Abstract
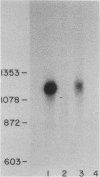
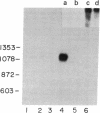
The levels of interferon mRNA as a function of interferon induction by poly(rI) . poly(rC) in human fibroblast cells were determined by RNA hybridization using a cloned beta interferon cDNA and by translation in Xenopus oocytes. Whereas previous studies analyzed mixtures of interferons, the availability of the cloned beta interferon cDNA and the antiserum to purified beta interferon enabled us to focus on the expression of only one class (beta) of interferon genes. The induction of interferon synthesis depends primarily on the accumulation of interferon beta mRNA in the cells, and the interferon beta mRNA rapidly disappears several hours after its appearance in the cytoplasm. No detectable interferon beta mRNA sequences are present in uninduced cells. The degradation of interferon beta mRNA in the induced cells requires ongoing protein synthesis; accumulation of interferon beta mRNA was observed in the continuous presence of cycloheximide. The interferon beta mRNA detected at the early stages of induction is 1100 nucleotides long and its size progressively decreases with time. By both the hybridization and the translational assay in Xenopus oocytes, only one size of interferon beta mRNA and one species of beta interferon could be identified.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. L., Cooper H. L. Very short-lived and stable mRNAs from resting human lymphocytes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3873–3877. doi: 10.1073/pnas.72.10.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Induction and decay of human fibroblast interferon mRNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4415–4419. doi: 10.1073/pnas.74.10.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Content J., DeClercq E., Volckaert G., Tavernier J., Devos R., Fiers W. Isolation and structure of a human fibroblast interferon gene. Nature. 1980 Jun 19;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- Field A. K., Tytell A. A., Lampson G. P., Hilleman M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1004–1010. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D. Kinetics of inactivation of histone mRNA in the cytoplasm after inhibition of DNA replication in synchronised HeLa cells. Nature. 1975 Sep 18;257(5523):247–248. doi: 10.1038/257247a0. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Shepard H. M., Yelverton E., Leung D., Crea R., Sloma A., Pestka S. Synthesis of human fibroblast interferon by E. coli. Nucleic Acids Res. 1980 Sep 25;8(18):4057–4074. doi: 10.1093/nar/8.18.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Jackson I. J., Porter A. G., Doel S. M., Catlin G. H., Barber C., Carey N. H. The absence of introns within a human fibroblast interferon gene. Nucleic Acids Res. 1981 Jan 24;9(2):247–266. doi: 10.1093/nar/9.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Spinelli G., Arnold E. Synthesis of histone messenger RNA of HeLa cells during the cell cycle. Cell. 1977 Sep;12(1):167–174. doi: 10.1016/0092-8674(77)90194-5. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Fernie B. F., Pitha P. M. Correlation between the induction of mouse interferon and the amount of its mRNA. Eur J Biochem. 1979 Jul;98(1):215–221. doi: 10.1111/j.1432-1033.1979.tb13179.x. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Interferon heterogeneity resulting from differences in glycosylation. J Interferon Res. 1981;1(4):595–599. doi: 10.1089/jir.1981.1.595. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Relationship between interferon production and interferon messenger RNA synthesis in human fibroblasts. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1483–1487. doi: 10.1073/pnas.74.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Synthesis of new proteins associated with the induction of interferon in human fibroblast cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4918–4922. doi: 10.1073/pnas.77.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. The messenger RNA sequences in human fibroblast cells induced with poly rI.rC to produce interferon. Nucleic Acids Res. 1980 Aug 11;8(15):3427–3437. doi: 10.1093/nar/8.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Premkumar E., Pitha P. M. Interferon activity produced by translation of human interferon messenger RNA in cell-free ribosomal systems and in Xenopus oöcytes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4881–4885. doi: 10.1073/pnas.72.12.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar A. D., Pickering L. A., Sussman-Berger P., Stewart W. E., 2nd, Sehgal P. B. Heterogeneity of interferon mRNA species from Sendai virus-induced human lymphoblastoid (Namalva) cells and Newcastle disease virus-induced murine fibroblastoid (L) cells. Nucleic Acids Res. 1981 Jan 10;9(1):149–160. doi: 10.1093/nar/9.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Gupta S. L. Regulation of the stability of poly(I)xpoly(C)-induced human fibroblast interferon mRNA: selective inactivation of interferon mRNA and lack of involvement of 2',5'-oligo(A) synthetase activation during the shutoff of interferon production. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3489–3493. doi: 10.1073/pnas.77.6.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Lyles D. S., Tamm I. Superinduction of human fibroblast interferon production: further evidence for increased stability of interferon mRNA. Virology. 1978 Aug;89(1):186–198. doi: 10.1016/0042-6822(78)90051-x. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Sagar A. D. Heterogeneity of poly(I) x poly(C)-induced human fibroblast interferon mRNA species. Nature. 1980 Nov 6;288(5786):95–97. doi: 10.1038/288095a0. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Stewart W. E., 2nd, Gosser L. B., Lockart R. Z., Jr Priming: a nonantiviral function of interferon. J Virol. 1971 Jun;7(6):792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Ohno S., Fujii-Kuriyama Y., Muramatsu M. The nucleotide sequence of human fibroblast interferon cDNA. Gene. 1980 Jun;10(1):11–15. doi: 10.1016/0378-1119(80)90138-9. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Derynck R., Fiers W. Evidence for a unique human fibroblast interferon (IFN-beta 1) chromosomal gene, devoid of intervening sequences. Nucleic Acids Res. 1981 Feb 11;9(3):461–471. doi: 10.1093/nar/9.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengris V. E., Stollar B. D., Pitha P. M. Interferon externalization by producing cell before induction of antiviral state. Virology. 1975 Jun;65(2):410–417. doi: 10.1016/0042-6822(75)90046-x. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A., Kohase M. Superinduction of interferon with metabolic inhibitors: possible mechanisms and practical applications. J Infect Dis. 1976 Jun;133 (Suppl):A22–A29. doi: 10.1093/infdis/133.supplement_2.a22. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Ng M. H., Friedman-Kien A. E., Krawciw T. Induction of interferon synthesis by synthetic double-stranded polynucleotides. J Virol. 1968 Jun;2(6):648–650. doi: 10.1128/jvi.2.6.648-650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U., Wallach D., Perricaudet M., Tiollais P., Revel M. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia coli cloning studies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]