Abstract
The roles of several small GTPases in the expression of an endogenous potassium current, Ito,f, in adult rat ventricular myocytes have been investigated. The results indicate that forward trafficking of newly synthesized Kv4.2, which underlies Ito,f in these cells, requires both Rab1 and Sar1 function. Expression of a Rab1 dominant negative (DN) reduced Ito,f current density by roughly one-half relative to control, mCherry-transfected myocytes. Similarly, expression of a Sar1DN nearly halved Ito,f current density. Rab11 is not essential to trafficking of Kv4.2, as expression of a Rab11DN had no effect on Ito,f over the time frames investigated here. In a process dependent on intact endoplasmic reticulum (ER)-to-Golgi transport, however, overexpression of wild-type Rab11 resulted in a doubling of Ito,f density; block of ER-to-Golgi traffic by Brefeldin A completely abrogated the effect. Also implicated in the trafficking of Kv4.2 are Rab5 and Rab4. Rab5DN expression increased endogenous Ito,f by two- to threefold, nonadditively with inhibition of dynamin-dependent endocytosis. And, in a phenomenon similar to that previously reported for myoblast-expressed Kv1.5, Rab4DN expression roughly doubled endogenous peak transient currents. Colocalization experiments confirmed the involvement of Rab4 in postinternalization trafficking of Kv4.2. There was little role evident for the lysosome in the degradation of internalized Kv4.2, as overexpression of neither wild-type nor DN isoforms of Rab7 had any effect on Ito,f. Instead, degradation may depend largely on the proteasome; the proteasome inhibitor MG132 significantly increased Ito,f density.
Keywords: cardiomyocyte, Rab GTPase, Kv4.2
voltage-gated k+ channels (Kv channels) are essential to the repolarization phase of the action potential in cardiac cells. Because they determine the duration of the action potential and of the refractory period, minor differences in Kv channel functional expression can have dramatic effects on cardiac electrophysiology. Cardiac ion channel functional expression may be modulated by phosphorylation and other modifications, by association with accessory subunits, and, as has been demonstrated in cardiomyoblast cell lines and heterologous systems (31, 45, 66), via control of channel trafficking into and out of the plasma membrane. This trafficking is regulated by a dynamic interplay between anterograde and retrograde trafficking pathways. Surface expression of Kv1.5 in a myoblast cell line, for example, requires a specific kinesin isoform (Kif5b), which is essential for the forward trafficking of the channel (66) and like several other potassium channels (29), the numbers remaining at the plasma membrane are modulated via a dynein-dependent process (10).
To date, the internalization and trafficking of already plasma membrane resident potassium channels has been best studied. Several Rab GTPases have been implicated in the internalization and recycling process of Kv1.5 (31, 66), KCNQ1 (45), and human ether-à-go-go related gene product (hERG) (21). Rab GTPases are intimately involved in the regulation of vesicle trafficking in all eukaryotic cells (67), including budding, delivery, tethering, and fusion (reviewed in Ref. 20), and they define the various intracellular trafficking vesicular compartments. They regulate the formation of these compartments, recruiting the various effectors required for their function (35, 40, 49, 50).
With regard to the trafficking of cardiac ion channels, however, there is some controversy on precisely which of the Rab GTPases are involved, and with which individual channel types. Rab5, localized to the early endosomes, acts as a primary effector of the rapid endocytosis of KCNQ1/KCNE1 (45) and of Kv1.5 in at least some cell types (66). Rab4 associates with a subset of early endosomes and is involved mainly in fast recycling of internalized membrane proteins back to the plasmalemma. The involvement of Rab4 in postinternalization trafficking of Kv1.5 is also established (31), although there are differences in the effects of a dominant negative (DN) Rab4 isoform in different cell lines.
After internalization, channels may also be delivered into Rab11-associated perinuclear recycling endosomes, and slowly recycled back to cell surface. To lesser or greater extents, this GTPase appears to be involved in the trafficking of cardiac potassium channels (1, 3, 45). Internalized channels may also be shunted for degradation either in the lysosome, via a Rab7-dependent pathway, or by the proteasome. In the case of Kv1.5, Rab7 wild-type (WT) overexpression in H9c2 cardiomyoblasts reduces Kv1.5 surface expression, very probably by enhancing the lysosomal degradation of the channel (66). hERG degradation, under different conditions, has been demonstrated to occur both in lysosomes (21) and by the proteasome (19).
Less well studied in heterologous and cardiomyoblast cell line systems has been the forward trafficking of potassium channels. While roles for retention signals and chaperones have been demonstrated in the trafficking of several potassium channels out of the endoplasmic reticulum (ER) (reviewed in Ref. 48), the actual paths followed upon ER exit are less well understood. It is clear, nevertheless, that there is considerable variation in the pathways employed by different channels. While some channels traffic to the Golgi apparatus via COPII-coated vesicles (52, 59), others have been shown to traffic independently of these vesicles, at least in some cell types (15, 23). Trafficking through COPII vesicles is dependent on the Sar1 small GTPase, which regulates the formation of the COPII coat-associated protein complex (5, 6). Rab1 recruits tethering factors into the cis-SNARE complex and facilitates fusion between ER budded vesicles and Golgi compartments (2). However, it has been reported that H-Ras is trafficked independently of Sar1 (68) and cystic fibrosis transmembrane conductance regulator (CFTR) trafficking does not require Rab1 (65), indicating that it may traffic by an alternate ER-to-Golgi route. Yet another route may exist for Kv4.2 trafficking in some cell types. The ER-to-Golgi trafficking of newly synthesized Kv4.2 channels in HeLa cells also expressing Kv channel-interacting protein 1 (KChIP1) has been shown to involve KChIP1 and to be independent of Sar1 but nevertheless dependent on Rab1 function (15, 23). KChIP1 was shown by the same workers also to traffic with Kv4.2 in Neuro2A cells. KChIP2 is the predominant KChIP isoform in the heart, however (43); the involvement of Sar1 and Rab1 in the trafficking of a Kv4.2-KChIP2 complex in cardiac cells has to date not been established.
To definitively answer the question of how cardiac ion channels are trafficked in vivo, studies must be conducted in cardiomyocytes themselves. Therefore we have developed techniques to transfect freshly isolated cardiac myocytes (12). Here, we have used refinements of the lipofectamine-mediated transfection method to investigate the trafficking of an endogenous potassium channel in rat ventricular myocytes. We have studied the rapidly inactivating component of the transient outward current, Ito,f, which is responsible for the initial repolarization notch in the cardiac action potential. While Ito itself is made up of three different components, only the rapidly inactivating portion (Ito,f) with its distinct activation and inactivation kinetics is underlain by Kv4.2 in rodent cardiac myocytes (33, 43, 64). Ventricular myocyte transfection has been combined with electrophysiological and imaging techniques to identify some of the small GTPases that regulate Ito,f and, thus, the trafficking of endogenous Kv4.2. Our findings implicate both Rab1 and Sar1 in the anterograde trafficking of Kv4.2. Once at the sarcolemma, channel internalization involves Rab5 activity, and similar to previously reported findings on Kv1.5 expression, interference with Rab4 function by a Rab4DN also increased the Kv4.2-dependent Ito,f current.
METHODS
Ethical approval.
All animal use was in accordance with protocols approved by the Animal Care Committee of the University of British Columbia in accordance with Canadian Institutes of Health Research guidelines.
Myocyte isolation and transfection.
Rat ventricular myocytes were isolated from the hearts of male Wistar rats weighing 250–300 g using a conventional horizontal heart Langendorf apparatus as previously described (12). Rapidly beating hearts were excised from rats intraperitoneally (ip) anesthetized with 125 mg/kg pentobarbital sodium that had been dosed 10 min earlier with 500 units of heparin (ip). Cells were cultured and transfected as reported previously (12) or in the absence of fetal calf serum as described below: After 30 min, the overlaying Storage Buffer was replaced with 1 ml medium 199 (M199; Sigma-Aldrich), pH 7.4, supplemented with 2 mM EGTA, 0.6 μg/ml insulin, 5 mM creatine, 2 mM dl-carnitine, 2 mM glutamine, 5 mM taurine, 50 U/ml penicillin, and 50 μg/ml streptomycin. The cells were incubated at 37°C overnight in a 1% CO2 incubator. On the second day, before transfection, the media were replaced with 1 ml M199 containing 5% FBS, 1/1,000 GIBCO Insulin-Transferrin-Selenium-A Supplement (ITS). Lipofectamine 2000 (6 μl; Invitrogen) in 300 μl of Opti-MEM was mixed with 3 μg of relevant plasmid and added to the cells after replacing the media with 1 ml M199 containing 1/1,000 ITS. After 4 h, the medium was replaced with 1 ml M199 supplemented with 1/1,000 ITS, 50 U/ml penicillin, and 50 μg/ml streptomycin. The cells were then incubated overnight at 37°C in 5% CO2 incubator.
Plasmid constructs.
Plasmid constructs were generally as described previously (66). The wild-type and dominant negative Sar1 and Rab1 clones were kind gifts of Terry Hebert (McGill University, Montreal, ON, Canada). Sar1WT, Sar1DN, Rab1WT, and Rab1DN were amplified by PCR and fused NH2-terminally to mCherry in pcDNA3 as described previously (66). Plasmid DNA was prepared for transfection using the Qiagen Plasmid Midi Kit (Qiagen, Valencia, CA).
Electrophysiology.
Whole cell voltage-clamp experiments were performed at room temperature using an Axopatch 200B amplifier and pClamp software (Axon Instruments, Foster City, CA). Patch electrodes were fabricated using thin-walled borosilicate glass (World Precision Instruments, Sarasota, FL) and polished by heating. Patch pipettes had a resistance of 1 to 3 MΩ. The standard bath solution contained (in mmol/l) 135 NaCl, 5 KCl, 1 MgCl2, 2.8 sodium acetate, 10 HEPES, and 1 CaCl2, adjusted to pH 7.4 using NaOH. The standard pipette filling solution contained (in mmol/l) 130 KCl, 5 EGTA, 1 MgCl2, 10 HEPES, 4 Na2ATP, and 0.1 GTP, adjusted to pH 7.2 with KOH. Compensation for capacitance and series resistance was performed manually in all whole cell recordings. Currents were elicited by 500-ms pulses stepping from a holding potential of −80 mV to test potentials of −130 to +90 mV in 10-mV increments followed by 100-ms pulse to +60 mV. The final step to +60 mV allowed measurement of the steady-state inactivation properties of the current. The voltage dependence of Ito,f steady-state inactivation was measured at the test pulse to +60 mV and fit by a Boltzmann equation: G/Gmax = 1/{1 + exp[−(V − V1/2)/K]}, where G/Gmax is the normalized conductance, V is membrane potential, V1/2 is the potential for half-maximal inactivation of potassium currents, and K is the slope factor. All recordings were performed at room temperature (20–23°C). The magnitude of Ito,f was determined by subtracting the amplitude of the steady-state current, measured at the end of the depolarization pulse, from the peak current amplitude.
For experiments with dynamin inhibitory peptide (myristoylated DIP; Tocris Bioscience), 5 mM DIP stock solution was prepared in H2O and stored at 4°C. Cells were incubated with 50 μM DIP for 6 h before electrophysiological recordings. For experiments with Brefeldin A (BFA), 10 mg/ml BFA stock solution was prepared in ethanol and stored at −20°C. Cells were incubated with 0.5 μg/ml BFA for 6 h before electrophysiological recordings. For experiments with the proteasomal inhibitor MG132, 50 mM MG132 stock solution was prepared in DMSO and stored at −20°C. Cells were incubated with 5 μM MG132 for 6 h before electrophysiological recordings.
Kv4.2 colocalization assay.
In Rab4-Kv4.2 colocalization assays, myocytes were cotransfected with Kv4.2-hemagglutinin (HA) and Rab4 tagged with enhanced green fluorescent protein (EGFP) and incubated at 37°C for 24 h. Surface Kv4.2 was labeled with mouse anti-HA (Roche) on ice for 1 h, washed three times with cold culture medium, then incubated at 37°C for 5 h to allow internalization of the labeled Kv4.2-HA. The myocytes were then fixed with 2% paraformaldehyde and permeabilized in PBS containing 0.1% Triton X-100, and nonspecific binding was blocked by incubation in 2% BSA in PBS for 30 min at room temperature. Following this, the cells were labeled with Alexa594-conjugated goat anti-mouse antibody (Molecular Probes) for 1 h to detect Kv4.2-HA. Images were collected on an Olympus Fluoview 1000 laser scanning confocal microscope. EGFP and Alexa488 were excited using the 488 nm line of an argon laser set at 5% transmission, and emission was collected using the variable band-pass filter set at 510 nm. Alexa594 and mCherry were excited using a 543 nm He-Ne laser set at 25% transmission, and emission was collected using the variable long-pass filter set at 618 nm. A ×60, 1.35 numerical objective oil immersion objective was used for imaging. A digital zoom of ×2 was often used. Images were acquired at 512 × 512 resolution. The images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). Colocalization was estimated by counting the number of Kv4.2-positive vesicles, the number of Rab4-positive vesicles, and the number of vesicles positive for both Kv4.2 and Rab4 and calculating the percentage of Kv4.2-positive vesicles that were also positive for Rab4.
Data statistics.
Results are expressed as means ± SE. Statistical analyses were conducted using by one-way ANOVA for repeated measures followed by Tukey's multiple comparison test.
RESULTS
Lipofectamine 2000-mediated transfection of ventricular myocytes.
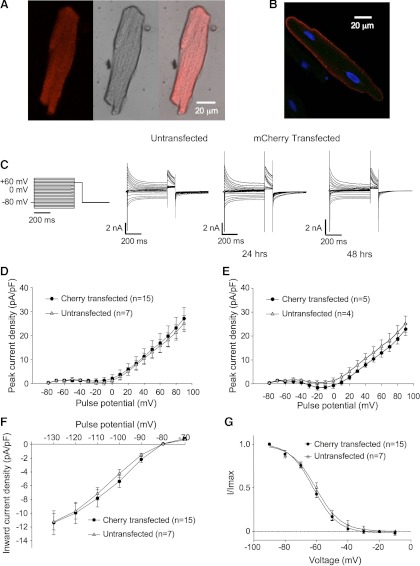
Lipofectamine-mediated transfection of rat ventricular myocytes produces myocytes that, at 24 h after transfection, are largely morphologically intact. Figure 1A shows fluorescent and bright field views of a ventricular myocyte transfected with mCherry. Figure 1B shows a myocyte transfected with an HA-tagged membrane protein, in this case Kir2.1. The channel is clearly targeted to the plasmalemma, as detected with anti-HA and Alexa568-conjugated secondary antibody in the permeabilized myocyte. The myocytes in Fig. 1B were costained with DAPI, identifying also the nuclei of the transfected and adjacent, evidently untransfected myocytes. Although some rounding of the myocytes occurs among the viable myocytes at time points beyond 24 h, myocytes transfected with the control mCherry construct retain current profiles typical of ventricular myocytes both at 24 and 48 h posttransfection (Fig. 1, C–G). Specifically illustrated are comparisons of peak transient currents (Fig. 1, D and E), steady-state inward rectifier currents (Fig. 1F), and steady-state inactivation (h-infinity) curves for Ito,f (Fig. 1G), all of which do not differ between the transfected and untransfected myocytes. We began our experiments to investigate the trafficking of Kv4.2 in intact myocytes with experiments testing whether the maintenance of Ito,f was dependent on the so-called conventional pathway from the endoplasmic reticulum to the Golgi apparatus.
Fig. 1.
Lipofectamine-mediated transfection of adult ventricular myocytes in the absence of fetal calf serum. A: fluorescent, bright field, and merged images of an adult rat ventricular myocyte transfected with mCherry using the modified lipofectamine-mediated transfection protocol described in methods. The myocyte was transfected 24 h before fixation and staining. B: fluorescent image of a ventricular myocyte transfected with a plasmalemma-targeted protein, in this case hemagglutinin (HA)-tagged Kir2.1. The myocytes were costained with DAPI to identify nuclei, and the transfected channel was detected in the permeabilized myocytes using mouse anti-HA and Alexa568-conjugated goat anti-mouse antibody. C: voltage protocol and representative currents from untransfected and mCherry-transfected rat ventricular cardiomyocytes recorded 12–24 or 36–48 h posttransfection or, in the case of traces from the untransfected myocyte shown, 24 h in culture. Transient outward currents (Ito) were elicited by 500-ms pulses stepping from a holding potential of −80 mV to test potentials of −130 to +90 mV in 10-mV increments followed by a 100-ms pulse to +60 mV. D and E: peak current density vs. voltage plots (−80 to +90 mV shown) for untransfected myocytes after 24 h in culture and for mCherry-transfected myocytes at 24 and 48 h posttransfection, respectively. Data are represented as means ± SE. F: sustained current density vs. voltage plots from −130 to −70 mV for the same cells as in D. Data are represented as means ± SE. G: h-infinity curves for the rapidly inactivating component of the transient outward current (Ito,f) calculated from data from the same cells as in D. Imax, maximum current.
Sar1 involvement in the maintenance of Ito,f in rat ventricular myocytes.
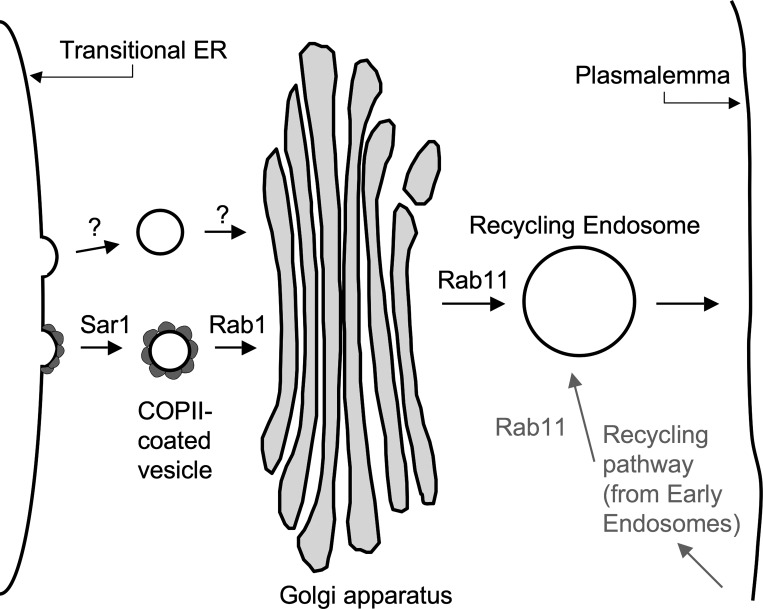
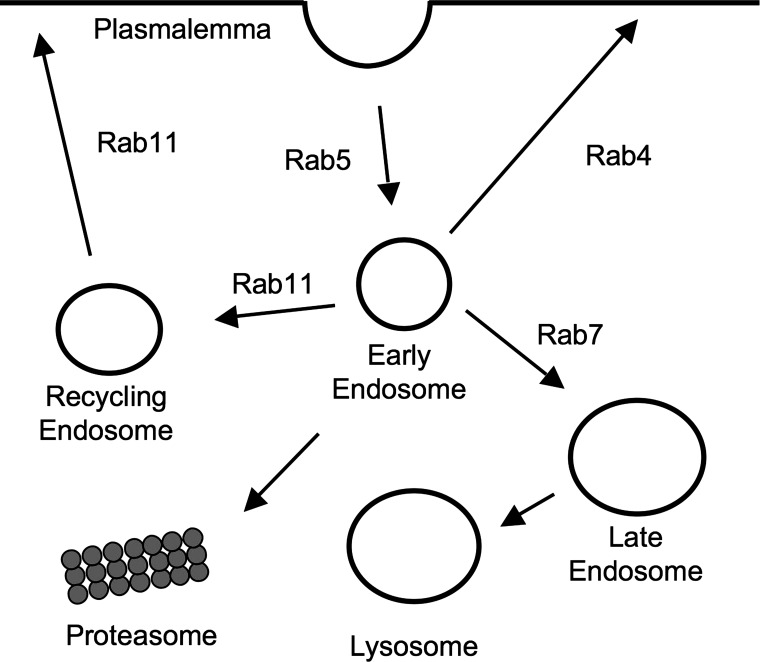
Sar1 is a small GTPase that controls the assembly of COPII complexes on ER exit membranes (28). It is essential for transport vesicle formation in the conventional ER-to-Golgi protein transport pathway. A schematic diagram illustrating Sar1's place, as well as those of Rab1 and Rab11, in the conventional ER to plasmalemma pathway is provided in Fig. 2. There is evidence that Kv4.2 does not traffic to the Golgi by this conventional pathway in some cells, however.
Fig. 2.
Schematic representation of the roles of various small GTPases in forward trafficking. Sar1 is required for the recruitment of coat-associated protein II (COPII) complexes to budding exit sites in the transitional endoplasmic reticulum (ER). Also illustrated exiting the ER is a non-COPII-coated vesicle, hypothetically involved in the exit of voltage-gated K+ channel Kv4.2 from the transitional ER. Rab1 is required for fusion between ER budded vesicles and the Golgi apparatus. Rab11 is involved in the trafficking of some proteins from the Golgi to the plasmalemma as well as in the recycling of endocytosed membrane proteins back to the plasmalemma.
When KChIP1 is coexpressed with the channel, Sar1 function is not required for Kv4.2 trafficking in heterologous cells (23). Hasdemir et al. (23) hypothesize that, rather than transiting the ER-Golgi divide via COPII-coated vesicles in these cells, Kv4.2 is instead carried in “KChIP vesicles.” Indeed, in their system, Kv4.2 and KChIP1 colocalize in apparent ER-to-Golgi bound vesicles lacking COPII. Instead, the process involves at least two SNARE proteins, Vtila and VAMP-7, that are not involved in the conventional ER-to-Golgi pathway (15). KChIP1 is not expressed in cardiomyocytes, however, where instead three splice variants of KChIP2 predominate (43). Although siRNAs against Vti1a and VAMP-7 did not block KChIP2-associated trafficking of Kv4.2 to the cell surface in heterologous cells (15), it is unknown whether KChIP2 can drive Kv4.2, underlying Ito,f, through a nonconventional pathway in the heart. Thus, it is of great interest to determine whether or not Kv4.2 ER-to-Golgi traffic is similarly independent of the conventional pathway in heart.
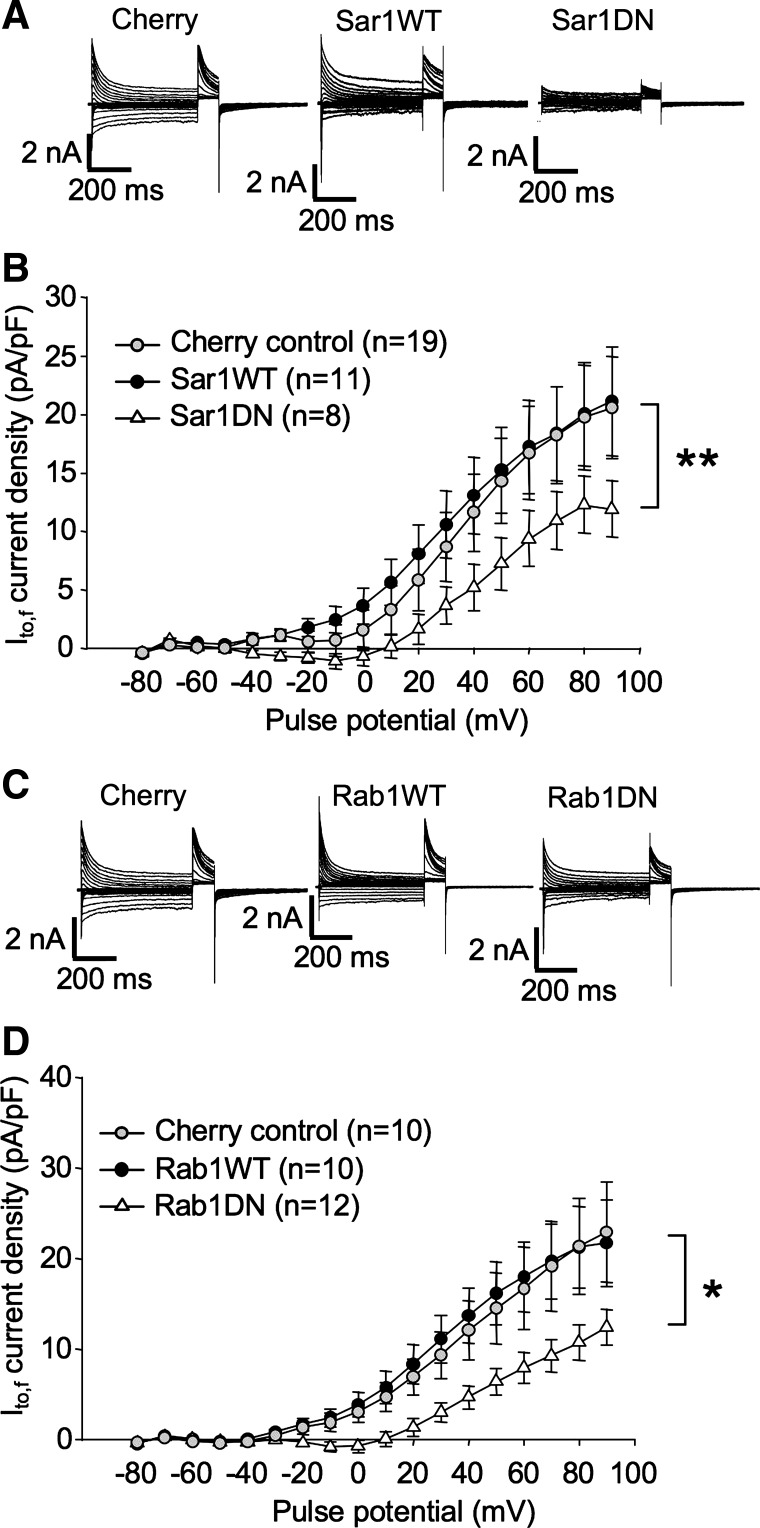
The involvement of small GTPases in ion channel trafficking is most commonly assayed in heterologous cells by testing the effects of expression of their wild-type and dominant negative isoforms on the localization and functional expression of ion channels coexpressed with them. Here we have employed a similar approach with the exception that our functional expression assays involve instead endogenous channels in rat ventricular myocytes. The dominant negative constructs that we have employed are well established to strongly interfere with the functioning of their endogenous wild-type homologs (14, 17, 37, 41, 56, 61). To test whether Sar1 influences the functional expression of endogenous Kv4.2 channels, the effects of transfection of ventricular myocytes with wild-type or a dominant negative isoform of Sar1 (Sar1 T39N; Sar1DN) on Ito,f were assayed. An mCherry construct was cotransfected with the individual Sar1 constructs to allow the ready identification of transfected myocytes. Expression of the Sar1DN roughly halved the peak transient outward current (Ito,f) to 11.9 ± 2.4 pA/pF at +90 mV (P < 0.01) by 36–48 h posttransfection from the 21.1 ± 4.6 pA/pF measured in myocytes transfected with mCherry alone (Fig. 3, A and B). Thus, while it remains possible that Kv4.2 traverses also an unconventional pathway out of the ER, Kv4.2 trafficking in cardiac myocytes is clearly dependent, at least in significant measure, on Sar1 function. Indicating that Sar1 wild-type expression is not limiting for the process, Ito,f density was unchanged in myocytes overexpressing the wild-type gene.
Fig. 3.
Involvement of Sar1 and Rab1 in maintenance of Ito,f in ventricular myocytes. A and B: normal expression of Ito,f requires intact Sar1 function. A: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry, mCherry + wild-type SAR1 (SAR1WT), or mCherry + dominant negative Sar1 (Sar1DN). Current protocols were as described in Fig. 1. Voltage protocols were as described in Fig. 1C. B: Ito,f current density vs. voltage plot for data from mCherry-, mCherry + Sar1WT-, and mCherry + Sar1DN (T39N)-transfected myocytes, respectively. Data are represented as means ± SE. **P < 0.01, comparing Sar1DN + mCherry to control, mCherry-transfected myocytes. C and D: normal expression of Ito,f requires intact Rab1 function. C: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry, mCherry + Rab1WT, or mCherry + Rab1DN. Voltage protocols were as described in Fig. 1. D: Ito,f current density vs. voltage plot for data from mCherry, mCherry + Rab1WT, and mCherry + Rab1DN (T39N)-transfected myocytes, respectively. Data are represented as means ± SE. *P < 0.05, comparing Rab1DN + mCherry to control, mCherry-transfected myocytes.
Rab 1DN significantly decreases Ito,f density in cardiac myocytes.
Another small GTPase important for conventional ER exit is Rab1. Similarly to Sar1, Rab1 is required for vesicular traffic from the ER to the cis-Golgi (Fig. 2), and for transport between the cis and medial compartments of the Golgi stack. It recruits tethering factors into the cis-SNARE complex and facilitates fusion between the vesicles budded from the ER and Golgi compartments (2), regulating the trafficking of COPII-coated vesicles from the ER to the cis-Golgi and, perhaps to intra-Golgi compartments as well (38, 53, 63). A Rab1DN has been reported to efficiently block trafficking of the Kir3.1/Kir3.4 complex to the plasma membrane when heterologously expressed in HEK293 cells (42). We tested whether such a Rab1 dominant-negative (Rab1 N124I) would similarly affect the trafficking of Kv4.2 in ventricular myocytes, assayed as an effect on endogenous Ito,f.
As illustrated in Fig. 3, C and D, the Rab1DN decreased Ito,f, by 36–48 h posttransfection, to 12.4 ± 2.0 pA/pF at +90 mV, significantly lower than 22.9 ± 5.6 pA/pF measured in myocytes transfected with mCherry alone (P < 0.05). This result is similar to that obtained in HeLa cells where, despite the fact that ER exit of Kv4.2 is independent of Sar1, Rab1 function was required for the process (15). Indicating that, like Sar1, endogenous Rab1 levels were not rate limiting in the process, current density in myocytes overexpressing Rab1WT averaged 21.7 ± 4.8 pA/pF at +90 mV, a value statistically indistinguishable from control.
A role for Rab11 in Kv4.2 trafficking?
Once a membrane protein traverses the Golgi apparatus, it must then travel to its final destination via Rab-regulated vesicles. Rab11 is one of the Rab-GTPases that can catalyze that traffic. Known largely for its role in the recycling of internalized membrane proteins to the plasma membrane (3, 18, 31, 45, 66), Rab11 is also involved in the forward trafficking of many newly synthesized proteins (9, 27). It localizes to the trans-Golgi network (TGN), to post-Golgi vesicles, as well as to pericentriolar recycling endosomes (9, 11, 54). We assayed whether Rab11 plays any role in the maintenance of Ito,f via its involvement in forward trafficking or recycling.
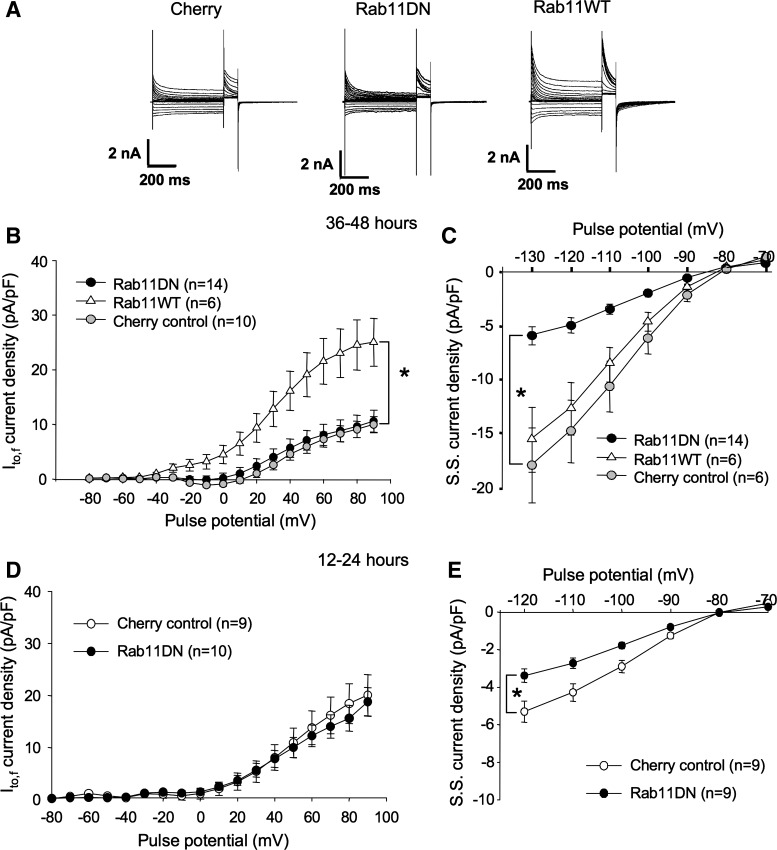
When transfected into ventricular myocytes, expression of a Rab11 dominant negative (Rab11 S25N) in rat ventricular myocytes had little effect on Ito,f 2 days posttransfection (Fig. 4, A and B). That this was not due to a failure of transfection was confirmed by the statistically significant decrease in inward rectifier currents measured in the same myocytes (Fig. 4, A and C). Although less strongly so, this effect was evident also at 12–24 h posttransfection (Fig. 4E), despite the fact that the Rab11DN had no effect on Ito,f in these myocytes either (Fig. 4D). Thus, if Rab11 function is important in maintaining normal Ito,f densities in ventricular myocytes, the processes for which it is essential to must be slow or other mechanisms must exist to compensate when Rab11 function is compromised. One possibility is that maintenance of Kv4.2 channel density may be largely controlled by internalization and rapid recycling of channel already resident in the plasma membrane (via, e.g., a Rab4-dependent process, see below), and that Rab11 functions instead mainly in the delivery of new channel to that membrane. Results from transfection with a Rab11 wild-type construct are consistent with this latter hypothesis.
Fig. 4.
Rab11DN expression has no effect on Ito,f but overexpression of Rab11WT substantially increases Ito,f in ventricular myocytes. A: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry, mCherry + Rab11DN, or mCherry + Rab11WT. Voltage protocols were as in Fig. 1C. B: Ito,f current density vs. voltage plot for data from −80 to +90 mV for mCherry-, mCherry + Rab11WT-, and mCherry + Rab11DN-transfected myocytes, respectively. Data are represented as means ± SE. *P < 0.05, comparing Rab11WT + mCherry to control, mCherry-transfected myocytes. C: sustained current density vs. voltage plots from −130 to −70 mV for the same cells as in B. SS, steady state. D: Ito,f current density vs. voltage plot for data from −80 to +90 mV recorded 12–24 h posttransfection in ventricular myocytes transfected via the modified, FCS-free protocol, with mCherry or mCherry + Rab11DN. E: sustained current density vs. voltage plots from −120 to −70 mV for the same cells as in D. Data are represented as means ± SE. *P < 0.05, comparing Rab11WT + mCherry to control, mCherry-transfected myocytes.
Overexpression of wild-type Rab11 increases Ito,f current density.
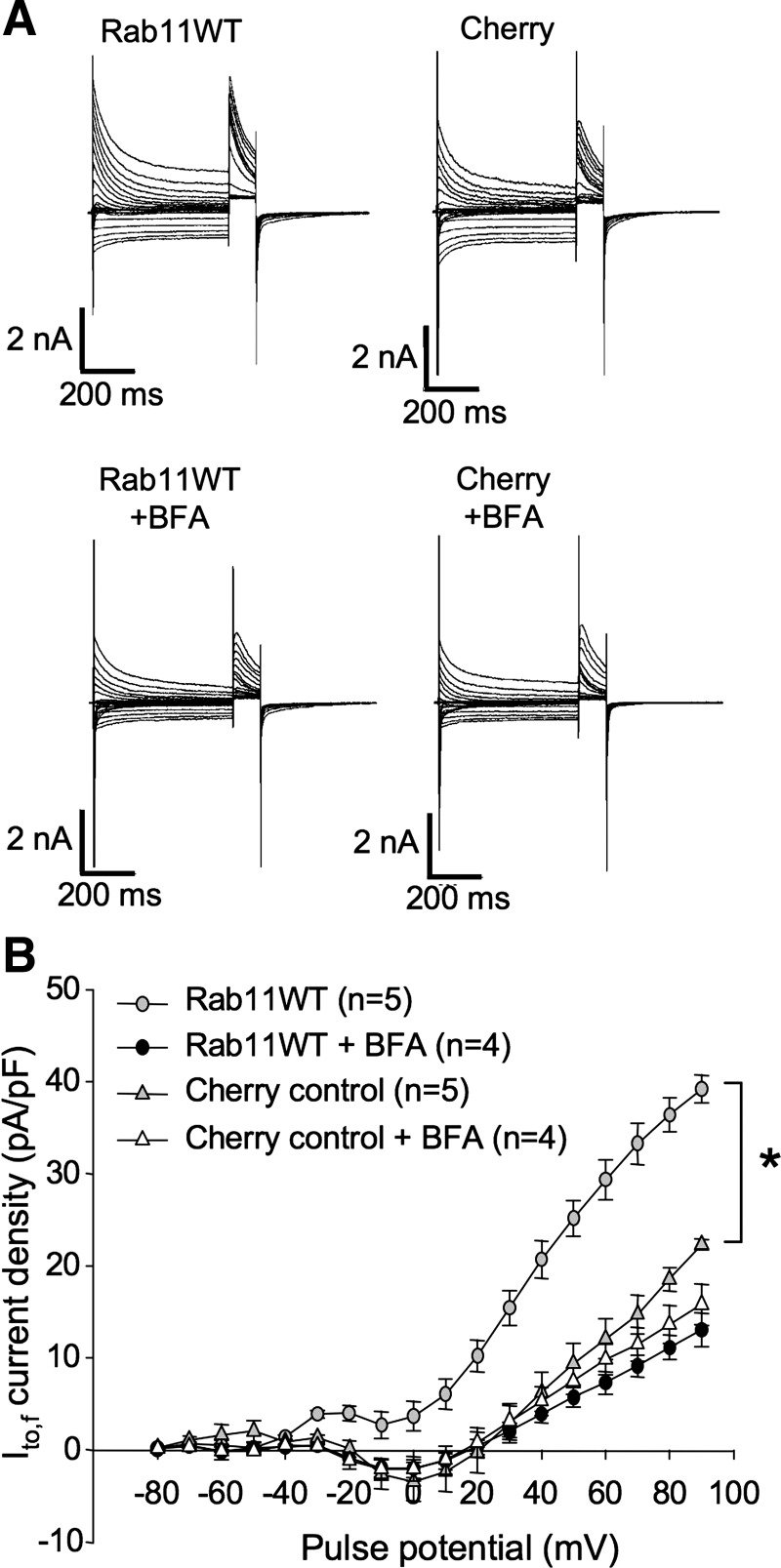
In contrast to all other Rab GTPases tested, overexpression of wild-type Rab11 substantially increased Ito,f in the cardiac myocytes. This overexpression of Rab11WT caused a significant increase of peak current density to 25.0 ± 4.4 pA/pF at +90 mV, much higher than the 10.0 ± 1.5 pA/pF present in control, mCherry-transfected myocytes (P < 0.05) (Fig. 4, A and B). Because Rab11 is involved in both recycling of endocytosed channels (3, 31, 45, 66) and in the trafficking of newly synthesized proteins to the cell surface (55), overexpression of this GTPase might increase Kv4.2 functional expression either by promoting the insertion of channel into the sarcolemmma from an internal pool in recycling endosomes or by promoting the forward trafficking of newly synthesized channel to that membrane (or both). To distinguish between these possibilities, we employed an inhibitor of ER to Golgi transport, BFA. BFA inhibits GTP exchange factors that catalyze the activation of Arf1, a small GTPase that is required for the formation of COPII-coated vesicles (24).Treatment of cells with BFA results in rapid collapse of the Golgi stacks and a complete redistribution of Golgi enzymes to the ER (44), evidently by allowing Golgi membrane fusion to the ER in the absence of vesicle formation (13). Export from the ER is very effectively blocked (16, 26, 61).
As illustrated in Fig. 5, A and B, BFA treatment of Rab11WT-transfected myocytes 6 h before electrophysiological analysis completely blocked the increase in Ito,f. Whereas Rab11WT overexpression in the absence of BFA increased Ito,f current density at +90 mV to 39.2 ± 1.5 pA/pF from the control 22.3 ± 0.6 pA/pF, no significant difference in current densities between Rab11WT-transfected cells (13.0 ± 1.8 pA/pF) and control cells (15.8 ± 2.2 pA/pF) was evident in the presence of BFA (Fig. 5B). Thus, overexpression of wild-type Rab11 very probably increases Ito,f in ventricular myocytes by increasing the forward trafficking of newly synthesized channels to the sarcolemma in these cells.
Fig. 5.
Brefeldin A (BFA) treatment blocks Rab11WT overexpression-associated increase in Ito,f. A: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry, or mCherry + Rab11WT incubated for 6 h with Brefeldin A as indicated. Voltage protocols were as in Fig. 1C. B: Ito,f current density vs. voltage plot for data from myocytes treated as described in A. Data are represented as means ± SE. *P < 0.05, relative to control, mCherry-transfected myocytes.
Rab5 is involved in Kv4.2 internalization.
Once delivered to the plasma membrane, a channel must eventually be reinternalized. To study the mechanisms by which Kv4.2 is internalized and trafficked thereafter in ventricular myocytes, we investigated the involvement of three additional Rab GTPases, Rab5, Rab4, and Rab7, shown previously to be involved in the endocytosis and postinternalization trafficking of other cardiac potassium channels in cardiomyoblast cell lines and heterologous expression systems (31, 45, 66). A simple schematic highlighting the roles of these GTPases in vesicle trafficking is provided in Fig. 6.
Fig. 6.
Schematic representation of postinternalization events. Rab5 is involved in endocytosis from the plasmalemma and the formation of early endosomes. Rab4 is involved in rapid recycling to the plasmalemma, and Rab11 is involved in slower recycling through the so-called recycling endosome as well as in the trafficking of some proteins from the Golgi to the plasmalemma. Rab7 directs traffic to the late endosome and, ultimately, the lysosome for degradation. Internalized proteins may also be shunted to the proteasome for degradation.
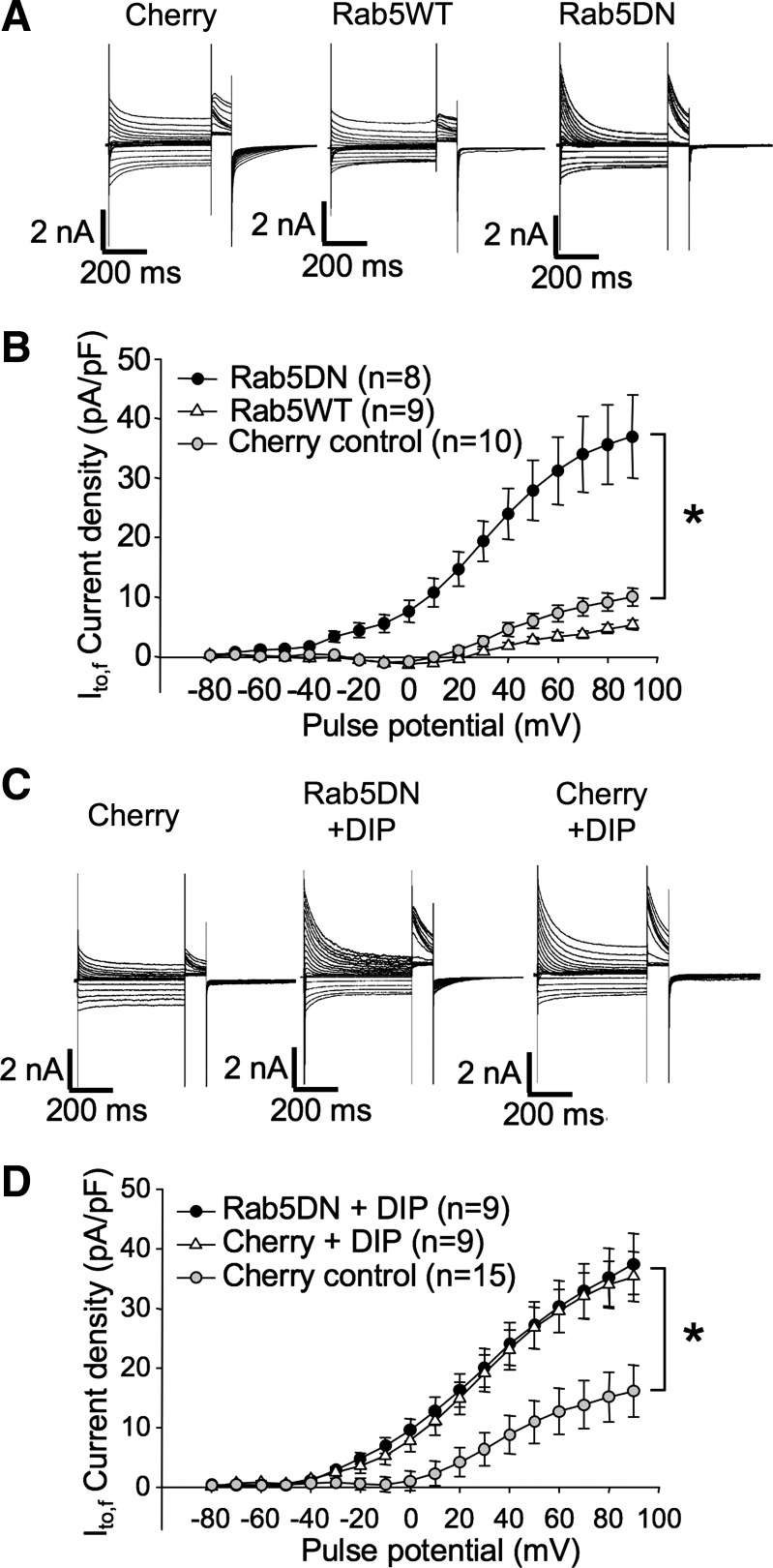
Rab5 is required for clathrin-mediated endocytosis and early endosome formation (7). In heterologous HEK293 cells and in the H9c2 cardiomyoblast line, Rab5 has been shown to be involved in Kv1.5 endocytosis; a Rab5 dominant negative (Rab5DN) mutant, Rab5S34N, increases Kv1.5 surface expression by interfering with endocytosis of the channel (66). In HL-1 cells, however, the Rab5 dominant negative had no detectable effect on this process (31), suggesting that Kv1.5 might internalize via a clathrin-independent manner in this cardiomyoblast line.
In myocytes transfected with Rab5DN (Rab5 S34N), Ito,f was significantly increased at 36–48 h posttransfection (Fig. 7, A and B). Peak current density at +90 mV was 37.0 ± 7.0 pA/pF in Rab5DN-expressing cells and 10.0 ± 1.5 pA/pF in mCherry-transfected control cells (Fig. 7B). Overexpression of Rab5WT did not significantly affect Ito,f in these myocytes, indicating that endogenous levels of this GTPase are not rate limiting for Kv4.2 endocytosis.
Fig. 7.
Interference with Rab5 function increases Ito,f in transfected ventricular myocytes. A: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry, mCherry + Rab5WT, or mCherry + Rab5DN. Voltage protocols were as in Fig. 1C. B: Ito,f current density vs. voltage plot for data from mCherry, mCherry + Rab5WT, and mCherry + Rab5DN-transfected myocytes, respectively. Data are represented as means ± SE. *P < 0.05, comparing Rab5DN + mCherry to control, mCherry-transfected myocytes. C: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry or Rab5DN + mCherry, incubated for 6 h with dynamin inhibitory peptide (DIP) as indicated. Voltage protocols were as described in Fig. 1. D: Ito,f current density vs. voltage plot for myocytes thus treated. Data are represented as means ± SE. *P < 0.05, comparing Rab5DN + mCherry + DIP or mCherry + DIP to control, mCherry-transfected myocytes.
To confirm that Rab5DN affects Ito,f by interfering with the endocytosis of the underlying Kv4.2 channel, we applied DIP to the transfected myocytes 6 h before performing electrophysiological experiments. An inhibitor of dynamin GTPase activity, DIP blocks endocytosis by competitively inhibiting the interaction of dynamin with amphiphysin (47, 62). In our experiment, when applied to ventricular myocytes, DIP treatment significantly increased peak current density at +90 mV from the 16.1 ± 4.4 pA/pF of control cells to 35.3 ± 4.2 pA/pF, but was not additive with Rab5DN (peak current density at +90 mV = 37.4 ± 5.1 mV) (Fig. 7, C and D). Thus, it is highly likely that both Rab5 DN and DIP are interfering with Kv4.2 trafficking by inhibiting endocytosis of the channel.
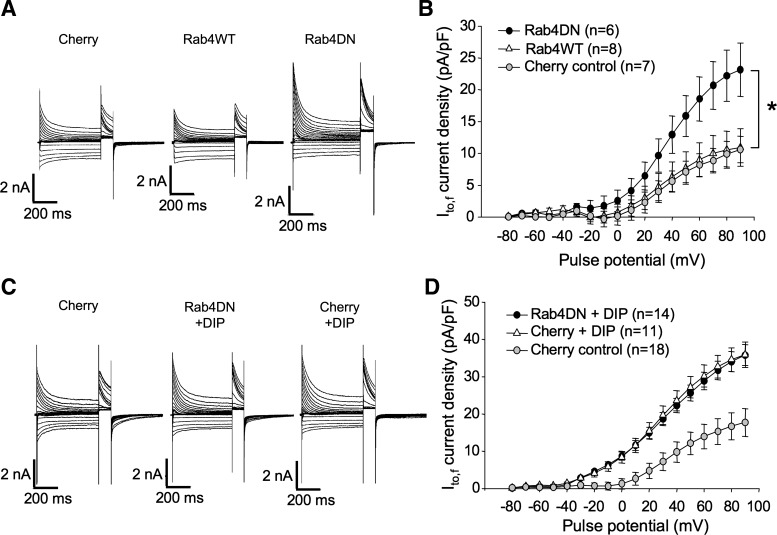
Rab4 involvement in Kv4.2 trafficking.
Rab4 associates with early endosomes shortly after their formation and regulates the rapid recycling of internalized surface proteins to the plasmalemma. Previous observations in cell culture systems indicate that Rab4 is involved in the rapid recycling of internalized Kv1.5 channels (31, 66). We investigated whether Rab4 is involved also in endogenous Kv4.2 trafficking. Rat ventricular myocytes were transfected with Rab4WT and dominant negative (N121I) constructs plus mCherry, or with mCherry alone according to the protocol described previously (12) and assayed electrophysiologically 36–48 h later. As illustrated in Fig. 8, A and B, peak current density in Rab4WT-overexpressing myocytes was 11.0 ± 2.9 mV at +90 mV, no different from the 10.6 ± 2.1 pA/pF measured in control myocytes transfected with mCherry alone (Fig. 8B). Expression of a Rab4DN in the myocytes, however, resulted in a significant increase in peak Ito,f, i.e., to 23.2 ± 4.2 pA/pF at +90 mV. This result is similar to our previously published finding that Kv1.5 surface expression is increased by this dominant negative construct (66). In that case, we demonstrated that the Rab4DN interfered with channel internalization, quite possibly by preventing maturation of early endosomes. To test whether the Rab4DN was interfering with endogenous Kv4.2 internalization, we tested whether its effects were additive with direct block of endocytosis by dynamin inhibitory peptide. As illustrated in Fig. 8, C and D, the effects of the two treatments were not additive, indicating that both likely operate on the same pathway. Peak current densities at +90 mV in DIP-treated mCherry-transfected and mCherry plus Rab4DN-transfected myocytes were statistically identical at 35.9 ± 3.4 and 35.8 ± 2.8 pA/pF, respectively, significantly higher than the control 17.7 ± 3.7 pA/pF measured in untreated mCherry-transfected myocytes. Thus, it is highly likely that Rab4DN is, like DIP, interfering with the endocytosis of endogenous Kv4.2.
Fig. 8.
Rab4DN expression also increases Ito,f in transfected ventricular myocytes. A: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry, mCherry + Rab4WT, or mCherry + Rab4DN. Voltage protocols were as in Fig. 1C. B: Ito,f current density vs. voltage plot for data from mCherry, mCherry + Rab4WT, and mCherry + Rab4DN-transfected myocytes, respectively. Data are represented as means ± SE. *P < 0.05, comparing Rab4DN + mCherry to control, mCherry-transfected myocytes. C: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry or Rab4DN + mCherry, incubated for 6 h with DIP as indicated. Current protocols were as described in Fig. 1. D: Ito,f current density vs. voltage plot for myocytes thus treated. Data are represented as means ± SE.
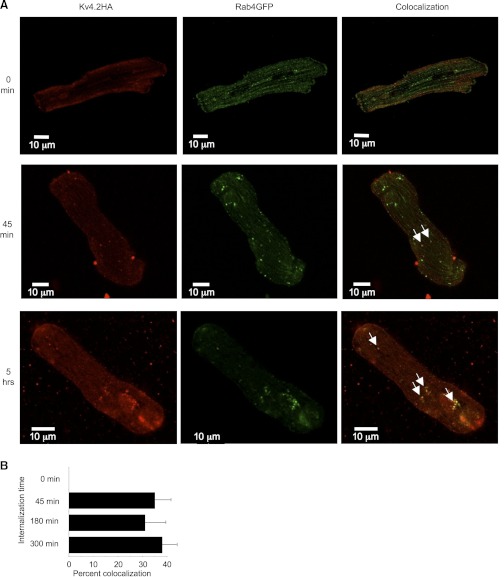
Nevertheless, the interference by the Rab4DN with the endocytosis of Kv4.2 does not guarantee that Rab4 is normally involved in the trafficking of the channel. It is possible that the dominant negative isoform nonspecifically interferes with endocytosis in general, and it is conceivable that Kv4.2 does not actually traverse a Rab4-positive compartment in its normal postinternalization trafficking. To determine whether the channel does indeed traffic through Rab4-positive vesicles, we looked for colocalization of the channel with wild-type Rab4 in ventricular myocytes. To accomplish this, freshly isolated ventricular myocytes were transfected with an extracellularly HA-tagged Kv4.2 construct (a kind gift of Dr. Robert Bähring, University of Hamburg, Hamburg, Germany) and Rab4WT tagged with GFP. The next day, the myocytes were incubated with anti-HA at 37°C for variable times to allow for internalization of the surface expressed channel before fixation and permeabilization and staining for the anti-HA with Alexa594. As illustrated in Fig. 9A, internalized Kv4.2 colocalized extensively with Rab4WT-GFP after as little as 45 min internalization time, and similar colocalization was evident also after allowing longer times for internalization of surface labeled Kv4.2. While the substantial majority of Rab4WT-GFP-positive vesicles did not harbor Kv4.2 signal, roughly one-third of the vesicles exhibiting internalized, surface-labeled Kv4.2 showed also Rab4WT-GFP fluorescence. At 45 min, 35.9 ± 6.7% (means ± SE) of Kv4.2-positive vesicles also showed the green fluorescence indicative of Rab4WT-GFP association with the vesicles. After 3- and 5-h internalization times, 31.3 ± 8.4 and 37.7 ± 6.5%, respectively, of vesicles harboring internalized, surface-labeled Kv4.2 also exhibited the Rab4-associated GFP signal. In cells fixed immediately after labeling, rather than being incubated at 37°C, virtually no Kv4.2-positive vesicles were in evidence and none colocalized with Rab4. These data are summarized in Fig. 9B. The data strongly indicate that internalized Kv4.2 does indeed traffic through Rab4-positive compartments. Very probably, under normal circumstances, the channel recycles via these vesicles to the cell surface.
Fig. 9.
Internalized Kv4.2 colocalizes with Rab4 in ventricular myocytes. A: colocalization of internalized Kv4.2 with enhanced green fluorescent protein-tagged Rab4WT (Rab4GFP). Arrows highlight some of the vesicles in which Kv4.2HA and Rab4-EGFP colocalize. Ventricular myocytes were transfected with externally HA-tagged Kv4.2 plus EGFP-tagged Rab4. Twenty-four hours later, surface Kv4.2 was labeled with mouse anti-HA (Roche) on ice for 1 h, then the myocytes were incubated at 37°C for the times indicated to allow internalization of the labeled Kv4.2-HA. The myocytes were then fixed and permeabilized, then labeled with Alexa594-conjugated goat anti-mouse antibody to detect Kv4.2HA. The myocytes were imaged using an Olympus Fluoview 1000 confocal microscope (see methods). B: quantitation of colocalization of internalized Kv4.2 with Rab4WT-GFP after various internalization times. Percent colocalizations of internalized HA-Kv4.2 with Rab4-GFP after internalization times of 0, 45, 180, and 300 min are indicated.
Kv4.2 degradation likely occurs in the proteasome.
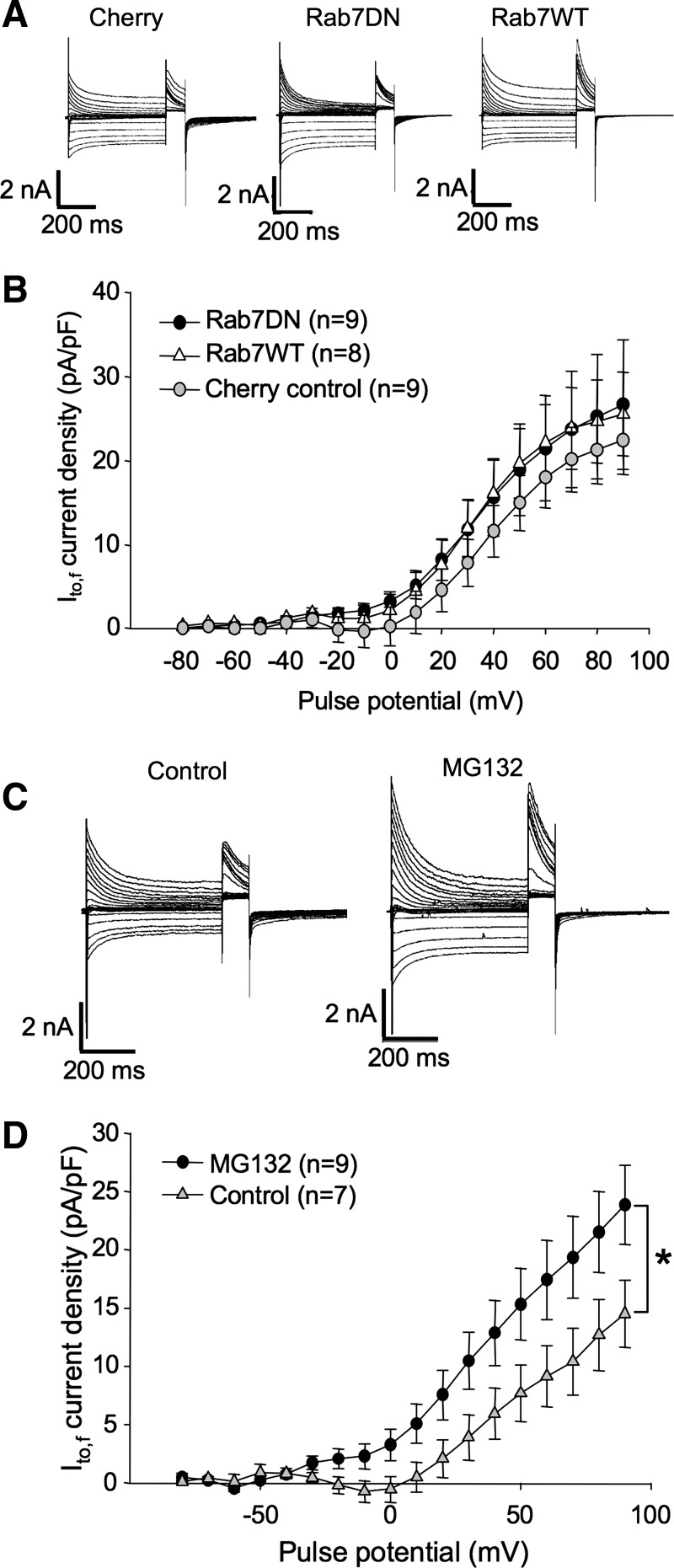
Recycled, or not, eventually channels must be degraded. One manner in which channels may be disposed of is through being shunted to the lysosome. Rab7, which reportedly localizes to early endosomes as they mature into late endosomes, has been shown to be critical for trafficking to the lysosome (8, 32). To determine whether Rab7 plays a significant role in the expression of endogenous Kv4.2 in ventricular myocytes, we tested the effects of overexpression of Rab7WT and DN (N125I) isoforms on Ito,f in this cell type. As shown in Fig. 10, A and B, at 36–48 h posttransfection, neither Rab7WT nor Rab7DN had a significant effect on Ito,f. Current densities in Rab7WT and DN-transfected myocytes were 25.6 ± 5.0 and 26.7 ± 7.7 pA/pF at +90 mV, respectively, statistically indistinguishable from the 22.4 ± 4.1 pA/pF measured in control myocytes transfected with mCherry alone. Thus, if internalized Kv4.2 is being shunted to the lysosome, the pathway is not essential for the maintenance of normal functional expression levels of the channel at the plasmalemma.
Fig. 10.
Internalized Kv4.2 is most likely degraded by the proteasome. A and B: expression of a Rab7DN does not affect endogenous Ito,f. A: representative currents from rat ventricular cardiomyocytes recorded 36–48 h posttransfection with mCherry, mCherry + Rab7DN, or mCherry + Rab7WT. Voltage protocols were as in Fig. 1C. B: Ito,f current density vs. voltage plot for data from mCherry, mCherry + Rab7WT, and mCherry + Rab7DN-transfected myocytes, respectively. Data are represented as means ± SE. C and D: incubation with proteasome inhibitor significantly increases the magnitude of Ito,f. C: representative currents from rat ventricular cardiomyocytes recorded ∼24 h postisolation and treated, where indicated, with MG132, for 6 h before recording. Current protocols were as described in Fig. 1. D: Ito,f current density vs. voltage plot for data from myocytes thus treated. Data are represented as means ± SE. *P < 0.05, comparing current densities in MG132-treated myocytes to control, untreated myocytes.
The major alternative pathway for membrane protein degradation is via the proteasome. We tested also whether inhibition of this complex would influence Ito,f current densities. Indicating that endogenous Kv4.2 in these myocytes may indeed be degraded by the proteosome, 6 h of incubation with the proteosome inhibitor MG132 increased peak current densities by roughly 60% from 14.5 ± 2.9 to 23.9 ± 3.4 pA/pF (Fig. 10, C and D). The precise cause of the increase in Ito,f density is, of course, unclear but very likely to be related to a failure of channel degradation. Perhaps an accumulation of internalized Kv4.2 is preventing further internalization of the channel or channel that would normally be shunted for degradation is instead being recycled to the plasmalemma under these conditions.
DISCUSSION
The trafficking of ventricular cardiomyocyte Kv4.2 has been investigated by measuring changes in the endogenous rapidly inactivating component of the transient outward current, Ito,f. For the most part, the trafficking of endogenous Kv4.2 in ventricular myocytes out of the ER, on to the Golgi and the sarcolemma, and, thereafter, its internalization from the sarcolemma and its recycling to that locale, appears to be conventional. The data indicate that Kv4.2 traffics out of the cardiac endoplasmic reticulum via a conventional pathway involving both Sar1 and Rab1 and that its trafficking to the sarcolemma can be enhanced by overexpression of wild-type Rab11. Once at the sarcolemma, Ito,f channels behave in much the same way as Kv1.5 was previously demonstrated to traffic in a myoblast cell line (66). Internalization appears dependent on Rab5 function, and block of Rab4 somehow inhibits this internalization. Unlike the results previously reported with Kv1.5, however, the evidence indicates that the channel may not traffic to the lysosome for degradation. Instead, proteosomal degradation of the channel appears to be operative.
That Kv4.2 traffics through a conventional ER-to-Golgi pathway in intact cardiomyocytes, involving both Rab1 and Sar1, is significant. This is quite different from the situation in a HeLa cells transfected with the channel and KChIP1 (15, 23). In those cells, Kv4.2/KChIP1 traffic has been shown to also depend on Rab1 but to be independent of Sar1, traveling from the ER to Golgi via vesicles not coated with COPII (15). In a majority of those cells, the Kv4.2/KChIP1 complex reached the plasmalemma even in the presence of a Sar1 dominant negative that completely blocked vesicular stomatitis virus glycoprotein (VSVG) traffic to that membrane (23). Hasdemir et al. (23) hypothesize that Kv4.2 traffics from the ER to Golgi in the heterologous system and, evidently, in hippocampal neurons—where Kv4.2 and KChIP1 colocalize extensively (23)—via “KChIP vesicles” that, in the case of KChIP1 at least, are demonstrably independent of Sar1 function. KChIP1 is predominant in brain (39) but not in the heart, where three splice variants of KChIP2 predominate (43). Thus, from our results it is highly likely that the trafficking of KChIP2-associated Kv4.2 is very different from that driven by KChIP1. Consistent with this, Flowerdew and Burgoyne (15) found that KChIP2-mediated Kv4.2 trafficking did not involve the same SNARE protein intermediaries (VAMP-7 and Vti1A) as that mediated by KChIP1. As they point out, others have shown that only KChIP1 targets to intracellular vesicles (34, 46, 57); KChIP2 reportedly targets directly to the plasma membrane even when expressed alone (51, 57).
Once out of the ER and past the Golgi apparatus, our data indicate that Rab11 plays a role in Kv4.2 trafficking, although precisely what role it plays is somewhat unclear. The increase in endogenous Ito,f when Rab11 wild-type is overexpressed in the ventricular myocytes is very different from that seen in a study of the expression of another Kv channel, Kv1.5, in heterologous cells and H9c2 myoblasts. In that case, currents were unaffected by overexpression of wild-type Rab11 and affected only mildly by the dominant negative and then only after prolonged coexpression (66). Nevertheless, Rab11 plays an important role in Kv1.5 trafficking in cardiomyocytes under at least some conditions. Balse et al. (3) found that the Rab11DN did block the increase in Kv1.5 associated with cholesterol depletion in atrial myocytes. In these ventricular myocytes, the effect of the dominant negative on Kv4.2, i.e., Ito,f, like its effect on Kv1.5 in the myoblasts, is negligible.
There are numerous pathways through which a channel can potentially traffic from the Golgi to the plasmalemma. In addition to traffic through Rab11-associated compartments, membrane proteins can traffic instead through several additional pathways, such as those controlled by Rab8 and Rab3 (reviewed in Ref. 49). Thus, it is not surprising that different channels are differentially affected by individual manipulations of one of those pathways. The failure of the dominant negative Rab11 mutant to significantly reduce Ito,f currents in the myocytes despite the increase in functional expression of the channel associated with Rab11WT overexpression, however, makes interpretation of the phenomenon more difficult. One possibility is that the delivery of newly synthesized Kv4.2 to the plasmalemma does not normally involve Rab11; overexpression of the GTPase would, in this scenario, artifactually recruit the channel into a Rab11-dependent pathway. More likely, though, Rab11 is involved in the normal delivery of newly synthesized Kv4.2 to the plasmalemma, although quite possibly in parallel with pathways dependent instead on Rab8 and Rab3, both of which are known to deliver cargo to the cell surface independently of Rab11 (reviewed in Ref. 49).
If this is true, however, the failure of the Rab11DN to reduce Ito,f in the myocytes must be explained. One possibility is that Rab11's dual roles intrinsically compensate for a loss in Rab11-driven Golgi-to-plasmalemma trafficking. Rab11 is involved also in the trafficking internalized membrane proteins out of early endosomes into recycling endosomes. Perhaps the removal of this latter pathway by expression of the dominant negative frees a population of endocytosed Kv4.2 to be rapidly recycled to the plasmalemma via the Rab4-dependent alternative fast recycling pathway. Or, perhaps, parallel pathways, as mentioned above, compensate for the loss of Rab11-driven Golgi-to-plasmalemma traffic. Further experiments involving Rab8 and Rab3 manipulations will be necessary to more fully understand these results.
Our results concerning the fates of Kv4.2 channels after delivery to the plasmalemma are more easily interpreted. The postinternalization trafficking of endogenous ventricular Kv4.2 appears to be conventional. That expression of the Rab5 dominant negative resulted in increased Ito,f in the ventricular myocytes was essentially as expected. Once the Kv4.2 channel is inserted in the plasmalemma, it is not surprising that expression of a Rab5 dominant negative would keep it there. Rab5 is required for clathrin-dependent endocytosis and early endosome formation (7). The internalization of CFTR (36), KCNQ1/KCNE1 (45), and Kv1.5 (66) have all been shown to depend on Rab5 function, albeit mainly in heterologous systems. Kv1.5 internalization has been also been shown to be dependent on Rab5 in one cardiomyoblast cell line (H9c2) (66), although no such dependence was found in a different cardiomyoblast (HL-1) line (31). hERG (21) and KCa2 (30) channels have also been shown to colocalize with Rab5 after internalization from the cell surface. As with most Rab GTPases tested, we found no effect of overexpression of Rab5 on endogenous Ito,f in the ventricular myocytes. Rab5 levels under normal conditions are very probably high enough that the wild-type is not rate limiting to the process. Baltaev et al. (4) similarly failed to find any effect of such overexpression on coexpressed Kv4.3/KChIP2b in Xenopus oocytes.
The similar increase in Ito,f in response to expression of a Rab4DN is also interesting. Similar to results previously obtained in a study of Kv1.5 trafficking in heterologous and H9c2 myoblast cells, expression of a Rab4 dominant negative in the ventricular myocytes resulted in an increase in the Kv4.2-dependent Ito,f. As previously shown for Kv1.5 trafficking, this phenomenon may well be due to an interference with channel internalization. Perhaps the Rab4 dominant negative effectively ties up Rab5 in early endosomes that cannot mature and be recycled to the plasmalemma. With the Rab5 so sequestered, it may no longer be available for the promotion of channel internalization. It will be very interesting to determine whether or not this is, in fact, the case. Whatever proves to underlie the phenomenon, it is clear from the Ito,f results and those previously obtained with Kv1.5 that the predicted effect(s) of interference with a Rab-catalyzed event do not necessarily hold. Very probably interference with one pathway can result in unexpected effects on pathways feeding into and deriving from that pathway.
Our data indicate that degradation of internalized Kv4.2 likely differs, at least in part, from that of Kv1.5 as well. Whereas Kv1.5 has been shown to traffic to the lysosome via Rab7-positive endosomes (66) and to be degraded also by the proteasome (25), endogenous Kv4.2 in ventricular myocytes very likely does not traffic in significant measure to the lysosome. Functional expression is unaffected by overexpression of wild-type or dominant negative Rab7 isoforms. That incubation with the proteasome inhibitor MG132 increases Ito,f indicates that Kv4.2 most likely is, like CFTR (60) and hERG channels (19), in large measure degraded by the proteasome. Quite possibly, of course, both pathways may be operative with the proteasomal pathway predominating under the conditions/time frames that existed in our experiments. There is substantial recent evidence that despite the fact that hERG is predominantly degraded by the proteasome under some physiological conditions (19), it is degraded via the lysosomal pathway under other conditions (22). As the proteasome is largely involved in the degradation of ubiquitinated proteins (58), it is reasonable to hypothesize that it is ubiquitination that drives Kv4.2 degradation in the cardiomyocyte.
In summary, we have implicated several small GTPases in the regulation of the cell surface current density of Ito,f in adult ventricular myocytes. It will be of great interest to determine how the trafficking of Kv4.2 intersects with that of other potassium channels in these myocytes and how these trafficking pathways are modulated both in health and disease.
GRANTS
This study was supported by funding to D. Fedida from the Canadian Institutes for Health Research and the Heart and Stroke Foundation of British Columbia and the Yukon.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.W., Y.C., Y.D., C.G., and J.-P.D. performed the experiments; T.W., Y.C., Y.D., C.G., J.-P.D., and D.F.S. analyzed the data; Y.D. and D.F.S. prepared the figures; Y.D., D.F.S., and D.F. approved the final version of the manuscript; D.F.S., C.H., and D.F. conception and design of the research; D.F.S., C.H., and D.F. interpreted results of experiments; D.F.S. and D.F. drafted the manuscript; D.F.S. and D.F. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank Kyung He Park for excellent technical assistance.
REFERENCES
- 1.Akyol M, Jalilzadeh S, Sinner MF, Perz S, Beckmann BM, Gieger C, Illig T, Wichmann HE, Meitinger T, Kaab S, Pfeufer A. The common non-synonymous variant G38S of the KCNE1-(minK)-gene is not associated to QT interval in Central European Caucasians: results from the KORA study. Eur Heart J 28: 305–309, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science 289: 444–448, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Balse E, El-Haou S, Dillanian G, Dauphin A, Eldstrom J, Fedida D, Coulombe A, Hatem SN. Cholesterol modulates the recruitment of Kv1.5 channels from Rab11-associated recycling endosome in native atrial myocytes. Proc Natl Acad Sci USA 106: 14681–14686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltaev R, Strutz-Seebohm N, Korniychuk G, Myssina S, Lang F, Seebohm G. Regulation of cardiac shal-related potassium channel Kv 4.3 by serum- and glucocorticoid-inducible kinase isoforms in Xenopus oocytes. Pflügers Arch 450: 26–33, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Barlowe C. COPII-dependent transport from the endoplasmic reticulum. Curr Opin Cell Biol 14: 417–422, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII - a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77: 895–907, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase Rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70: 715–728, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell 11: 467–480, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Feng Y, Chen DY, Wandinger-Ness A. Rab11 is required for trans-Golgi network to plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell 9: 3241–3257, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res 97: 363–371, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Deretic D, PuleoScheppke B, Trippe C. Effects of the synthetic peptides from small G proteins on post-Golgi trafficking of rhodopsin (Abstract). Mol Biol Cell 7: 2570, 1996 [Google Scholar]
- 12.Dou Y, Balse E, Zadeh AD, Wang TT, Goonasekara CL, Noble GP, Eldstrom J, Steele DF, Hatem SN, Fedida D. Normal targeting of a tagged Kv1.5 channel acutely transfected into fresh adult cardiac myocytes by a biolistic method. Am J Physiol Cell Physiol 298: C1343–C1352, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Elazar Z, Orci L, Ostermann J, Amherdt M, Tanigawa G, Rothman JE. ADP-ribosylation factor and coatomer couple fusion to vesicle budding. J Cell Biol 124: 415–424, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Press B, Wandingerness A. Rab-7 - an important regulator of late endocytic membrane traffic. J Cell Biol 131: 1435–1452, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flowerdew SE, Burgoyne RD. A VAMP7/Vti1a SNARE complex distinguishes a non-conventional traffic route to the cell surface used by KChIP1 and Kv4 potassium channels. Biochem J 418: 529–540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fullekrug J, Sonnichsen B, Schafer U, Van PN, Soling HD, Mieskes G. Characterization of brefeldin A induced vesicular structures containing cycling proteins of the intermediate compartment/cis-Golgi network. FEBS Lett 404: 75–81, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Galperin E, Sorkin A. Visualization of Rab5 activity in living cells by FRET microscopy and influence of plasma-membrane-targeted Rab5 on clathrin-dependent endocytosis. J Cell Sci 116: 4799–4810, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Gentzsch M, Chang XB, Cui LY, Wu YF, Ozols VV, Choudhury A, Pagano RE, Riordan JR. Endocytic trafficking routes of wild type and Delta F508 cystic fibrosis transmembrane conductance regulator. Mol Biol Cell 15: 2684–2696, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong QM, Keeney DR, Molinari M, Zhou ZF. Degradation of trafficking-defective long QT syndrome type II mutant channels by the ubiquitin-proteasome pathway. J Biol Chem 280: 19419–19425, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103: 11821–11827, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo J, Massaeli H, Xu JM, Jia ZC, Wigle JT, Mesaeli N, Zhang ST. Extracellular K+ concentration controls cell surface density of IKr in rabbit hearts and of the HERG channel in human cell lines. J Clin Invest 119: 2745–2757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J, Wang TZ, Yang TH, Xu JM, Li WT, Fridman MD, Fisher JT, Zhang ST. Interaction between the cardiac rapidly (IKr) and slowly (IKs) activating delayed rectifier potassium channels revealed by low K+-induced hERG endocytic degradation. J Biol Chem 286: 34664–34674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasdemir B, Fitzgerald DJ, Prior IA, Tepikin AV, Burgoyne RD. Traffic of Kv4 K+ channels mediated by KChlP1 is via a novel post-ER vesicular pathway. J Cell Biol 171: 459–469, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson CL, Casanova JE. Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol 10: 60–67, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Kato M, Ogura K, Miaki J, Sasaki N, Taniguchi S, Igawa O, Yoshida A, Hoshikawa Y, Murata M, Nanba E, Kurata Y, Kawata Y, Ninomiya H, Morisaki T, Kitakaze M, Isatome I. Evidence for proteasomal degradation of Kv1.5 channel protein. Biochem Biophys Res Commun 337: 343–348, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116: 1071–1080, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knodler A, Feng S, Zhang J, Zhang X, Das A, Peränen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA 107: 6346–6351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol 125: 51–65, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loewen ME, Wang ZR, Eldstrom J, Zadeh AD, Khurana A, Steele DF, Fedida D. Shared requirement for dynein function and intact microtubule cytoskeleton for normal surface expression of cardiac potassium channels. Am J Physiol Heart Circ Physiol 296: H71–H83, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Lu L, Timofeyev V, Li N, Rafizadeh S, Singapuri A, Harris TR, Chiamvimonvat N. alpha-Actinin2 cytoskeletal protein is required for the functional membrane localization of a Ca2+-activated K+ channel (SK2 channel). Proc Natl Acad Sci USA 106: 18402–18407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of KV1.5 in atrial myocytes. J Biol Chem 282: 29612–29620, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Meresse S, Gorvel JP, Chavrier P. The Rab7 GTPase resides on a vesicular compartment connected to lysosomes. J Cell Sci 108: 3349–3358, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward potassium current (Ito) expression and regulation. J Mol Cell Cardiol 48: 12–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Callaghan DW, Hasdemir B, Leighton M, Burgoyne RD. Residues within the myristoylation motif determine intracellular targeting of the neuronal Ca2+ sensor protein KChIP1 to post-ER transport vesicles and traffic of Kv4 K+ channels. J Cell Sci 116: 4833–4845, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol 11: 487–491, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol 285: C1009–C1018, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pind SN, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2+ are required for the fusion of carrier vesicles mediating endoplasmic reticulum to Golgi transport. J Cell Biol 125: 239–252, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plutner H, Cox AD, Pind S, Khosravifar R, Bourne JR, Schwaninger R, Der CJ, Balch WE. Rab1B regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol 115: 31–43, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pourrier M, Schram G, Nattel S. Properties, expression and potential roles of cardiac K+ channel accessory subunits: MinK, MiRPs, KChIP, and KChAP. J Membr Biol 194: 141–152, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Pucadyil TJ, Schmid SL. Conserved functions of membrane active GTPases in coated vesicle formation. Science 325: 1217–1220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren MD, Xu GX, Zeng JB, Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA 95: 6187–6192, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robitaille M, Ramakrishnan N, Baragli A, Hebert TE. Intracellular trafficking and assembly of specific Kir3 channel/G protein complexes. Cell Signal 21: 488–501, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Schultz JH, Janzen C, Volk T, Ehmke H. Kv4.2 and KChIP2 transcription in individual cardiomyocytes from the rat left ventricular free wall. J Mol Cell Cardiol 39: 269–275, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Sciaky N, Presley J, Smith C, Zaal KJM, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of Brefeldin A visualized in living cells. J Cell Biol 139: 1137–1155, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, Marks D, Pagano RE, Attali B, Pfeufer A, Kass RS, Sanguinetti MC, Tavare JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res 100: 686–692, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC, Carroll KI, Sweatt JD, Rhodes KJ, Trimmer JS. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem 278: 36445–36454, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, DeCamilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science 276: 259–263, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Steele DF, Eldstrom J, Fedida D. Mechanisms of cardiac potassium channel trafficking. J Physiol 582: 17–26, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 81: 153–208, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Takimoto K, Yang EK, Conforti L. Palmitoylation of KChIP splicing variants is required for efficient cell surface expression of Kv4.3 channels. J Biol Chem 277: 26904–26911, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Taneja TK, Mankouri J, Karnik R, Kannan S, Smith AJ, Munsey T, Christesen HBT, Beech DJ, Sivaprasadarao A. Sar1-GTPase-dependent ER exit of K-ATP channels revealed by a mutation causing congenital hyperinsulinism. Hum Mol Genet 18: 2400–2413, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Tisdale EJ, Bourne JR, Khosravifar R, Der CJ, Balch WE. GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol 119: 749–761, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135: 913–924, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urbe S, Huber LA, Zerial M, Tooze SA, Parton RG. Rab11, a small Gtpase associated with both constitutive and regulated secretory pathways in PC12 cells. FEBS Lett 334: 175–182, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Vandersluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein Rab4 controls an early sorting event on the endocytic pathway. Cell 70: 729–740, 1992 [DOI] [PubMed] [Google Scholar]
- 57.Venn N, Haynes LP, Burgoyne RD. Specific effects of KChIP3/calsenilin/DREAM, but not KChIPs 1, 2 and 4, on calcium signalling and regulated secretion in PC12 cells. Biochem J 413: 71–80, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68: 1015–1068, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Wang XD, Matteson J, An Y, Moyer B, Yoo JS, Bannykh S, Wilson IA, Riordan JR, Balch WE. COPII-dependent export of cystic fibrosis transmembrane conductance regulator from the ER uses a di-acidic exit code. J Cell Biol 167: 65–74, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell 83: 121–127, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Ward TH, Polishchuk RS, Caplan S, Hirschberg K, Lippincott-Schwartz J. Maintenance of Golgi structure and function depends on the integrity of ER export. J Cell Biol 155: 557–570, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wigge P, Kohler K, Vallis Y, Doyle CA, Owen D, Hunt SP, McMahon HT. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol Biol Cell 8: 2003–2015, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson BS, Nuoffer C, Meinkoth JL, Mccaffery M, Feramisco JR, Balch WE, Farquhar MG. A Rab1 mutant affecting guanine-nucleotide exchange promotes disassembly of the Golgi apparatus. J Cell Biol 125: 557–571, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeola SW, Snyders DJ. Electrophysiological and pharmacological correspondence between Kv4.2 current and rat cardiac transient outward current. Cardiovasc Res 33: 540–547, 1997 [DOI] [PubMed] [Google Scholar]
- 65.Yoo JS, Moyer BD, Bannykh S, Yoo HM, Riordan JR, Balch WE. Non-conventional trafficking of the cystic fibrosis transmembrane conductance regulator through the early secretory pathway. J Biol Chem 277: 11401–11409, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Zadeh AD, Xu HJ, Loewen ME, Noble GP, Steele DF, Fedida D. Internalized Kv1.5 traffics via Rab-dependent pathways. J Physiol 586: 4793–4813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117, 2001. Erratum. Nat Rev Mol Cell Biol 2: 107–117, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Zheng H, Mckay J, Buss JE. H-ras does not need COP I- or COP II-dependent vesicular transport to reach the plasma membrane. J Biol Chem 282: 25760–25768, 2007 [DOI] [PubMed] [Google Scholar]