogen content and atom% 15N of dried tissues were determined using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (UC Davis, Davis, CA). Leaf 15N background levels were measured in three replicate samples.
Nitrate reductase and glutamine synthetase activity
The incubations described in the previous section were repeated for the determination of nitrate reductase (NR) and glutamine synthetase (GS) activity. In these incubations, the leaf media were enriched with 30 μM of nonlabeled NH4Cl and KNO3. The leaf incubations at different CO2 level were performed simultaneously, whereas replicate incubations (n = 3) were performed sequentially. NR activity was measured in vivo using the method described by Corzo and Niell (1991), optimized for Z. noltii (Alexandre et al. 2004). This method is based on the colorimetric measure of nitrite, formed after the reduction in nitrate by NR. Leaf tissue (0.12 g fresh weight) was incubated in 50 mM KNO3, 0.1M K2HPO4 (pH 8.0), 0.5 mM EDTA, and 0.5% 1- propanol, in a final assay medium volume of 10 mL, flushed with N2 for 2 min to remove oxygen. Incubations lasted 30 min at 30°C. The nitrite produced was measured spectrophotometrically (540 nm) after adding 1 mL of sulfanilamide and 1 mL of naphtyl-etylenediamine to the assay medium. The in vivo assay was chosen because it yielded consistently higher activity than the in vitro assay, which also failed to provide reproducible results (see also Touchette and Burkholder 2007 and references therein). GS activity was measured in vitro, using the method described by Sagi et al. (2002), optimized for Z. noltii. The normal biologic activity of GS combines ammonium with glutamate to yield glutamine. This reaction is mimicked in the synthetase assay, in which hydroxylamine is substituted for ammonium to yield the product γ-glutamyl-hydroxamate, which can be quantitated spectrophotometrically. Leaf tissue samples (0.12 g FW) were extracted in 1.6 mL of buffer containing 200 mM Tris buffer (pH 7.8), 2 mM EDTA, 3 mM dithiothreitol (DTT), 10 μM flavin adenine dinucleotide (FAD), 10 mM MgCl, 2% (w/v) casein, 10% (v/v) glycerol, and 0.1 g polyvinylpyrrolidone (PVP). The homogenized plant material was centrifuged at 30,000 g, at 4°C for 15 min. A quantity of 100 μL of the enzyme extract was added to 250 μL of assay medium containing 18 mM ATP, 45 mM MgCl2·6H2O, 25 mM hydroxylamine, 92 mM l-glutamate, and 50 mM imidazole HCl (pH 7.2), at 30°C. After 20 min, the reaction was stopped by the addition of 0.5 mL of ferric chloride reagent (0.37 M ferric chloride, 0.67 M HCl, and 0.2 M trichloroacetic acid). The reaction solution was then centrifuged, and the absorbance of the supernatant was read at 540 nm.
Data analysis
The effects of CO2 enrichment on net photosynthesis, leaf growth rates, and enzymatic activity were tested using t-tests. Differences in electron transport rates between CO2 levels were tested using Mann–Whitney nonparametric test because data were not normally distributed, even after transformation. Two-way analysis of variance was used to test significant effects of CO2 level and nutrient concentration on the ammonium uptake rates. A nonparametric Mann–Whitney test was used to detect significant effects of CO2 level on the nitrate uptake rate for each nutrient concentration. Effects were considered statistically significant at a level of P = 0.05.
Results
Seawater chemistry
The daily fluctuation in the CO2 level and pH in the mesocosm was similar in both CO2 treatments (Fig. 1). CO2 increased during the night to a maximum at dawn decreasing throughout the day, whereas pH showed the opposite pattern. The concentration of CO2 and pH in the control-CO2 treatment averaged 360 ± 128 ppm and 8.13 ± 0.12, whereas in the enriched-CO2 treatment it averaged 695 ± 167 ppm and 7.91 ± 0.08, respectively.
Figure 1.
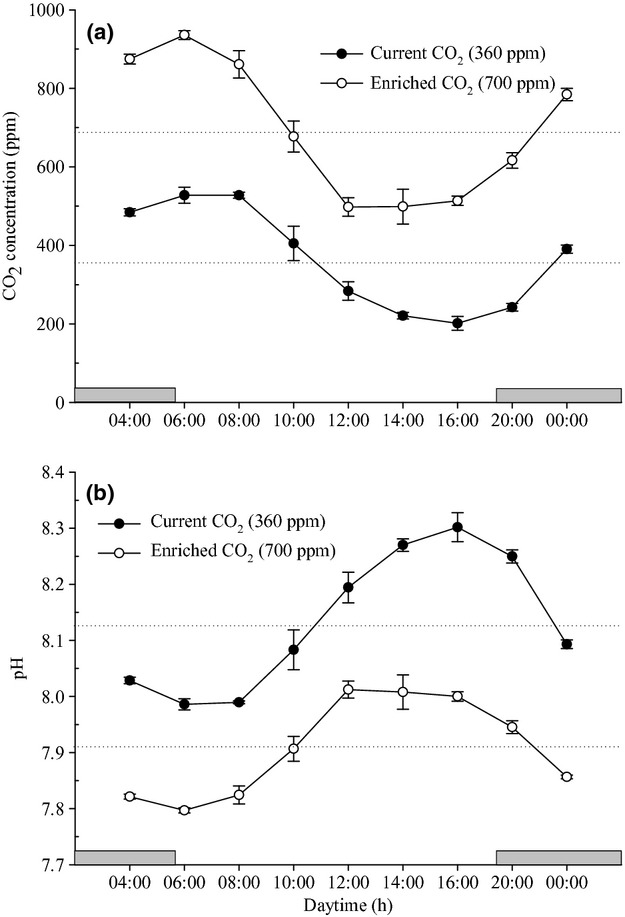
Daily fluctuation of (a) CO2 concentration (ppm) and (b) pH of the seawater in the control (open circle) and the CO2-enriched (closed circle) treatments. Dark areas represent nighttime. Values are mean ± SD (n = 6).
As a consequence of CO2 addition to the system, the concentration of CO2 and HCO3− increased in the enriched-CO2 treatment compared with the control, whereas the concentration of CO32− was reduced (Table 1). Total alkalinity was not significantly different between treatments and did not vary much along the day. The concentration of ammonium and nitrate in both treatments was nearly undetectable throughout the daily cycle (<0.01 μM) suggesting that all the available inorganic nitrogen was being taken up by the plants. Phosphate concentration in the control and CO2-enriched treatment averaged 0.18 ± 0.03 and 0.24 ± 0.04 μM, respectively.
Table 1.
Daily fluctuation of the seawater carbonate speciation in the two experimental CO2 levels (360 and 700 ppm). Values of total carbon (TC), bicarbonate (HCO3−), and carbonate (CO32−) were calculated using total alkalinity (TA), pH, salinity, and temperature of the seawater (Pelletier et al. 1997). Values are mean ± SD (n = 6) and represent pooled data from the two replicate mesocosm units. Units are μmol/Kg
| 360 ppm | 700 ppm | |||||||
|---|---|---|---|---|---|---|---|---|
| Daytime (h) | TA | TC | HCO3− | CO32− | TA | TC | HCO3− | CO32− |
| 04:00 | 2722 ± 17 | 2340 ± 16 | 2040 ± 15 | 285 ± 3 | 2760 ± 18 | 2510 ± 17 | 2288 ± 16 | 198 ± 2 |
| 06:00 | 2629 ± 34 | 2278 ± 40 | 2015 ± 36 | 255 ± 3 | 2765 ± 14 | 2529 ± 13 | 2314 ± 12 | 190 ± 2 |
| 08:00 | 2656 ± 21 | 2295 ± 13 | 2032 ± 19 | 259 ± 2 | 2738 ± 24 | 2488 ± 20 | 2266 ± 20 | 198 ± 7 |
| 10:00 | 2661 ± 19 | 2260 ± 39 | 1926 ± 54 | 305 ± 17 | 2697 ± 10 | 2398 ± 9 | 2153 ± 17 | 227 ± 10 |
| 12:00 | 2653 ± 9 | 2121 ± 24 | 1730 ± 36 | 376 ± 16 | 2697 ± 15 | 2300 ± 22 | 2001 ± 26 | 286 ± 7 |
| 14:00 | 2622 ± 14 | 2035 ± 12 | 1613 ± 20 | 411 ± 6 | 2660 ± 16 | 2277 ± 24 | 1986 ± 35 | 277 ± 15 |
| 16:00 | 2627 ± 15 | 2028 ± 29 | 1582 ± 45 | 428 ± 14 | 2680 ± 14 | 2307 ± 13 | 2020 ± 13 | 273 ± 5 |
| 19:30 | 2702 ± 25 | 2145 ± 20 | 1695 ± 57 | 400 ± 18 | 2743 ± 29 | 2409 ± 26 | 2141 ± 24 | 251 ± 6 |
| 00:00 | 2743 ± 15 | 2238 ± 4 | 1878 ± 17 | 360 ± 5 | 2774 ± 42 | 2456 ± 39 | 2192 ± 35 | 244 ± 4 |
Photosynthesis and growth
The electron transport rates (ETR) of Z. noltii plants both under present and increased CO2 values, showed a typical variation, increasing during the morning, peaking at midday, and decreasing in the afternoon (Fig. 2). At peak irradiance values (13:00, PAR ≍ 300 μmol/m2/s), the ETR of control plants showed a sharp decline that suggests a dynamic downregulation of photosynthesis, whereas the CO2-enriched plants did not. However, the whole ETR values of control plants were not significantly different from those of plants exposed to CO2-enriched conditions (P = 0.39) (Fig. 2). The irradiance-saturated photosynthetic rate (Pm) of plants exposed to CO2-enriched conditions (1061.5 ± 60.5 μmol O2/m2/s) was 1.3-fold higher than the rate of plants exposed to current CO2 concentration (799.4 ± 36.2 μmol O2/m2/s) (Fig. 3). Similarly, the photosynthetic rates at limiting irradiances (α), expressed as photosynthetic efficiency, were much higher in CO2-enriched plants (17.3 ± 2.7 μmol O2/μmol quanta) than in plants exposed to current CO2 concentration (4.1 ± 0.4 μmol O2/μmol quanta). On the other hand, no significant effect of CO2 enrichment was detected on the leaf growth rate of Z. noltii. The leaf growth rate of plants exposed to elevated CO2 concentration was 1.12 ± 0.27 cm/d/shoot, whereas the rate of plants grown at current CO2 conditions was 1.18 ± 0.21 cm/d/shoot.
Figure 2.
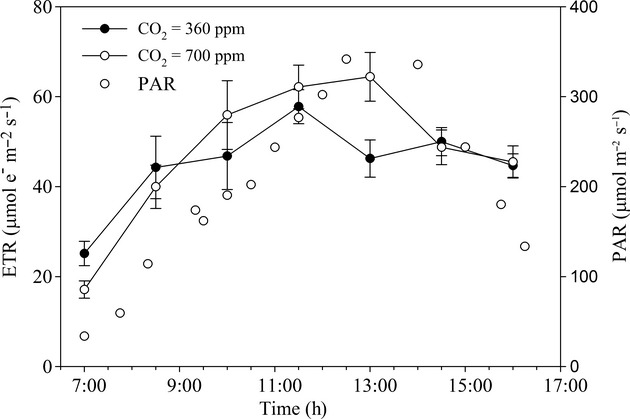
Zostera noltii. Diurnal variation in electron transport rate (ETR, μmol e−/m2/s) and available photosynthetic active radiation (PAR) in plants exposed to current (360 ppm) and elevated (700 ppm) CO2 concentrations.
Figure 3.
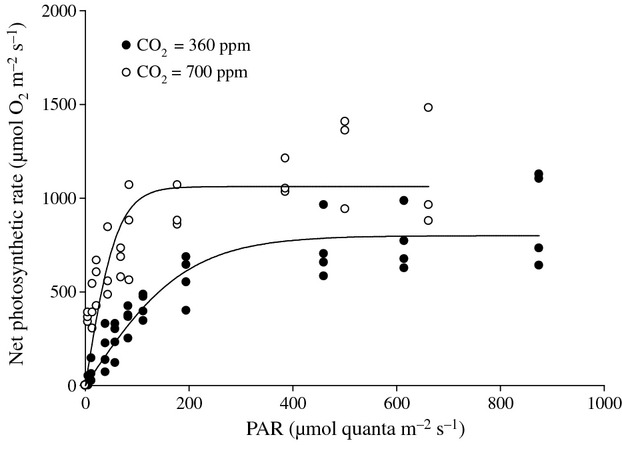
Zostera noltii. Net photosynthetic rate (μmol O2/m2/s) versus photosynthetic active radiation (PAR; μmol quanta/m2/s) measured following oxygen evolution determined at 20°C in leaf segments of plants exposed at 360 ppm (closed circles) and 700 ppm (open circles). Values are mean ± SD (n = 3–4).
Nitrogen uptake
The ammonium uptake rates of leaves exposed to higher CO2 concentration, either incubated with 5 μM (2.42 ± 0.18 μmol/g DW/h−1) or 30 μM 15NH4Cl (10.27 ± 0.83 μmol/g DW/h), were not significantly different from the rates of plants exposed to the current CO2 conditions (2.69 ± 0.44 and 9.07 ± 0.64 μmol/g DW/h, respectively, P = 0.427) (Fig. 4, Table 2). Similarly, both the nitrate uptake rates of CO2-enriched leaves incubated at 5 μM (0.02 ± 0.01 μmol/g DW/h) and at 30 μM (0.05 ± 0.03 μmol/g DW/h) were not significantly different from the control (0.08 ± 0.02 μmol/g DW/h, P = 0.065, and 0.16 ± 0.04 μmol/g DW/h, P = 0.240, respectively), but the leaf nitrate uptake rate of plants exposed to CO2-enriched conditions was fourfold lower than the uptake of plants exposed to current CO2 level.
Figure 4.
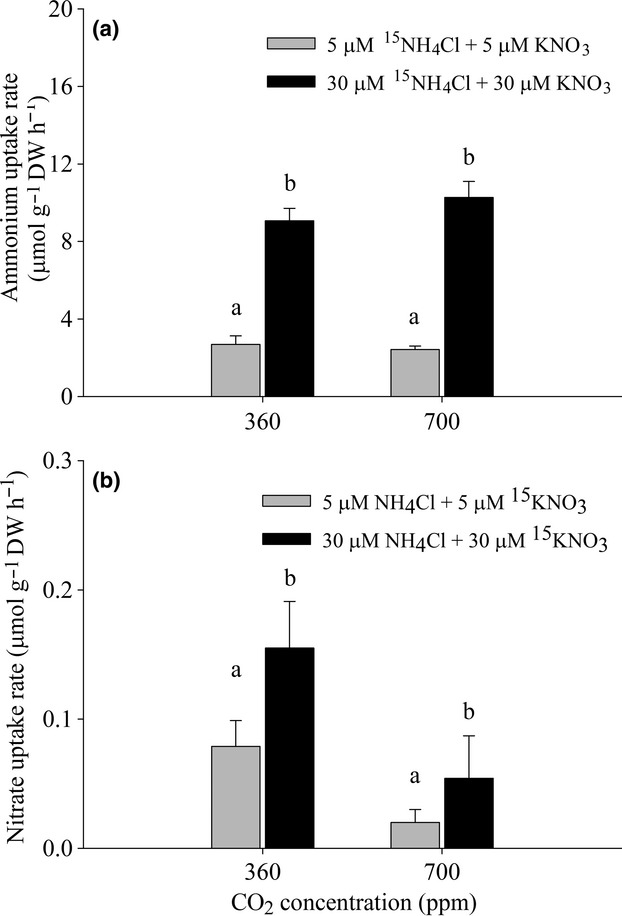
Zostera noltii. Ammonium (a) and nitrate (b) uptake rates (μmol/g DW/h) of plants leaves exposed to CO2 concentrations of 360 and 700 ppm when incubated at 5 and 30 μM of NH4Cl + KNO3. Values are mean ± SE (n = 6). Different letters denote significant differences.
Table 2.
Combined effects of CO2 and dissolved inorganic nitrogen on the ammonium uptake rates of Zostera noltii, as determined by two-way analysis of variance
| df | MS | F | P | |
|---|---|---|---|---|
| Ammonium uptake | ||||
| CO2 | 1 | 1.307 | 0.658 | 0.427 |
| N concentration | 1 | 303.599 | 152.85 | <0.001 |
| CO2 × N concentration | 1 | 3.229 | 1.626 | 0.217 |
Enzymatic activity
The activity of the enzyme glutamine synthetase of plant leaves grown under CO2 enrichment (923 ± 58 μmol glutamil-hydroxamate/g DW/h) was not significantly different from that of plants from the control treatment (907 ± 48 μmol glutamil-hydroxamate/g DW/h) (Fig. 5). On the other hand, the activity of nitrate reductase was threefold higher (16.4 ± 4.4 μmol NO2−/g DW/h) in the leaves of plants exposed to higher CO2 concentration than in control plants (4.7 ± 1.2 μmol NO2−/g DW/h).
Figure 5.
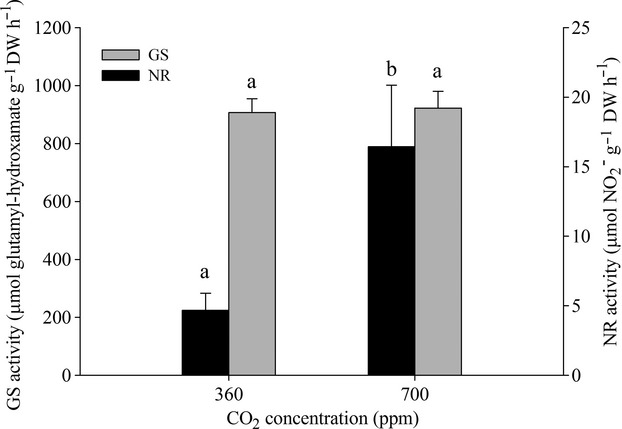
Zostera noltii. Effect of CO2 enrichment on the activity of the enzymes glutamine synthetase (GS) (gray bars) and nitrate reductase (NR) (black bars). Values are mean ± SE (n = 3). Different letters denote significant differences.
Discussion
This study showed that the net photosynthetic rate of Z. noltii is positively affected by the CO2 enrichment of the seawater. Plants exposed to CO2-enriched conditions showed higher photosynthetic rates at saturating irradiances and were photosynthetically more efficient at limiting light intensities (higher α) when compared with plants exposed to the current CO2 concentration. This was probably the result of a higher carboxylation activity relatively to oxygenation activity from RuBisCO in the presence of a higher CO2/O2 ratio, as CO2 and O2 are competitive inhibitors for RuBisCO's active site (Taiz and Zeiger 2002). The higher carboxylation activity relatively to oxygenation activity results in lower photorespiration, which decreases the energy cost for CO2 fixation and increases the photochemical quantum yield (Furbank 1998; Taiz and Zeiger 2002). CO2-enriched plants showed higher ETR values at peak light intensity contrasting with the control plants that showed much lower values, suggesting a dynamic downregulation of photosynthesis. This indicates that photosynthesis of Z. noltii is Ci-limited at the current inorganic carbon concentration of seawater, confirming the conclusions previously obtained for the same species (Silva et al. 2005), and for other seagrass species (Beer and Koch 1996; Zimmerman et al. 1997; Invers et al. 2001). These results also suggest that Z. noltii may benefit from future CO2 enrichment by enhancing the photosynthetic rates at higher CO2 concentrations. The CO2-stimulated increase in photosynthesis found here for Z. noltii is consistent with the findings reported for the temperate and tropical seagrass species Z. marina and Thalassia hemprichii, where positive photosynthetic responses to CO2 enrichment were also found (Beer and Koch 1996; Thom 1996; Zimmerman et al. 1997; Jiang et al. 2010).
Our results showed that CO2 enrichment did not stimulate the growth rate of Z. noltii leaves exposed to increased CO2 for 5 months. This finding is consistent with Palacios and Zimmerman (2007) observations for Z. marina, but contrary to Thom (1996) and Jiang et al. (2010) studies, where CO2 enrichment enhanced leaf growth rates of Z. marina and T. hemprichii, respectively. However, CO2 enrichment did have an effect on belowground growth rates of Z marina (Palacios and Zimmerman 2007). Observations of enhanced seaweed growth rates under high-CO2 levels have also been reported (Gao et al. 1991, 1993; Gordillo et al. 2001; Zou 2005; Xu et al. 2010). However, these studies investigated the effects of CO2 enrichment using only short-term experiments (days). Longer term experiments (months) with seagrass species found no significant differences in leaf growth rates of plants exposed to current and elevated CO2 concentrations (Palacios and Zimmerman 2007; this study). In some terrestrial plant species short-term exposure to elevated CO2 resulted in decreased photorespiration and even inhibition of dark respiration (Amthor 1991; Cousins et al. 2001). In the long term, the inhibition of the respiratory metabolism may result in a decrease in starch catabolism and available energy, with possible consequences for plant growth (Yelle et al. 1990). On the other hand, there is evidence that the stimulation of growth by elevated CO2 concentrations can be strongly curtailed in plants grown under nitrogen-limited conditions (Stitt and Krapp 1999 and references therein; Liu et al. 2010). Nitrogen may thus become the limiting factor of plant production in the enriched CO2 future. This was previously reported for the seaweed Ulva sp., which more than doubled its growth rate when cultivated at CO2-enriched conditions, whereas under nitrogen-limited conditions its growth was only slightly increased (Gordillo et al. 2001). The nitrogen status of the plants, which is a consequence of the nitrogen growth conditions, has been used to determine the expression of CO2 enrichment effects on growth rates (Andría et al. 1999; Gordillo et al. 2001). The leaf nitrogen content of Z. noltii plants from both experimental CO2 treatments (1.4%) was below the critical level of 1.8% reported as indicative of low nitrogen supply (Duarte 1990), suggesting that the nitrogen available for the plants in the mesocosm (the natural concentration available in Ria Formosa lagoon) was insufficient to fully meet the species nitrogen requirements for growth. Therefore, we hypothesize that the growth rates of Z. noltii plants were primarily controlled by the low nitrogen availability rather than by the elevated CO2 concentration in the mesocosm. An important corollary of this is that the global effects of CO2 on seagrass growth may not be spatially homogeneous and will depend on the specific availability of nitrogen, as well as other nutrients, such as P and Fe, of each system. Under conditions of elevated CO2 and nitrogen limitation, it is possible that the additional fixed C from higher photosynthetic rates remains stored until nitrogen availability is restored to levels that meet the nitrogen requirements for growth. On the other hand, there is the possibility that the additional fixed carbon is exudated in the form of DOC, or that it was used for belowground growth and shoot proliferation (Palacios and Zimmerman 2007), which were not assessed in this study.
Surprisingly, there were no significant effects of CO2 enrichment on the nitrate and ammonium uptake rates. These findings do not corroborate our initial hypothesis that the lower pH of CO2-enriched seawater would increase the nitrate uptake rates because in higher plants nitrate is cotransported along with H+ through the membrane (Vessey et al. 1990) and that the ammonium uptake rates would decrease, as the activity of H+-ATPase (involved in the cation transport into the cells) is reduced by the higher H+ content (Marschner 1995). Research on the nitrate transport system of Z. marina leaves (García-Sánchez et al. 2000) suggested that nitrate uptake in this species is probably not coupled with H+, as it is in other angiosperms (Ullrich 1992). A similar situation may occur in Z. noltii.
Factors other than pH might be involved in the decrease in the nitrate uptake rates of Z. noltii observed at high CO2. A nitrogen-limited seaweed Ulva lactuca also showed much lower nitrate uptake rates when exposed to elevated CO2 conditions (Magnusson et al. 1996). The authors concluded that the decreasing effect of CO2 enrichment on the nitrate uptake rates was not related to the pH level of the seawater and suggested that uncontrolled CO2 entrance in the cellular compartments may affect regulatory mechanisms and enzyme functioning with consequences for the nutrient uptake rates.
The nitrate assimilatory capacity of Z. noltii was positively affected by the CO2 enrichment, as revealed by the higher nitrate reductase activity of plant leaves grown under CO2-enriched conditions, while nitrate uptake rate was reduced. These results indicate that nitrate uptake and reduction are uncoupled when Z. noltii is grown at high CO2. Some alteration in the production of ATP relative to NADPH might also explain the imbalance between nitrate reductase activity and assimilation of nitrogen found in Z. noltii grown at high-CO2 conditions, as suggested by Mercado et al. (1999). The CO2-driven stimulation of this enzyme's activity was also reported for terrestrial plants and seaweeds (Fonseca et al. 1997; Mercado et al. 1999; Gordillo et al. 2001; Zou 2005). In terrestrial plants, it has been suggested that elevated CO2 controls nitrate assimilation indirectly through the amount of accumulated carbohydrates (Fonseca et al. 1997). Increased accumulation of carbohydrates, such as soluble sugars and starch, has been observed in both seagrass and seaweed species grown at elevated CO2 concentrations as a consequence of limiting nitrogen regimes (Zimmerman et al. 1995, 1997; Andría et al. 1999; Jiang et al. 2010). Under nitrogen limitation, the increased photosynthetic activity observed in Z. noltii may have caused an imbalance between the carbon supply and its utilization for growth, leading to an accumulation of carbohydrates. We hypothesize that Z. noltii plants exposed to elevated-CO2 concentrations may have accumulated higher levels of carbohydrates, which contributed to increase the nitrate reductase activity by supplying energy and carbon skeletons for the nitrate reduction process. On the other hand, it has also been suggested that the CO2-driven increase in the maximum nitrate reductase activity is not regulated by the carbohydrate level or internal carbon content but rather through a direct action on the enzyme synthesis, which is triggered by nitrate signaling (Gordillo et al. 2001). This is an interesting topic that deserves further investigation.
In conclusion, the photosynthetic rate of Z. noltii increased under high-CO2 conditions, but no effects were detected on growth probably because plants were nutrient limited, as revealed by the low total nitrogen content of the plants at the end of the experiment. Under a CO2 increase scenario, the natural levels of nutrients will probably become limiting for Z. noltii. This potential limitation becomes more relevant because the expected positive effect of CO2 increase on nitrate uptake rate was not confirmed.
Acknowledgments
The authors are grateful to G. Graça, B. Claro, M. Costa, A. Mejia, and S. Albano for their assistance in the mesocosm setup and field sampling. We thank the five anonymous reviewers who greatly improve the manuscript. The mesocosm facility at Ramalhete field station of CCMAR (Centre of Marine Sciences of Algarve) was provided by the EU FP7 research infrastructure initiative, ASSEMBLE – Association of European Marine Biological Laboratories. This study is part of a collaborative research with the University of Stockholm in the ambit of the COST Action ES0906 “Seagrass productivity: from genes to ecosystem management”. A. A. was funded by a Ph.D. grant from Fundação para a Ciência e a Tecnologia (SFRH/BD/21487/2005), cofunded by POCI 2010 and FSE.This publication is supported by COST. ESF provides the COST Office through an EC contract. COST is supported by the EU RTD Framework programme.
Conflict of Interest
None declared.
References
- Alexandre A, Silva J, Santos R. The maximum nitrate reductase activity of the seagrass Zostera noltii (Hornem.) varies along its vertical distribution. J. Exp. Mar. Biol. Ecol. 2004;307:127–135. [Google Scholar]
- Alexandre A, Silva J, Santos R. Inorganic nitrogen uptake and related enzymatic activity in the seagrass Zostera noltii. Mar. Ecol. 2010;31:539–545. [Google Scholar]
- Amthor JS. Respiration in a future, higher-CO2 world. Plant Cell Environ. 1991;14:13–20. [Google Scholar]
- Andría JR, Vergara JJ, Pérez-Lloréns JL. Biochemical responses and photosynthetic performance of Gracilaria sp. (Rodophyta) from Cádiz, Spain, cultured under different inorganic carbon and nitrogen levels. Eur. J. Phycol. 1999;34:497–504. [Google Scholar]
- Beer S, Koch E. Photosynthesis of marine macroalgae and seagrasses in globally changing CO2 environments. Mar. Ecol. Prog. Ser. 1996;141:199–204. [Google Scholar]
- Beer S, Björk M, Hellblom F, Axelsson L. Inorganic carbon utilization in marine angiosperms (seagrasses) Funct. Plant Biol. 2002;29:349–354. doi: 10.1071/PP01185. [DOI] [PubMed] [Google Scholar]
- Cabaço S, Machás R, Vieira V, Santos R. Impacts of urban wastewater discharges on seagrass meadows (Zostera noltii. Estuar. Coast. Shelf Sci. 2008;78:1–13. [Google Scholar]
- Caldeira K, Wickett ME. Oceanography: anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Corzo A, Niell FX. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method. J. Exp. Mar. Biol. Ecol. 1991;146:181–191. [Google Scholar]
- Cousins AB, Adam NR, Wall GW, Kimball BA, Pinter PJ, Jr, Leavitt SW, et al. Reduced photorespiration and increased energy-use efficiency in young CO2-enriched sorghum leaves. New Phytol. 2001;150:275–284. [Google Scholar]
- Duarte CM. Seagrass nutrient content. Mar. Ecol. Prog. Ser. 1990;67:201–207. [Google Scholar]
- Duarte CM, Chiscano CL. Seagrass biomass and production: a reassessment. Aquat. Bot. 1999;65:159–164. [Google Scholar]
- Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, et al. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305:362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- Fonseca F, Bowsher C, Stulen I. Impact of elevated atmospheric carbon dioxide on nitrate reductase transcription and activity in leaves and roots of Plantago major. Physiol. Plant. 1997;100:940–948. [Google Scholar]
- Furbank RT. C4 pathway. In: Raghavendra AS, editor. Photosynthesis, a comprehensive treatise. Cambridge, U.K: Cambridge Univ. Press; 1998. pp. 123–135. [Google Scholar]
- Gao K, Aruga Y, Asada K, Ishihara T, Akano T, Kiyohara M. Enhanced growth of the red alga Porphyra yezoensis Ueda in high CO2concentrations. J. Appl. Phycol. 1991;3:355–362. [Google Scholar]
- Gao K, Aruga Y, Asada K, Kiyohara M. Influence of enhanced CO2 on growth and photosynthesis of the red algae Gracilaria sp. and G. chilensis. J. Appl. Phycol. 1993;5:563–571. [Google Scholar]
- García-Sánchez MJ, Jaime MP, Ramos A, Sanders D, Fernández JA. Sodium-dependent nitrate transport at the plasma membrane of leaf cells of the marine higher plant Zostera marina L. Plant Physiol. 2000;122:879–885. doi: 10.1104/pp.122.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattuso J-P, Frankignoulle M, Bourge I, Romaine S, Buddemeier RW. Effect of calcium carbonate saturation of seawater on coral calcification. Glob. Planet. Change. 1998;18:37–46. [Google Scholar]
- Genty B, Briantais J, Baker N. The relationship between the quantum yields of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 1989;990:87–92. [Google Scholar]
- Gordillo FJL, Niell FX, Figueroa FL. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta) Planta. 2001;213:64–70. doi: 10.1007/s004250000468. [DOI] [PubMed] [Google Scholar]
- Guinotte JM, Fabry VJ. Ocean acidification and its potential effects on marine ecosystems. Ann. N. Y. Acad. Sci. 2008;1134:320–342. doi: 10.1196/annals.1439.013. [DOI] [PubMed] [Google Scholar]
- Hall-Spencer JM, Rodolfo-M R, etalpa, Martin S, Ransome E, Fine M, Turner SM, et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 2008;454:96–99. doi: 10.1038/nature07051. [DOI] [PubMed] [Google Scholar]
- Hemminga MA, Duarte CM. Seagrass ecology. Cambridge, U.K: Cambridge Univ. Press; 2000. [Google Scholar]
- Invers O, Zimmerman RC, Alberte RS, Pérez M, Romero R. Inorganic carbon sources for seagrass photosynthesis: an experimental evaluation of bicarbonate use in species inhabiting temperate waters. J. Exp. Mar. Biol. Ecol. 2001;265:203–217. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) Climate change 2007 synthesis report. New York: Cambridge Univ. Press; 2007. [Google Scholar]
- Jassby AD, Platt T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976;21:540–547. [Google Scholar]
- Jiang ZJ, Huang XP, Zhang JP. Effects of CO2 enrichment on photosynthesis, growth and biochemical composition of seagrass Thalassia hemprichii (Ehrenb.) Aschers. J. Integr. Plant Biol. 2010;52:904–913. doi: 10.1111/j.1744-7909.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Ge YM, Zhou YF, Tian GM. Effects of elevated CO2 on growth and nutrient uptake of Eichhornia crassipe under four different nutrient levels. Water Air Soil Pollut. 2010;212:387–394. [Google Scholar]
- Magnusson G, Larsson C, Axelsson L. Figueroa FL, Jiménez C, Pérez- Llorens JL, Niell FX, editors. Effects of high CO2 treatment on nitrate and ammonium uptake by Ulva lactuca grown in different nutrient regimes. Underwater light and algal photobiology. Sci. Mar. 1996;60(Suppl. 1):179–189. [Google Scholar]
- Marschner M. Mineral nutrition of higher plants. 2nd ed. Lond: Academic Press Limited; 1995. [Google Scholar]
- Mercado JM, Javier F, Gordillo L, Niell FX, Figueroa FL. Effects of different levels of CO2 on photosynthesis and cell components of the red alga Porphyra leucosticta. J. Appl. Phycol. 1999;11:455–461. [Google Scholar]
- Mercado JM, Niell FX, Silva J, Santos R. Use of light and inorganic carbon acquisition by two morphotypes of Zostera noltii Hornem. J. Exp. Mar. Biol. Ecol. 2003;297:71–84. [Google Scholar]
- Palacios SL, Zimmerman RC. Eelgrass (Zostera marina L.) response to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar. Ecol. Prog. Ser. 2007;344:1–13. [Google Scholar]
- Parsons TR, Maita Y, Lalli CM. A manual of chemical and biological methods for seawater analysis. Oxford, U.K: Pergamon Press; 1984. [Google Scholar]
- Pelletier G, Lewis E, Wallace D. CO2 sys.xls (version 1.0). A calculator for the CO2 system in seawater for Microsoft Excel/VBA. Olympia, WA: Washington State Department of Ecology; 1997. [Google Scholar]
- Peralta G, Pérez-Lloréns JL, Hernández I, Brun F, Vergara JJ, Bartual A, et al. Morphological and physiological differences between two morphotypes of Zostera noltii Hornem. from the south-western Iberian Peninsula. Helgol. Mar. Res. 2000;54:80–86. [Google Scholar]
- Pörtner HO. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar. Ecol. Prog. Ser. 2008;373:203–217. [Google Scholar]
- Porzio L, Buia MC, Hall-Spencer JM. Effects of ocean acidification on macroalgal communities. J. Exp. Mar. Biol. Ecol. 2011;400:278–287. [Google Scholar]
- Riebesell U, Schulz KG, Bellerby RGJ, Botros M, Fritsche P, Meyerhöfer M, et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature. 2007;450:545–548. doi: 10.1038/nature06267. [DOI] [PubMed] [Google Scholar]
- Sagi M, Scazzocchio C, Fluhr R. The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J. 2002;31:305–317. doi: 10.1046/j.1365-313x.2002.01363.x. [DOI] [PubMed] [Google Scholar]
- Santos R, Silva J, Alexandre A, Navarro R, Barrón C, Duarte CM. Ecosystem metabolism and carbon fluxes of a tidally-dominated coastal lagoon. Estuaries. 2004;27:977–985. [Google Scholar]
- Semesi IS, Beer S, Björk M. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Mar. Ecol. Prog. Ser. 2009;382:41–47. [Google Scholar]
- Silva J, Santos R. Daily variation patterns in seagrass photosynthesis along a vertical gradient. Mar. Ecol. Prog. Ser. 2003;257:37–44. [Google Scholar]
- Silva J, Santos R, Calleja ML, Duarte CM. Submerged versus air-exposed intertidal macrophyte productivity: from physiological to community-level assessments. J. Exp. Mar. Biol. Ecol. 2005;317:87–95. [Google Scholar]
- Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ. 1999;22:583–621. [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. 3rd ed. Sunderland, MA: Sinauer Associates, Inc., Publishers; 2002. [Google Scholar]
- Thom RM. CO2-enrichment effect on eelgrass (Zostera marina L.) and bull kelp (Nereocystis luetkeana (MERT.) P. & R.) Water Air Soil Pollut. 1996;88:383–391. [Google Scholar]
- Touchette BW, Burkholder JM. Carbon and nitrogen metabolism in the seagrass, Zostera marina L.: environmental control of enzymes involved in carbon allocation and nitrogen assimilation. J. Exp. Mar. Biol. Ecol. 2007;350:216–233. [Google Scholar]
- Ullrich WR. Transport of nitrate and ammonium through plant membranes. In: Mengel K, Pilbeam DJ, editors. Nitrogen metabolism of plants. Oxford, U.K: Clarendon Press; 1992. pp. 121–137. [Google Scholar]
- Vessey JK, Henry LT, Chaillou S, Raper CD., Jr Root-zone acidity affects relative uptake of nitrate and ammonium from mixed nitrogen sources. J. Plant Nutr. 1990;13:95–116. doi: 10.1080/01904169009364061. [DOI] [PubMed] [Google Scholar]
- Xu ZG, Zou DH, Gao KS. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rodophyta) Bot. Mar. 2010;53:123–129. [Google Scholar]
- Yelle S, Beeson RC, Gosselin MJ, Trudel A., Jr Duration of CO2 enrichment influences growth, yield, and gas exchange of two tomato species. J. Am. Soc. Hortic. Sci. 1990;115:52–57. [Google Scholar]
- Zieman JC. Methods for the study of the growth and production of the turtlegrass Thalassia testudinum König. Aquaculture. 1974;4:139–143. [Google Scholar]
- Zimmerman RC, Kohrs DG, Alberte RS. Sucrose partitioning in Zostera marina L. in relation to photosynthesis and the daily light-dark cycle. Plant Physiol. 1995;108:1665–1671. doi: 10.1104/pp.108.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RC, Kohrs DG, Steller DL, Alberte RS. Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant Physiol. 1997;115:599–607. doi: 10.1104/pp.115.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed Hizikiafusiforme (Sargassaceae, Phaeophyta) Aquaculture. 2005;250:726–735. [Google Scholar]


