Abstract
Collagen XXIV (Col24α1) is a recently discovered fibrillar collagen. It is known that mouse Col24α1 is predominantly expressed in the forming skeleton of the mouse embryo, as well as in the trabecular bone and periosteum of the newborn mouse. However, the role and mechanism of Col24α1 in osteoblast differentiation and mineralization remains unclear. By analyzing the expression pattern of Col24α1, we confirmed that it is primarily expressed in bone tissues, and this expression gradually increased concomitant with the progression of osteoblast differentiation. Through the use of a lentivirus vector-mediated interference system, silencing Col24α1 expression in MC3T3-E1 murine preosteoblastic cells resulted in significant inhibition of alkaline phosphatase (ALP) activity, cell mineralization, and the expression of osteoblast marker genes such as runt-related transcription factor 2 (Runx2), osteocalcin (OCN), ALP, and type I collagen (Col I). Subsequent overexpression not only rescued the deficiency in osteoblast differentiation from Col24α1 silenced cells, but also enhanced osteoblastic differentiation in control cells. We further revealed that Col24α1 interacts with integrin β3, and silencing Col24α1 up-regulated the expression of Smad7 during osteoblast differentiation while at the same time inhibiting the phosphorylation of the Smad2/3 complex. These results suggest that Col24α1 imparts some of its regulatory control on osteoblast differentiation and mineralization at least partially through interaction with integrin β3 and the transforming growth factor beta (TGF-β) /Smads signaling pathway.
Keywords: COLLAGEN XXIV, Osteoblast differentiation, Bone mineralization, SMAD, Integrin.
Introduction
Collagen, the most abundant protein in the body, is a main component of the extracellular matrix (ECM) forming unique networks in the interstitial spaces between cells. The superfamily of collagens now includes more than 20 types with at least 38 distinct polypeptide chains, as well as more than 15 additional proteins that have collagen-like domains 1. Fibrillar collagens traditionally have been classified into major (types I, II, and III) and minor (types V and XI) collagen types based on their relative abundance in connective tissues. During mammalian development, the different types of collagens are expressed in unique spatiotemporal patterns 2, 3 ultimately providing mechanical strength to skin, bone, and other tissues. It has been shown that aberrations in collagen biosynthesis, or mutations in collagen genes, can result in various bone disorders. Examples include osteogenesis imperfecta 4 resulting from a mutation in type I collagen genes (ColA1 or Col1A2), Achondrogenesis II from a mutation of the Col2Α1 gene, and Kniest and Stickler syndromes similarily with a mutation in the collagen type II gene (Col2Α1) 5-9, to name a few.
Structurally, collagens are characterized by a triple helical region containing Gly-x-y repeats 10, and interact with three types of receptors: integrins, discoidins, and glycoprotein VI 11. Specifically, the integrin family of adhesion molecules has been shown to mediate interactions between cells and their ECM ligands, such as collagen, through direct binding with Arg-Gly-Asp (RGD) or Lys-Gly-Asp (KGD) sites, ultimately regulating cell migration, proliferation, and differentiation 12-15. Previous studies demonstrate that RGD- and KGD-containing peptides are specific and potent competitive inhibitors of integrin β3 function 16, 17 the results of which include blocked platelet activation and platelet PDGF-AB release, as well as negatively regulating Smad7 expression. Additionally, accumulating evidence shows that there is crosstalk between integrins and TGF-β signaling 18, 19, with TGF-β regulating the expression of integrins, their ligands, and integrin-associated proteins, as well as affecting cell adhesion and migration. Conversely, some integrins directly regulate TGF-β activation 12. Asano et al 20, 21 observed an increased expression of both integrin αvβ3 and αvβ5 in the dermis of scleroderma patients, which subsequently elicited autocrine TGF-β signaling in patient fibroblasts in vitro. Additionally, mutation of the RGD site of latency-associated peptide (LAP) causes defects similar to those showed in TGF-β1-null mice 22.
Col24α1 is a recently defined minor type V collagen containing two clusters of Gly-x-y collagenous repeats and three non-collagenous domains 10. Having 6 repeat KGD motifs, which are binding sites for integrins 23, Col24α1 was found to be initially expressed in the emerging skeletal elements of the head and appendicular skeleton at embryonic day 15 with expression at later embryonic stages detected in the cornea, the otic capsule, and skeleton where it coincided with the formation of primary ossification centers and sites of type I collagen expression 24. This suggests that Col24α1 may participate in the control of important physiological processes in bone and cartilage, however the role and mechanism of Col24α1 in osteoblast differentiation and mineralization remains unclear.
We hypothesize that Col24α1 regulates osteoblast differentiation and mineralization through interaction with integrins, which leads to the activation of the TGF-β/Smads signaling pathway. In this study, we first confirmed that Col24α1 is predominantly expressed in bone tissues and during osteoblast differentiation. We then analyzed the effect of Col24α1 on osteoblast differentiation and mineralization by performing loss of function and gain of function studies. Finally, we looked into the specific interaction with integrin β3 and the TGF-β/Smads signaling pathway that Col24α1 uses to impart its regulatory control. We believe that our findings provide novel insight into collagen research as well as osteoblast differentiation and mineralization.
Materials and methods
Cells and cell culture
A murine preosteoblastic cell line derived from murine calvaria (MC3T3-E1 clone 4) 25, and a human embryonic kidney cell line (HEK293T) were obtained from the American Type Culture Collection (ATCC). MC3T3-E1 cells were seeded at a density of 2×104 cells/cm2 and maintained in α-MEM complete media (α-modified Eagle's Minimum Essential Medium [α-MEM] with 10% fetal bovine serum [FBS, Gibco], 100U/ml penicillin and 100mg/ml streptomycin). HEK293T cells were cultured in DMEM complete media (Dulbecco's Modified Eagle Media with 10% FBS, 100U/ml penicillin and 100mg/ml streptomycin). Mouse mensenchymal stem cells (MSCs) were prepared and cultured as previous described 26. For osteoblast differentiation, MC3T3-E1 cells or MSCs were induced with osteogenic medium (OS media), which is α-MEM complete media with 50μg/ml ascorbic acid, 10mM β-glycerol-phosphate and 10nM dexamethasone (Sigma) 27.
Reverse transcription PCR (RT-PCR) and quantitative real-time RT-PCR (qPCR) analysis
All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee, University at Buffalo, and were performed in accordance with the institutional guidelines and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council). Total RNAs were isolated from 14-day-old c57BL/6J mouse tissues and OS media-induced MC3T3-E1 cells using Trizol reagent (Invitrogen) according to the manufacturer's instructions. In regards to isolation of total RNA from long bone it was performed as described 28, 29 with minor modifications. Briefly, long bones, which had been snap frozen, were freed of periosteum and bone marrow, ground with a mortar and pestle, and then total RNA was extracted using Trizol reagents. Reverse transcription of total RNA was carried out for 50 mins at 42°C and then 15 mins at 70°C, using the SuperScriptTM First Strand Synthesis System for RT-PCR (Invitrogen). The resulting single-strand cDNA molecules were used to perform qPCR. The primers were designed as follows: Col24α1 (forward primer, 5'-GAAGCCACCCACACCATCAC-3'; reverse primer, 5'-TTCTTCAAAAATCTGACCA TTCCAA-3'), Smad2 (forward primer, 5'-GAGGAGCAGCTCGCCAA-3'; reverse primer, 5'-CTGTCAAGGTCCGGCCAGCG-3'), Smad3 (forward primer, 5'- GTGA CCCTTCGGTGCCAGCC-3'; reverse primer, 5'-GGGGCTCAATGCCAGCAGGG-3'), Smad7 (forward primer, 5'-CTGCAGCGGCCAATGACCA-3'; reverse primer, 5'-AT GAGCCTCTCAGCCGGGGG-3'), ALP (forward primer, 5'-GC AGCTTGGTGCACACCTAG-3'; reverse primer, 5'-GAGACATTTTCCCGTTCACC-3'.), Runx2 (forward primer, 5′-CCGGCAAGATGAGCGAGGTCA-3′; reverse primer, 5′-GTGGGTT GGAGAAGCGGCTCT-3′), OCN (forward primer, 5'-ATGAGGACCCTCTCTCTGC T-3'; reverse primer, 5'-GGAGCTGCTGTGACATCCAT-3'), bone sialo protein (BSP) (forward primer, 5'-CAGGGAGGCAGTGACTCTTC-3'; reverse primer, 5'-AGTGT GGAAAGTGTGGCGTT-3') and GAPDH (forward primer, 5'-ACCACAGTCCATGCCATCAC-3'; reverse primer, 5'-TCCACCACCCTGTTGCTGTA-3'). Reactions were performed on an ABI PRISM 7500 sequence detection system with SYBR GREEN PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions. The PCR conditions were 94°C for 1 min followed by 95°C for 30 secs and 58°C for 40 secs for a total of 35 cycles. All of the reactions were run in triplicate and normalized to the housekeeping gene GAPDH. The relative differences in PCR results were calculated using the comparative cycle threshold method.
Col24α1 shRNA lentivirus packaging, tittering, and cell infection
To identify the role of the Col24α1 gene in osteoblast differentiation as well as the osteoblastic signaling pathway, we used a lentivirus vector-mediated interference system. The Col24α1 shRNA sequences used can be found in Table 1. Five individual pLB-Col24α1 shRNA (Col-S) vectors and a control pLB-scramble shRNA (PLB) vector (Open Biosystems) were co-transfected with the packaging plasmids, pCMV-dRr8.2 and pCMV-VSV-G (Addgene) 30, into HEK293T cells using a calcium phosphate co-precipitation method. The medium was replaced with fresh complete media after co-transfection for 8 hrs. The lentiviral supernatant was harvested after 48-72 hrs, and titers were determined by infecting HEK293T cells with serial dilutions of lentivirus in the presence of 4μg/ml polybrene (Sigma). The viral supernatant was then used to infect MC3T3-E1 cells and after 24 hrs the virus-containing media was removed and replaced with fresh complete media. After incubation for 48 hrs, the cells were analyzed by qPCR, western blot, and immunostaining to test the silence efficiency of the Col24α1 gene. For osteoblast differentiation, the infected cells were induced with OS media for the indicated times based on different experiments.
Table 1.
The shRNA sequences of Col24α1.
| 1. Hairpin sequence for TRCN0000089943 |
| CCGGCCCTAGAACTGAAATTGTATACTCGAGTATACAATTTCAGTTCTAGGGTTTTTG |
| Mature Sense for TRCN0000089943 CCCTAGAACTGAAATTGTATA |
| Mature Antisense for TRCN0000089943 TATACAATTTCAGTTCTAGGG |
| 2. Hairpin sequence for TRCN0000089944 |
| CCGGCCTCTCAGATGACTGCAAGATCTCGAGATCTTGCAGTCATCTGAGAGGTTTTTG |
| Mature Sense for TRCN0000089944 CCTCTCAGATGACTGCAAGAT |
| Mature Antisense for TRCN0000089944 ATCTTGCAGTCATCTGAGAGG |
| 3. Hairpin sequence for TRCN0000089945 |
| CCGGCCACAGTTCAGACATATTCAACTCGAGTTGAATATGTCTGAACTGTGGTTTTTG |
| Mature Sense for TRCN0000089945 CCACAGTTCAGACATATTCAA |
| Mature Antisense for TRCN0000089945 TTGAATATGTCTGAACTGTGG |
| 4. Hairpin sequence for TRCN0000089947 |
| CCGGGTACAAGGTTTCAGATGGAAACTCGAGTTTCCATCTGAAACCTTGTACTTTTTG |
| Mature Sense for TRCN0000089947 GTACAAGGTTTCAGATGGAAA |
| 5. Mature Antisense for TRCN0000089947 TTTCCATCTGAAACCTTGTAC |
| Hairpin sequence for TRCN0000089946 |
| CCGGGTTGGAGTTTGGAGTCAGCAACTCGAGTTGCTGACTCCAAACTCCAACTTTTTG |
| Mature Sense for TRCN0000089946 GTTGGAGTTTGGAGTCAGCAA |
| Mature Antisense for TRCN0000089946 TTGCTGACTCCAAACTCCAAC |
Note: Reference Sequence for: NM_027770.
Ectopic expression of Col24α1
Retroviral vector pBMN-Col24α1 was constructed by inserting a full-length 6.18kb Col24α1 cDNA (access no. NM_027770) into the EcoRI and Not I site of pBMN-I-GFP (Addgene). Packaging was performed as in the protocol from the Dr. Garry Nolan Laboratory, Stanford University. Briefly, the retrovirus vector pBMN-I-GFP (Control) and experimental pBMN-Col24α1 vector (Col-O) were separately transfected into the Phoenix-Eco packaging cells using a calcium phosphate co-precipitation method 31. Following transfection, the cells were placed in a 32°C humidified incubator for 48 hrs (32°C aids in stabilizing the virus). The virus containing supernatant was harvested and filtered through a 0.45 µm filter for tittering assay. The Control and Col-O retroviruses were then used to infect 70-80% subconfluent MC3T3-E1 cells in the presence of 8μg/ml polybrene. After incubation for 48 hrs, the cells were analyzed by western blot and immunostaining. For osteoblast differentiation, the infected cells were induced with OS media for the indicated times based on different experiments.
ALP activity measurement
ALP activity was determined using the ALP assay kit in keeping with the manufacturer's instructions (Sigma). Briefly, cells were washed with ice-cold PBS, lysed with 0.5% Triton X-100, and centrifuged. ALP assay was performed in alkaline buffer solution (1.5 M, pH 10.3) containing 10mM p-nitrophenyl phosphate as a substrate. Following the addition of the stop solution (3M NaOH), the optical density was measured in a microplate reader at 405nm. ALP activity was normalized with the value of DNA content, measured according to the method of Schneider 32, and expressed as nmol of p-nitrophenol produced per minute per mg of total DNA 29.
Alizarin red staining
To measure bone nodule formation, extracellular matrix calcium deposits were stained using Alizarin red dye as previously described 33, 34. Briefly, at 14 days following the induction with OS media, cells were fixed with 2.0% formaldehyde and stained with 40mmol/L of Alizarin red solution (pH 4.4) for 40 mins at room temperature. The images of stained cells were captured using a phase contrast microscope with a digital camera (IM50, Leica, Germany). The cells were then destained for 15 mins with 10% (w/v) cetylpyridinium chloride (Sigma) in 10mM sodium phosphate (pH 7.0). The extracted stain was transferred to a 96-well plate and the absorbance measured at 562nm. The mineralization values were normalized to the relative value of the control (PLB or Control) 35.
Von Kossa staining
Because calcium coprecipitates with phosphate ions in the matrix, Von Kossa staining was also used for determining mineralization in the cultures 36, 37. Cells were fixed with 2.0% formaldehyde for 15 mins. After washing with deionized water 3 times, cells were incubated in 5% silver nitrate solution at room temperature under ultraviolet light for 1 hr.
Immunofluorescence staining
The cells were plated on 24-well plates, incubated overnight, and then infected with Col-S or Col-O for 24 hrs. After induction with OS media for 14 days, the cells were fixed with 100% methanol for 10 mins, blocked with 5% (v/v) normal rabbit serum for 60 mins, then correspondingly incubated with mouse anti-Col24α1 antibody (1µg/ml, Sigma) in TBS containing 1.5% normal rabbit serum for 60 mins. Following this, cells were incubated in Texas red conjugated anti-mouse IgG (1µg/ml, Santa Cruz Biotechnology) with 1.5% normal rabbit serum for 60 mins, washed three times in PBS, and examined with fluorescence microscopy 31.
Western blot analysis
Western blots were performed as described 38. Briefly, the cells were lysed with NP40 buffer (1% NP-40, 0.15M NaCl, 50mM Tris, pH 8.0) containing protease inhibitors (Sigma). Equal amounts of protein were separated on an 8-10% polyacrylamide-SDS gel. Proteins were transferred to polyvinylidene difluoride membranes. Activation of Smad2/3 was detected using anti-phospho-Smad2/3 antibody (Santa Cruz Biotechnology, CA). Expression of Col24α1 or GAPDH was detected using anti-Col24α1 antibody (Sigma), and anti-GAPDH antibody (Cell Signaling Biotechnology, CA), respectively.
Co-immunoprecipitation (Co-IP)
Cells were lysed in NP40 buffer containing protease inhibitors (Sigma). Lysates were incubated for 6 hrs at 4°C with anti-integrin β3 antibody (Abcam, USA) coupled to Protein A-Agarose beads (Santa Cruz Biotechnology, CA). To approximately 1ml of whole cell lysates, 0.25µg of the appropriate control IgG was added with 20µl of the appropriate suspended (25% v/v) agarose conjugate (Protein A-Agarose). Cell lysates were then incubated at 4°C on a rotating device overnight. Centrifugation at 3,000 rpm for 30 secs at 4°C was performed to collect the cell pellet. After washing 2 times, the pellet was resuspended and boiled for 5 mins. The proteins were identified by western blot analysis using the anti-integrin β3 and anti-Col24α1 antibodies as described 39.
Statistical analysis
Where indicated, experimental data are reported as mean ± SD of triplicate independent samples. Statistical analysis was performed using the software SPSS-17.0. Statistical significance for two groups was assessed using Student's t-test. All other data was analyzed using one-way analysis of variance 40, and the Tukey HSD was applied as a post hoc test if significance was found. The probability level (P) at which differences were considered significant was P<0.05.
RESULTS
Col24α1 is predominantly expressed in bone tissues and during osteoblast differentiation
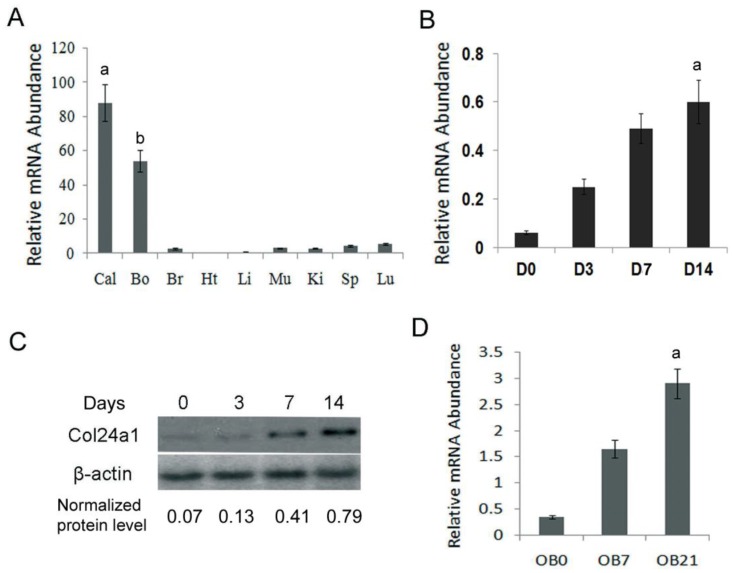
To identify the expression pattern of Col24α1 in different tissues, qPCR analysis was performed in 14-day-old c57BL/6J mouse tissues. The results showed that Col24α1 was predominantly expressed in calvaria and long bone. There was also the expression observed in the brain, muscle, kidneys, spleen, liver, and lung but to a far lesser extent (Fig. 1A). To characterize the time course of Col24α1 expression during osteoblast differentiation, qPCR and western blot analysis were performed. As shown in Fig. 1B-C, Col24α1 was expressed at low levels in MC3T3-E1 cells prior to OS media induction. Following OS media induction, Col24α1 expression increased concomitant with osteoblast differentiation. Finally, we looked at the expression of Col24α1 in OS media induced MSCs by qPCR. As shown in Fig. 1D, the expression of Col24α1 gradually increased during osteoblast differentiation in those cells as well.
Fig 1.
Col24α1 is predominantly expressed in pre-osteoblasts, osteoblasts, and bone tissue. (A) Quantitative real-time RT-PCR analysis of Col24α1 mRNA expression in 14-day-old c57BL/6J mouse tissues. Total RNA was extracted from calvaria, long bone, brain, heart, liver, muscle, kidney, spleen, and lung. N=6, p < 0.01; a: calvaria vs other tissues, b: long bone vs other tissues. (B) Quantitative real-time RT-PCR analysis of Col24α1 mRNA in MC3T3-E1 cells induced with OS media for 0, 3, 7, 14 days show that the expression of Col24α1 gradually increases during osteoblast differentiation. N=6, p < 0.01; a: between all groups. (C) Western blot analysis of Col24α1 protein expression in MC3T3-E1 cells induced with OS media for 0, 3, 7, and 14 days confirms the results in B. Normalization performed using β-actin levels. (D) Quantitative real-time RT-PCR analysis of Col24α1 expression in MSCs induced with OS media for 0, 7, and 21 days. The results show that Col24α1 expression increases during osteoblast differentiation. N=6, p < 0.01; a: between all groups. Unless indicated otherwise normalization was performed against GAPDH levels for control in the same reaction.
Silencing Col24α1 inhibits osteoblastic ALP activity and cell mineralization
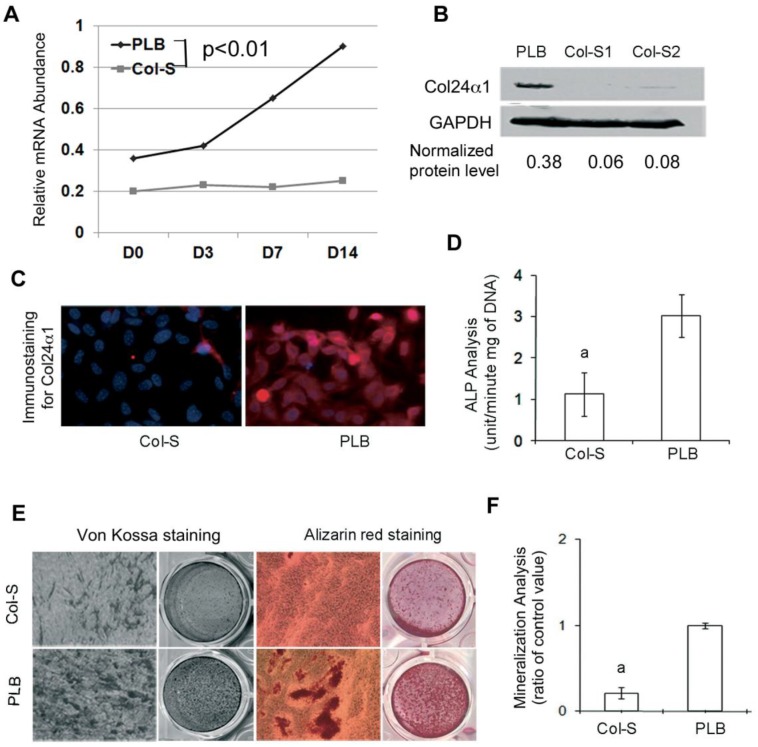
To demonstrate the importance of Col24α1 function in osteoblast differentiation and activation, a lentivirus vector-mediated interference system was used to silence Col24α1 expression in MC3T3-E1 cells. We found that both Col24α1 mRNA and protein expression were blocked in Col-S cells as compared with that of PLB cells (Fig. 2A-B). Consistent with these results, immunofluorescent staining showed the diminished Col24α1 protein expression in silenced cells as compared to control (Fig. 2C).
Fig 2.
Silencing Col24α1 blocks osteoblast differentiation and mineralization. (A) Quantitative real-time RT-PCR analysis. MC3T3-E1 cells were infected with Col-S or PLB lentiviruses for 48 hrs, and then induced with OS media for 0, 3, 7, 14 days. The mRNA levels of Col24α1 gradually increase in control cells over time as compared to control cells, which are significantly lower. N=6, p < 0.05; PLB vs Col-S at all time points. (B) Western blot analysis of Col24α1 protein expression. The cells were treated as described in A. The level of Col24α1 in the silenced groups was 5.6 fold (Col-S1) and 4.6 fold (Col-S2) lower than that in the control. (C) Immunofluorescence staining revealed that the expression of Col24α1 was diminished in Col-S cells at day 7 after infection. (D) ALP activity. Cells at 70-80% confluence were infected with Col-S or PLB lentiviruses and then induced with OS media for 7 days. ALP activity in the Col-S group was significantly lower than the PLB group. N=6, p<0.05; a: PLB vs Col-S. (E) Alizarin red and Von Kossa staining. MC3T3-E1 were infected with Col-S or PLB lentiviruses for 24 hrs and then induced with OS media for 14 days. Silencing Col24α1 significantly reduces extracellular matrix mineralization. (F) The quantitative analysis of Alizarin red staining as seen in E confirms these results. N=6, p < 0.05; a: PLB vs Col-S. Unless indicated otherwise normalization was performed against GAPDH levels for the control group in the same reaction.
ALP hydrolyzes pyrophosphate and provides inorganic phosphate to promote mineralization in osteoblasts 41. In Fig. 2D we found that ALP activity was significantly reduced in Col24α1 silenced MC3T3-E1 cells after induction with OS media for 7 days as compared to the control. Looking further into the importance of Col24α1 on bone mineralization, MC3T3-E1 cells infected with either Col-S or PLB were induced with OS media for 14 days and then analyzed using Alizarin red and Von Kossa staining assays. Silencing Col24α1 resulted in a reduction of both calcium and phosphate deposits (Fig. 2E). More so, the quantitative mineralization level, based on Alizarin red staining, was 3.3-fold lower in silenced cells than control (Fig. 2F).
Ectopic expression of Col24α1 promotes osteoblast differentiation and cell mineralization
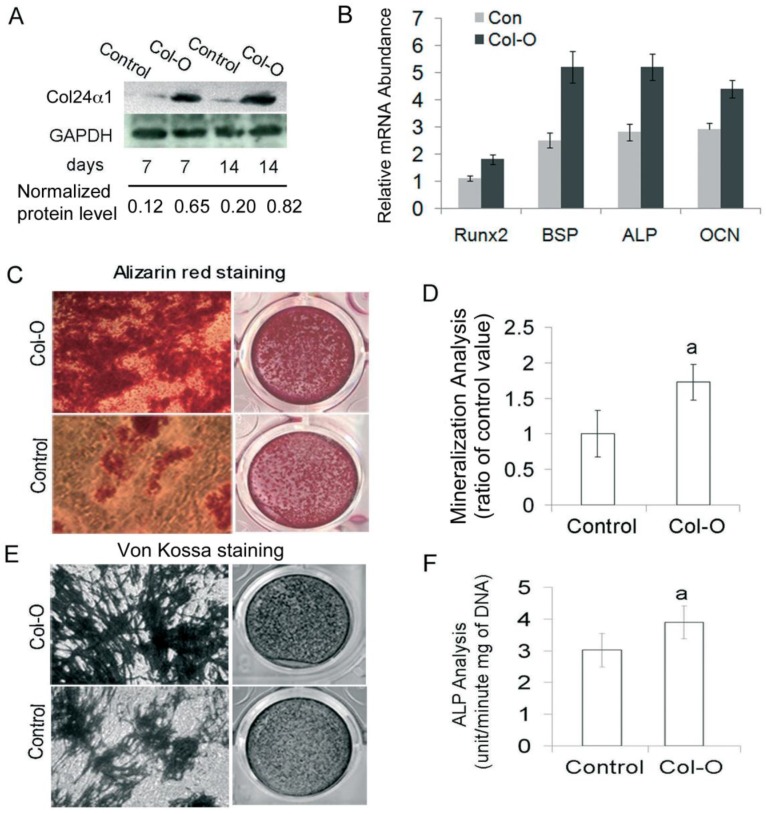
To gain further insight into the function of Col24α1, we characterized the effect of its ectopic expression on osteoblast differentiation and mineralization in MC3T3-E1 cells induced with OS media. Following infection with either the Col-O or Control retroviruses, we detected Col24α1 protein expression by western blot. As shown in Fig. 3A, protein levels were significantly increased in Col24α1 overexpressed cells compared with the control. Using qPCR to detect expression levels of osteoblast marker genes Runx2, BSP, ALP and OCN, we demonstrated that overexpression of Col24α1 dramatically increased their expression (Fig. 3B). To analyze the effect of Col24α1 overexpression on cell mineralization, infected MC3T3-E1 cells were induced with OS media for 14 days, following which Alizarin red and Von Kossa staining assays were performed. Notably, we found that overexpression of Col24α1 significantly increased the production of mineralized matrix (Fig. 3C-E). Lastly, to evaluate the effects of overexpression of Col24α1 on osteoblast differentiation, the cells were induced with OS media for 7 days following infection with either Col-O or Control retroviruses and then ALP activity was measured. As shown in Fig 3F, overexpression of Col24α1 significantly increases ALP activity.
Fig 3.
Overexpression of Col24α1 significantly increases osteoblast differentiation and mineralization. MC3T3-E1 cells were infected with Control or Col-O retroviruses for 24 hrs and then induced with OS media for 7 and 14 days. (A) Western blot analysis. The expression level of Col24α1 in the Col-O group is increased 5.4 fold (7 days) and 4.1 fold (14 days) than that in the control. (B) Quantitative real-time RT-PCR analysis of osteoblast marker genes Runx2, BSP, ALP, and OCN in MC3T3-E1 cells induced with OS media for 7 days. For all genes the mRNA levels were significantly increased in Col-O group as compared with the Control. N=6, p < 0.05; Control vs Col-O for all genes. (C) Alizarin red staining. There is an increase in calcium deposits for the overexpression group as compared to the control. (D) The quantitative analysis of Alizarin red staining as seen in C. N=6, p<0.05; a: Control vs Col-O. (E) Von Kossa staining method shows increased phosphate ion deposits in mineralized matrix. (F) ALP activity. Following infection of MC3T3-E1 cells with Col-O or Control retroviruses they were induced with OS media for 7 days. The Col-O group shows an increase in activity as compared to the Control group. N=6, p<0.05; a: Control vs Col-O. Unless indicated otherwise normalization was performed against GAPDH levels for the control group in the same reaction.
Ectopic expression of Col24α1 rescues impaired osteoblast differentiation and mineralization in Col24α1 silenced cells
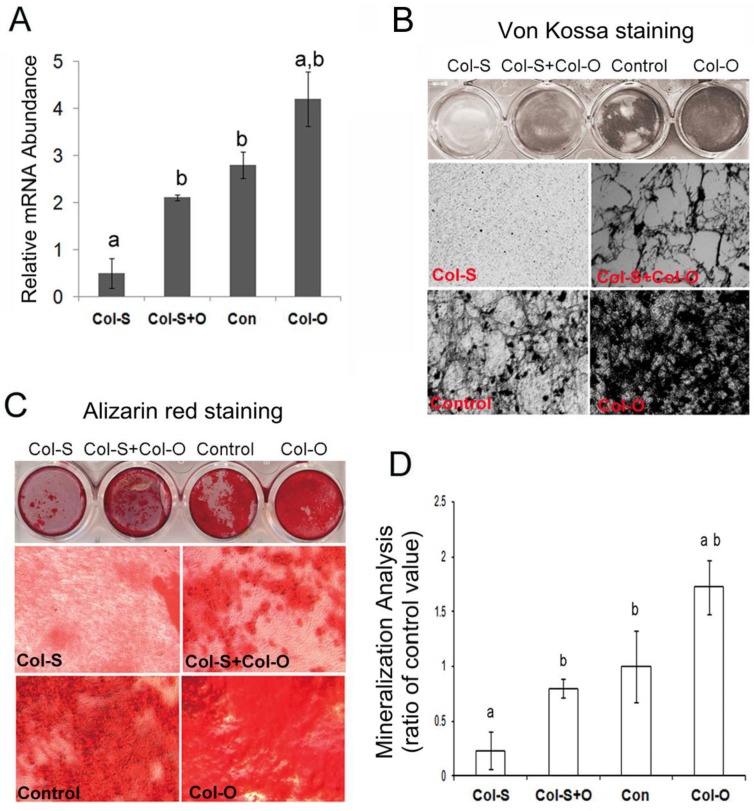
MC3T3-E1 cells were infected with Col-S or PLB lentivirus for 24 hrs before infecting with the Col-O or Control retrovirus for an additional 24 hrs. Following culture for 48 hrs, cells were harvested and the expression level of Col24α1 was analyzed with qPCR. As expected, Col-O was able to rescue the expression of Col24α1 in the silenced cells almost to the level of the control (Fig 4A). Additionally, infected cells were induced with OS media for 14 days for Von Kossa (Fig 4B) and Alizarin red staining (Fig 4C). We found that overexpression of Col24α1 could rescue the impaired cell mineralization resulting from silencing. Quantitative analysis of Alizarin red stain showed the levels of mineralization in Col-S+Col-O and Col-O cells increased 3.43-fold and 7.41-fold, respectively, as compared to Col-S cells. There was no significant difference between Col-S+Col-O and control cells (Fig. 4D).
Fig 4.
Ectopic expression of Col24α1 rescues impaired osteoblast differentiation and mineralization resulting from Col24α1 silencing. MC3T3-E1 cells were first infected with Col-S or PLB lentiviruses for 48 hrs and then infected with Col-O or Control retroviruses for an additional 48 hrs. Fresh media was then added and the cells were cultured for the appropriate amount of time based on the given experiment. (A) Quantitative real-time RT-PCR. At 48 hrs cells were harvested and Col24α1 mRNA levels were analyzed. The Col-S group was significantly lower than all other groups. There was no significant difference found between the Col-S+O and Control groups. The Col-O group was significantly greater than all other groups. N=12, p < 0.05; a: Col-S vs all other groups, b: Col-O vs all other groups. (B) Von Kossa staining following induction with OS media for 14 days. There is minimal phosphate ion deposits in the Col-S group, similar levels of mineralization between the Col-S+O and Control groups, and a dramatic increase in the Col-O group. (C) Alizarin red staining following induction with OS media for 14 days. As one might expect, identical results can be seen here as in B. (D) The quantitative analysis of Alizarin red staining as seen in C. Compared to the Col-S group there is a significant increase in mineralization of 3.43 fold and 7.41 fold for the Col-S+O and Col-O groups, respectively. There is no significant difference between Col-S+O and Control groups. N=12, p < 0.05; a: Col-S vs all other groups, b: Col-O vs all other groups. Unless indicated otherwise normalization was performed against GAPDH levels for the control group in the same reaction.
Col24α1 interacts with the integrin β3 chain to regulate TGF-β/Smads signaling pathway
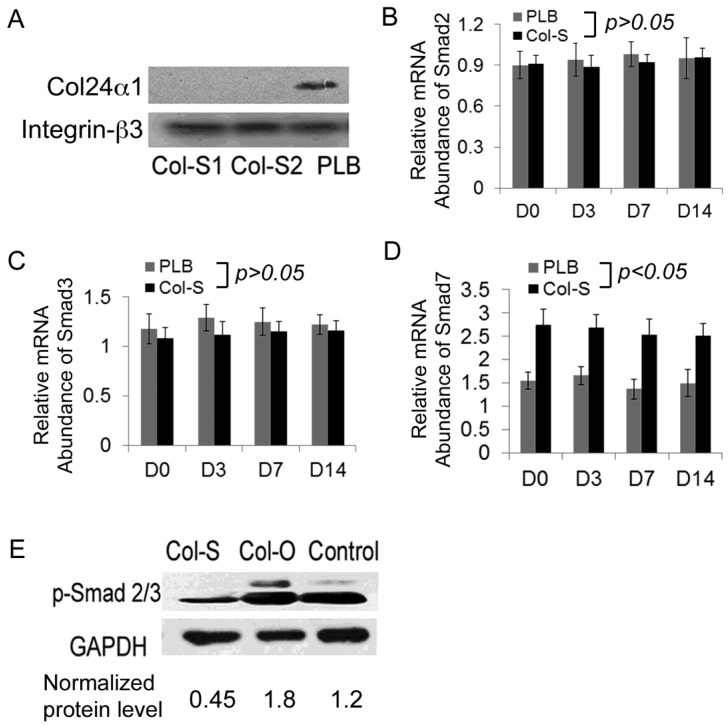
Bone mineralization and osteoblast differentiation are modulated by integrins 42, 43. Furthermore, previous reports have shown that Arg-Gly-Asp (RGD) and Lys-Gly-Asp (KGD) are putative integrin binding motifs 12, 14, 23. Knowing that MC3T3-E1 cells express integrin chains such as αv, α2, β1, and β3 44-46 it is likely that Col24α1, which contains 6 repeat KGD motifs, imparts some of its regulatory control at these sites. Since KGD binds with much better affinity to integrin β3 chain than to others, such as β1 and αv 47, we choose to characterize its interaction with Col24α1. Our results indicated that not only is there specific binding between Col24α1 and the integrin β3 chain in osteoblasts, but silencing Col24α1 has no effect on the expression level of the integrin β3 chain (Fig. 5A).
Fig 5.
Col24α1 interacts with the integrin β3 chain and regulates the TGF-β/Smads signaling pathway. MC3T3-E1 cells were infected with Col-S or PLB lentiviruses for 48 hrs and then induced with OS media for appropriate time based off of the requirements for the given experiment. (A) Co-immunoprecipitation assay. The infected cells were induced with OS media for 7 days, harvested for precipitation of integrin β3, and then western blot was performed using antibodies against the Col24α1 as well as integrin β3 proteins. It is evident that Col24α1 directly interacts with the integrin β3 subunit in OS media-induced cells. (B-D) Quantitative real-time RT-PCR analysis of the transcription of Smad2 (B), Smad3 (C) and Smad7 (D) in MC3T3-E1 cells induced with OS media for 0, 3, 7, 14 days. There is a significant increase in Smad7 mRNA in the Col-S group as compared to the PLB group at all time points. N=12, p < 0.05. There is no significant difference between the groups for either Smad2 or Smad3 mRNA levels. N=12, p > 0.05. (E) Western blot analysis of phospho-Smad2/3 protein expression. Following infection with either Col-S or Col-O viruses, MC3T3-E1 cells were induced with OS media for 7 days. The levels of phospho-Smad2/3 in the Col-S and Col-O groups are 0.38 fold and 1.52 fold that of the Control group, respectively. Unless indicated otherwise normalization was performed against GAPDH levels for the control group in the same reaction.
Accumulating evidence indicates that integrins directly mediate TGF-β activation 12. Additionally, Smad proteins are critical components of the TGF-β signaling pathway, critically regulating osteoblast differentiation. Using qPCR we found that silencing Col24α1 had no effect on Smad2 and Smad3 expression, however it did result in increased expression of the inhibitory Smad7 (Fig. 5B-D). To further investigate whether Col24α1 affects Smads activation, we examined the protein level of phospho-Smad2/3 by western blot. As shown in Fig. 5E, there was a significant difference in the level of phospho-Smad2/3 between the Col-O and control group, as well as between Col-S and the control group. The level of phospho-Smad2/3 in the Col-O and Col-S groups were 1.52-fold and 0.38-fold that of the control group, respectively, clearly indicating that both silencing and overexpression of Col24α1 affected Smad2/3 activation. This suggests that Col24α1 regulates osteoblast differentiation and mineralization through the TGF-β/Smads signaling pathway.
Discussion
Col24α1 is predominantly expressed in the forming bone elements of the mouse embryo 24 as well as in the trabecular bone and periosteum of the newborn mouse 48. Consistent with these previous studies, we found that Col24α1 was highly expressed in the calvaria and bone tissues of 14-day-old c57BL/6J mice. There was also expression observed in the brain, muscle, kidneys, spleen, liver, and lung but to a far lesser extent.
During early stages of osteoblast differentiation, cells in the osteoblast lineage synthesize type I collagen (Col-I) and other matrix proteins, followed by the production of ALP and other osteoblastic differentiation markers, ultimately leading to the induction of ECM calcification 49, 50. Matsuo et al 48 found that Col24α1 transcription is activated at about the same time as that of the osteocalcin gene and gradually increases to eventually plateau as osteoblasts begin to deposit a mineralizing matrix. Our results showed that Col24α1 expression was detected in the early stages and increased concomitant with osteoblast differentiation.
Several studies have demonstrated that the components of the ECM play an important role not only in osteoblast differentiation but also in bone mineralization 51-53. In this study, we found that silencing Col24α1 in MC3T3-E1 cells suppressed the expression of osteoblast marker genes ALP, OCN, Runx2, and BSP, as well as significantly blocked ALP activity and cell mineralization. Subsequent overexpression of Col24α1 was able to rescue this deficit in silenced cells, and significantly promoted osteoblast differentiation and mineralization in the control cells. Collectively, these above findings confirm that Col24α1 is not only selectively expressed in bone tissues, but it appears to have an integral role in osteoblast gene expression, differentiation, and the formation of mineralized bone matrix. So the question remains, what the exact role and mechanism by which Col24α1 imparts this regulatory control on osteoblast differentiation and subsequent mineralization?
Integrins are a family of heterodimeric transmembrane glycoproteins consisting of an α- and β- chain, and are the major cell surface receptors mediating ECM interactions 12. They act as transducers by relaying information from the ECM to the cell interior or vice versa and have diverse roles in mediating cell adhesion, migration, proliferation, differentiation, and survival 13, 14. Osteoblasts express integrins, which interact with bone matrix proteins (e.g. collagen) to transduce signals from the ECM and regulate their commitment. Previous reports have shown that Arg-Gly-Asp (RGD) and Lys-Gly-Asp (KGD) are putative integrin binding motifs 12, 14, 23. We know that Col24α1 contains 6 repeat KGD motifs and our Co-IP results demonstrated the presence of a Col24α1-integrin β3 complex in control cells but not in silenced cells. This indicates that Col24α1 directly interacts with the integrin β3 chain during osteoblast differentiation most likely through binding at the KGD motifs.
TGF-β signaling plays a crucial role in osteoblast differentiation 54 and mineralization 55-57, and integrins are the main modulators of this cascade 58, 59. TGF-β elicits its effects by interacting with TGF-β receptors to recruit and activate, through phosphorylation, the intracellular effectors Smad2 and Smad3. The phosphorylated Smad2/3 complex subsequently binds to Smad4 and translocates to the nucleus affecting gene expression and ultimately cell behavior 54, 60. We showed that silencing Col24α1 inhibited the phosphorylation of the Smad2/3 protein complex and resulted in the decreased expression of the osteoblast marker genes Runx2, ALP, OCN, and BSP. At the same time, the expression of Smad7 was up-regulated. In contrast to this, overexpression of Col24α1 significantly increased phosphorylation of the Smad2/3 complex. It is known that Smad7 inhibits TGF-β signaling by associating with its receptor and thus preventing Smad2 and Smad3 access for phosphorylation 61, 62. Given this evidence it seems that Col24α1 regulates osteoblast differentiation and mineralization in some form or another through the TGF-β/Smads signaling pathway.
Col24α1 undoubtedly has a role in osteoblast differentiation and mineralization. Synthesizing the evidence provided here one explanation is that Col24α1 binds with the integrin β3 chain at one or more of its six KGD motifs. Subsequent binding with TGF-β leads to the negative regulation of Smad7 signaling, which has the effect of freeing up the TGF-β receptor to allow the Smad2/3 complex access for phosphorylation resulting in an increase in osteoblast gene expression, differentiation, and cell matrix mineralization. Current studies in our lab are looking into whether Col24α1 interacts with other integrins as well as further elucidating how it is working through the TGF-β/Smads signaling pathway. We are also in the process of generating a conditional knockout model to look at its affects in vivo. This research may provide a platform for the development of new pharmacologic strategies for the treatment of bone disorders associated with mutations in collagen synthesis, accumulation, and degradation. Additionally, there is the potential to use Col24α1 as an osteogenic factor for promoting bone regeneration and healing.
Acknowledgments
We thank Drs. Elizabeth (Betty) A. Smith and David Hadbawnik for their critical reading of the manuscript, and Dr. Wade J. Sigurdson for his technical assistance with fluorescence microscopy and image analysis. This study was supported by the grant AR055678 (S. Yang) and AR061052 (S. Yang) from National Institutes of Health.
Grant sponsor
NIAMS/NIH AR055678 (S. Yang) and AR061052 (S. Yang).
References
- 1.Ricard-Blum S, Ruggiero F. The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol (Paris) 2005;53:430–42. doi: 10.1016/j.patbio.2004.12.024. doi:S0369-8114(04)00313-X [pii] 10.1016/j.patbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Golub EE. Role of matrix vesicles in biomineralization. Biochim Biophys Acta. 2009;1790:1592–8. doi: 10.1016/j.bbagen.2009.09.006. doi:S0304-4165(09)00268-2 [pii] 10.1016/j.bbagen.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson HC, Garimella R, Tague SE. The role of matrix vesicles in growth plate development and biomineralization. Front Biosci. 2005;10:822–37. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 4.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C. et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52. doi: 10.1038/382448a0. doi:10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 5.Chung KS, Jacenko O, Boyle P, Olsen BR, Nishimura I. Craniofacial abnormalities in mice carrying a dominant interference mutation in type X collagen. Dev Dyn. 1997;208:544–52. doi: 10.1002/(SICI)1097-0177(199704)208:4<544::AID-AJA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Savontaus M, Rintala-Jamsa M, Morko J, Ronning O, Metsaranta M, Vuorio E. Abnormal craniofacial development and expression patterns of extracellular matrix components in transgenic Del1 mice harboring a deletion mutation in the type II collagen gene. Orthod Craniofac Res. 2004;7:216–26. doi: 10.1111/j.1601-6343.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 7.Spranger J, Menger H, Mundlos S, Winterpacht A, Zabel B. Kniest dysplasia is caused by dominant collagen II (COL2A1) mutations: parental somatic mosaicism manifesting as Stickler phenotype and mild spondyloepiphyseal dysplasia. Pediatr Radiol. 1994;24:431–5. doi: 10.1007/BF02011911. [DOI] [PubMed] [Google Scholar]
- 8.Wang WZ, Guo X, Duan C, Ma WJ, Zhang YG, Xu P. et al. Comparative analysis of gene expression profiles between the normal human cartilage and the one with endemic osteoarthritis. Osteoarthritis Cartilage. 2009;17:83–90. doi: 10.1016/j.joca.2008.05.008. doi:S1063-4584(08)00170-2 [pii] 10.1016/j.joca.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Guo X, Chen J, Xu P, Lammi MJ. Morphology and phenotype expression of types I, II, III, and X collagen and MMP-13 of chondrocytes cultured from articular cartilage of Kashin-Beck Disease. J Rheumatol. 2008;35:696–702. doi:08/13/0313 [pii] [PubMed] [Google Scholar]
- 10.Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339:247–57. doi: 10.1007/s00441-009-0844-4. doi:10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunsmore SE, Geoffrey JL, Steven DS. Extracellular matrix: Collagens; Encyclopedia of Respiratory Medicine. Oxford: Academic Press; 2006. pp. 168–75. [Google Scholar]
- 12.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105. doi: 10.1038/embor.2009.276. doi:embor2009276 [pii] 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. doi:S0092867402009716 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. doi:0092-8674(92)90115-S [pii] [DOI] [PubMed] [Google Scholar]
- 15.Schaffner P, Dard MM. Structure and function of RGD peptides involved in bone biology. Cell Mol Life Sci. 2003;60:119–32. doi: 10.1007/s000180300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R. et al. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest. 2008;118:965–74. doi: 10.1172/JCI33538. doi:10.1172/JCI33538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. doi:10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 18.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–15. doi: 10.1016/j.ejcb.2008.01.012. doi:10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Scotton CJ, Krupiczojc MA, Konigshoff M, Mercer PF, Lee YC, Kaminski N. et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119:2550–63. doi: 10.1172/JCI33288. doi:10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126:1761–9. doi: 10.1038/sj.jid.5700331. doi:10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- 21.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–18. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J. et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–93. doi: 10.1083/jcb.200611044. doi:10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nykvist P, Tasanen K, Viitasalo T, Kapyla J, Jokinen J, Bruckner-Tuderman L. et al. The cell adhesion domain of type XVII collagen promotes integrin-mediated cell spreading by a novel mechanism. J Biol Chem. 2001;276:38673–9. doi: 10.1074/jbc.M102589200. doi:10.1074/jbc.M102589200 M102589200 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Koch M, Laub F, Zhou P, Hahn RA, Tanaka S, Burgeson RE. et al. Collagen XXIV, a vertebrate fibrillar collagen with structural features of invertebrate collagens: selective expression in developing cornea and bone. J Biol Chem. 2003;278:43236–44. doi: 10.1074/jbc.M302112200. doi:10.1074/jbc.M302112200 M302112200 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. Journal of Bone and Mineral Research. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. doi:10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 26.He X, Dziak R, Mao K, Genco R, Swihart M, Li C. et al. Integration of a novel injectable nano calcium sulfate/alginate scaffold and BMP2-gene modified MSCs for bone regeneration. Tissue Eng Part A. 2012 doi: 10.1089/ten.tea.2012.0244. doi:10.1089/ten.TEA.2012.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang SY, Wei DY, Wang D, Phimphilai M, Krebsbach PH, Franceschi RT. In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation. Journal of Bone and Mineral Research. 2003;18:705–15. doi: 10.1359/jbmr.2003.18.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Tang W, Fang J, Ren J, Li H, Xiao Z. et al. Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol. 2009;23:1505–18. doi: 10.1210/me.2009-0085. doi:10.1210/me.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Wang C. The intraflagellar transport protein IFT80 is required for cilia formation and osteogenesis. Bone. 2012;51:407–17. doi: 10.1016/j.bone.2012.06.021. doi:10.1016/j.bone.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS. et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S, Li YP. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 2007;21:1803–16. doi: 10.1101/gad.1544107. doi:gad.1544107 [pii] 10.1101/gad.1544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider GB, Whitson SW, Cooper LF. Restricted and coordinated expression of beta3-integrin and bone sialoprotein during cultured osteoblast differentiation. Bone. 1999;24:321–7. doi: 10.1016/s8756-3282(99)00007-1. doi:S8756-3282(99)00007-1 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Ovchinnikov D. Alcian blue/alizarin red staining of cartilage and bone in mouse. Cold Spring Harb Protoc. 2009. doi:2009/3/pdb.prot5170 [pii] 10.1101/pdb.prot5170. [DOI] [PubMed]
- 34.Yamakawa K, Iwasaki H, Masuda I, Ohjimi Y, Honda I, Saeki K. et al. The utility of alizarin red s staining in calcium pyrophosphate dihydrate crystal deposition disease. J Rheumatol. 2003;30:1032–5. doi:0315162X-30-1032 [pii] [PubMed] [Google Scholar]
- 35.Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN. et al. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–7. [PubMed] [Google Scholar]
- 36.Rungby J, Kassem M, Eriksen EF, Danscher G. The von Kossa reaction for calcium deposits: silver lactate staining increases sensitivity and reduces background. Histochem J. 1993;25:446–51. doi: 10.1007/BF00157809. [DOI] [PubMed] [Google Scholar]
- 37.McCauley LK, Koh AJ, Beecher CA, Cui Y, Decker JD, Franceschi RT. Effects of differentiation and transforming growth factor beta 1 on PTH/PTHrP receptor mRNA levels in MC3T3-E1 cells. Journal of Bone and Mineral Research. 1995;10:1243–55. doi: 10.1002/jbmr.5650100815. doi:10.1002/jbmr.5650100815. [DOI] [PubMed] [Google Scholar]
- 38.Yang SY, Chen W, Stashenko P, Li YP. Specificity of RGS10A as a key component in the RANKL signaling mechanism for osteoclast differentiation. Journal of Cell Science. 2007;120:3362–71. doi: 10.1242/jcs.008300. doi:Doi 10.1242/Jcs.008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamidouche Z, Fromigue O, Ringe J, Haupl T, Vaudin P, Pages JC. et al. Priming integrin alpha5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci U S A. 2009;106:18587–91. doi: 10.1073/pnas.0812334106. doi:0812334106 [pii]10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steenhard BM, Isom K, Stroganova L, St John PL, Zelenchuk A, Freeburg PB. et al. Deletion of von Hippel-Lindau in glomerular podocytes results in glomerular basement membrane thickening, ectopic subepithelial deposition of collagen {alpha}1{alpha}2{alpha}1(IV), expression of neuroglobin, and proteinuria. Am J Pathol. 2010;177:84–96. doi: 10.2353/ajpath.2010.090767. doi:ajpath.2010.090767 [pii] 10.2353/ajpath.2010.090767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010;77:4–12. doi: 10.1272/jnms.77.4. doi:JST.JSTAGE/jnms/77.4 [pii] [DOI] [PubMed] [Google Scholar]
- 42.Kim DS, Jeon OH, Lee HD, Yoo KH. Integrin alphavbeta3-mediated transcriptional regulation of TIMP-1 in a human ovarian cancer cell line. Biochem Biophys Res Commun. 2008;377:479–83. doi: 10.1016/j.bbrc.2008.10.010. doi:S0006-291X(08)01960-8 [pii] 10.1016/j.bbrc.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Reed RK, Rubin K. Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc Res. 2010;87:211–7. doi: 10.1093/cvr/cvq143. doi:cvq143 [pii]10.1093/cvr/cvq143. [DOI] [PubMed] [Google Scholar]
- 44.Hughes DE, Salter DM, Dedhar S, Simpson R. Integrin expression in human bone. Journal of Bone and Mineral Research. 1993;8:527–33. doi: 10.1002/jbmr.5650080503. doi:10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- 45.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. Journal of Bone and Mineral Research. 1997;12:1189–97. doi: 10.1359/jbmr.1997.12.8.1189. doi:10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 46.Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. Journal of Bone and Mineral Research. 1994;9:487–96. doi: 10.1002/jbmr.5650090408. doi:10.1002/jbmr.5650090408. [DOI] [PubMed] [Google Scholar]
- 47.Scarborough RM, Rose JW, Hsu MA, Phillips DR, Fried VA, Campbell AM. et al. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991;266:9359–62. [PubMed] [Google Scholar]
- 48.Matsuo N, Tanaka S, Yoshioka H, Koch M, Gordon MK, Ramirez F. Collagen XXIV (Col24a1) gene expression is a specific marker of osteoblast differentiation and bone formation. Connect Tissue Res. 2008;49:68–75. doi: 10.1080/03008200801913502. doi:791857477 [pii] 10.1080/03008200801913502. [DOI] [PubMed] [Google Scholar]
- 49.Beresford JN, Graves SE, Smoothy CA. Formation of mineralized nodules by bone derived cells in vitro: a model of bone formation? Am J Med Genet. 1993;45:163–78. doi: 10.1002/ajmg.1320450205. doi:10.1002/ajmg.1320450205. [DOI] [PubMed] [Google Scholar]
- 50.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–23. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 51.Shi S, Kirk M, Kahn AJ. The role of type I collagen in the regulation of the osteoblast phenotype. Journal of Bone and Mineral Research. 1996;11:1139–45. doi: 10.1002/jbmr.5650110813. doi:10.1002/jbmr.5650110813. [DOI] [PubMed] [Google Scholar]
- 52.Lynch MP, Stein JL, Stein GS, Lian JB. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995;216:35–45. doi: 10.1006/excr.1995.1005. doi:S0014-4827(85)71005-1 [pii] 10.1006/excr.1995.1005. [DOI] [PubMed] [Google Scholar]
- 53.Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143:213–21. doi: 10.1002/jcp.1041430203. doi:10.1002/jcp.1041430203. [DOI] [PubMed] [Google Scholar]
- 54.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–88. doi: 10.7150/ijbs.2929. doi:10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito Y, Yeo JY, Chytil A, Han J, Bringas P Jr, Nakajima A. et al. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–80. doi: 10.1242/dev.00708. doi:10.1242/dev.00708 130/21/5269 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Chai Y, Ito Y, Han J. TGF-beta signaling and its functional significance in regulating the fate of cranial neural crest cells. Crit Rev Oral Biol Med. 2003;14:78–88. doi: 10.1177/154411130301400202. doi:14/2/78 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Bio. 2000;1:169–78. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 58.Munger JS, Sheppard D. Cross talk among TGF-beta signaling pathways, integrins, and the extracellular matrix. Cold Spring Harbor perspectives in biology. 2011;3:a005017.. doi: 10.1101/cshperspect.a005017. doi:10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–13. doi: 10.1074/jbc.M106176200. doi:10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 60.Seo HS, Serra R. Tgfbr2 is required for development of the skull vault. Dev Biol. 2009;334:481–90. doi: 10.1016/j.ydbio.2009.08.015. doi:10.1016/j.ydbio.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R. et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–5. doi: 10.1038/39369. doi:10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW. et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–73. doi: 10.1016/s0092-8674(00)80303-7. doi:S0092-8674(00)80303-7 [pii] [DOI] [PubMed] [Google Scholar]