Abstract
The normal contractile, electrical, and energetic function of the heart depends on the synchronization of biological oscillators and signal integrators that make up cellular signaling networks. In this review we interpret experimental data from molecular, cellular, and transgenic models of cardiac signaling behavior in the context of established concepts in cell network architecture and organization. Focusing on the cellular Ca2+ handling machinery, we describe how the plasticity and adaptability of normal Ca2+ signaling is dependent on dynamic network configurations that operate across a wide range of functional states. We consider how (mal)adaptive changes in signaling pathways restrict the dynamic range of the network such that it cannot respond appropriately to physiologic stimuli or perturbation. Based on these concepts, a model is proposed in which pathologic abnormalities in cardiac rhythm and contractility (e.g., arrhythmias and heart failure) arise as a consequence of progressive desynchronization and reduction in the dynamic range of the Ca2+ signaling network. We discuss how a systems-level understanding of the network organization, cellular noise, and chaotic behavior may inform the design of new therapeutic modalities that prevent or reverse the disease-linked unraveling of the Ca2+ signaling network.
Keywords: heart oscillation, calcium, network, chaos, synchronization, plasticity
our present mechanistic understanding of cardiac cell signaling has largely come from experimental approaches that range from genetic and biophysical reductionism through to integrated perspectives from whole animal and human physiology studies. However, these advances have required the conceptual and practical segregation of cellular components to the extent that even “integrative” assessments of cardiac cell function are considered within the confines of discrete processes (e.g., excitation-contraction coupling) (32, 35, 55). Thus, although we have a reasonably detailed molecular perspective on the architecture of signaling pathways, we do not fully understand the coherence of multiple processes at the cell and tissue level.
Cell signals are channeled through complex networks that exhibit emergent behavior (15, 27, 145), and a more complete understanding of signal transmission through these networks inevitably requires the synergy of experimental and computational approaches (20, 69, 141). This drive towards a “systems-level” understanding of biological phenomena (widely termed systems biology) permeates contemporary studies on molecular architecture (138), predictive metabolic fluxes in cellular networks (“fluxomics”) (110) through to developments in personalized medicine (63). Systems biology has even been grandly positioned as “the culmination of biology” (19).
Cardiac cell signaling is underpinned by the coupling of biological oscillators, multitasking signal integrators, and parallel-processing pathways and thus lends itself to a systems-level interrogation. In this review, we interpret findings from experimental models of cardiac (dys)function in the context of established principles of cell networks to illustrate the intricate organization of molecular and cellular events that control normal cardiac cell behavior. We extend these concepts into pathological scenarios and propose a model of cardiac dysfunction that is linked to the progressive desynchronization of cellular processes and the reduced plasticity1 of the signaling network. Finally, we consider how our emerging understanding of Ca2+ signaling from a network perspective may be useful in guiding new therapeutic approaches for heart disease.
Dynamic Control of Signal Transmission in Cell Signaling Networks
Cell signaling networks are constructed from the hierarchical organization of molecular components, termed nodes, into modules that can perform higher-order functions such as switching, information storage, and amplification (58, 67, 88, 89, 101, 108, 152). In a cardiac cell context, nodes [that include cAMP- and Ca2+-dependent protein kinases (PKA and PKC), Ca2+/calmodulin-dependent kinase (CaMKII), and ryanodine receptor type 2 (RyR2)] are assembled into modules [e.g., the macromolecular complex that regulates RyR2-dependent Ca2+ release (13, 28, 32, 94)] via protein-protein interaction and compartmentalization in specialized environments (11, 29, 171). From the precise configuration of these nodes and modules emerge “small world” networks that are characterized by delay, robustness, plasticity, synchronization, and enhanced signal-speed propagation (3, 145, 162) (Table 1).
Table 1.
Glossary of terms
| Definition | |
|---|---|
| Complexity (dynamic) | Total degrees of freedom available to a system in terms of functional nodes (i.e., elementary mechanisms) and type of interconnections. Functionally quantified as the observable independent degrees of freedom that remain unaffected by synchronization events. (Range of output available to a system for a given parametric configuration. Quantified by phase-space filling characteristics at various scales of observation.) |
| Configuration (dynamic) | Architecture employed in the connectivity of systems components. (Observable behavior of a system, characterized by conformation to universal/hallmark patterns of dynamic activity.) |
| Dynamic range | The set of dynamical configurations simultaneously available to a system. In nonlinear systems available configurations are reached asymptotically and successively due to trajectory divergence. |
| Entrainment | Reduction of the potential complexity of a system of interconnected components (identified as the total number of degrees of freedom available) due to local and global synchronization events. |
| Hierarchical structure (horizontal and vertical connectivity) | A spatially extended set of coupled primary mechanisms constitutes a horizontal layer. Interaction of such layers via either a feedback or unidirectional coupling can result in vertical connectivity in hierarchical dynamical structures. |
| Maladaptation | The potential of a system to reach alternative steady states/dynamical configurations, through combinations of parametric variations. The original/“healthy” and resulting configurations may be functionally related, they correspond, however, to distinct equilibria/basins of attraction within the parametric/phase-space. |
| Plasticity | The capacity of a system to assume and maintain various dynamical configurations. |
| Pseudo-stable state | A homeostatic configuration that maintains apparent features of a primary/“healthy” behavior but is compromised in terms of robustness. |
| Robustness | Insensitivity of a system to parametric variations. |
| Synchronization | The ability of coupled oscillators to establish and maintain a constant phase shift (in phase or out of phase periodicities). Large arrays of interacting oscillatory systems can exhibit a single emergent periodic mode via nonequilibrium phase transitions. |
Tools such as topological mapping and graph theory have enabled the architecture of cell networks to be visualized as biological circuit diagrams in which signaling information is transmitted through densely clustered, highly interconnected pathways (39, 42, 157, 160, 174). However, such biological circuitry is not “hardwired” but rather is distinguished by the plasticity of molecular interaction that can be reconfigured on an activity-dependent basis in response to defined signal inputs (2, 68, 96). Thus cellular networks evolve under the influence of local (e.g., intracellular) and long-distance (e.g., intercellular) factors that include electrical and metabolic cues, hormonal exposure, hemostatic environment, and their functional coupling to neighboring cells.
The proper routing of signals through cell networks depends on the exquisite interactivity of nodes and modules yet paradoxically these signaling hubs must operate relatively independently of each other. Recent work has shown that the relative abundance of proteins within a network is an important factor that determines the functional independence of modules despite them being embedded in a “globally connected topology” (96, 97, 175).
Protein abundance is the product of synthetic and degradative pathways and is synchronized with other aspects of cardiac signaling network via mechanisms such as excitation-transcription coupling (ETC), in which gene transcription is regulated by the cellular contractile machinery (7, 33, 170), transcription-translation feedback looping (TTFL) (128), and transcriptional reinforcement (180). These mechanisms effectively act as cellular surveillance systems that ensure the responsive tuning of protein abundance, such that proteins are not overexpressed (and thus damp signal transmission), nor are they underrepresented thereby compromising their biological utility. For example, the expression levels of proteins that act as scaffolds for signal transduction complexes are typically kept low to prevent promiscuous nonspecific interactions with cytoplasmic proteins (62).
Consequently, altered protein abundance or the derangement of protein localization within cells would be anticipated to profoundly reroute signal flow in the signaling network. In a cardiac setting, there is substantial evidence supporting the role of altered cellular abundance and/or distribution of Ca2+ signaling proteins in both chronic and acute heart disease. In end-stage heart failure, severe contractile abnormalities are associated with grossly perturbed Ca2+-handling dysfunction resulting from changes in the cellular abundances of L-type Ca2+ channels (LTCC), phospholamban (PLB), RyR2, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), and Na+/Ca2+ exchanger (NCX) (65). Elsewhere, the altered interaction of regulatory co-proteins in the RyR2 macromolecular complex [e.g., FKBP12.6, phosphodiesterase 4D (PDE4D), and protein phosphatase 1 (PP1)] is controversially proposed as an early causative event in atrial fibrillation, heart failure, and stress-induced ventricular tachycardia (VT) (14, 44, 64, 79, 95, 121, 156, 163, 164). In the context of monogenic arrhythmia syndromes, long-QT syndrome type 4 (LQT4) is caused by mutations in the cytoplasmic adaptor protein ankyrin B that result in the sarcolemmal disordering of otherwise functional Na-K-ATPase and NCX (102).
These examples of perturbed signaling serve to highlight the inherent limitations of any experimental system that involves the alteration of protein levels or the modification of their biological activities (e.g., transgenic overexpression or knockdown/knockout strategies) (26). In these scenarios, the alteration of protein abundance or activity may fundamentally “rewire” the physical (spatial aspect of a component) and logical (route of signal transmission) topological status of the network to a configuration very different to that normally occurring in situ. Consequently, when devising experimental approaches to interrogate cell signaling networks, the utility and appropriateness of animal models in which network structure may have been reconfigured (for example, following the targeted ablation of a phosphorylation motif) should be borne in mind. We expand upon this concept in the section Towards an Understanding of Reduced Dynamic Range, Maladaptation, and the Disease-Linked Network State.
Synchronization of Cellular Processes and Uncoupling as a Pathogenic Event
In the section Dynamic Control of Signal Transmission in Cell Signaling Networks, we briefly described some of the mechanisms controlling protein expression to illustrate synchronization at a horizontal level i.e., synchronization of mechanisms that converge on a common process. However, network complexity emerges from the layering of multiple horizontal levels (e.g., protein expression, fluxes through ion channels) into vertical network structures that coordinate more diverse processes (e.g., ion fluxes feeding into intercellular communication or protein expression governing multicellular susceptibility to apoptosis). In the biological context, in principle each layer of connectivity can be associated with a specific set of pathologies, e.g., defects in contractile, electrical, or energetic behavior in the diseased heart.
One of the best known examples of horizontal network structure in cardiac signaling is the synchronization of membrane and intracellular Ca2+ oscillations (75). The sarcoplasmic reticulum (SR), the main intracellular Ca2+ reservoir, is inherently predisposed to spontaneous RyR2-dependent Ca2+ release and functions as an internal Ca2+ oscillator (termed the Ca2+ clock) (78, 158, 172). In normal ventricular myocytes the Ca2+ clock is suppressed by entrainment with sarcolemmal ion fluxes (membrane clock) mediated by the hyperpolarization-activated cyclic nucleotide (HCN) channels (“funny” current, If) (76, 77). Interestingly, in sinoatrial node cells (SANC) the heightened activities of PKA and CaMKII increase RyR2 phosphorylation (among other downstream targets) and partially uncouple the Ca2+ and membrane clocks thus driving spontaneous oscillatory behavior (which manifests as pacemaker activity) (76, 77).
The horizontal coupling of SR and sarcolemmal oscillators is embedded in a network organization with mitochondrial oscillation, the synchronization of which is dependent on both the physical coupling of mitochondria (71, 124) and the Ca2+ microenvironment at the SR/mitochondrial interface (34, 122, 126, 147). In turn, synchronized SR, sarcolemmal, and mitochondrial oscillations are connected to hubs of cellular metabolism such as the mammalian target of rapamycin resistance (mTOR)/ AMP-activated protein kinase (AMPK) axis that integrates nutrient availability, gene expression, and protein synthesis (5, 41, 87, 91, 139). In this example of vertical network structure, energy-dependent processes at the SR (e.g., SERCA-dependent Ca2+ uptake into the SR) can be matched to energy production (e.g., mitochondrial ATP synthesis) with both processes being responsive to the cellular metabolic environment (e.g., via mTOR/AMPK “surveillance”).
Consequently, the desynchronization of horizontal or vertical network structures is considered a pivotal event in cardiac disease (18). With reference to the SR/sarcolemmal entrainment of Ca2+ oscillation, failing ventricular cardiomyocytes exhibit abnormally high levels of RyR2 phosphorylation (a pathogenic mimic of the situation in SANC) that uncouples the SR and surface membrane clocks (i.e., results in a “de-repressed” Ca2+ clock) and predisposes the system to electrical instabilities (12). This idea of pathogenic desynchronization is reinforced by the demonstration that the physical and functional uncoupling of mitochondria is arrhythmogenic (21). Alternatively, it is plausible that arrhythmias may arise as a consequence of a loss of entrainment between SR, mitochondrial, and cytoplasmic events.
The negative consequences of uncoupling vertical network structure is also illustrated by the loss of cardiomyocytes by apoptosis, now recognized as a causal driver of cardiac dysfunction (130, 140). It has recently been shown that the susceptibility of a multicellular population to apoptosis is linked to molecular and cellular divergence within the population (16, 22, 104, 120, 137). Consequently, cellular uncoupling, which is also accelerated by the desynchronization of dynamic Ca2+-modulated processes (59), disrupts both the electrical connectivity in the myocardium (31, 161) and the intercellular synchronization of signal pathways. Thus in the context of cardiac disease, apoptosis may be triggered in response to an irrecoverable loss of synchronization between multiple cellular processes and/or between populations of cells. In another example of the pathogenic unraveling of vertical network structure, the uncoupling of cellular metabolism from sarcolemmal Na, K+, and Ca2+ ionic fluxes exacerbates the rhythm and contractile perturbations in heart failure (66, 106, 144, 155).
To further explore such disease-linked network “unraveling,” experimental approaches to untangle the complex web of nonlinear interactions that exist within and between cellular processes should not consider events in terms of linear “cause-and-effect”-type scenarios (23). Indeed, network complexity emerges from enmeshing discrete processes in a global topological network and thus the ability to experimentally track causal events that trigger successive network dysfunction requires a deep knowledge as to how each process is functionally integrated in the network structure (see section Dynamic Control of Signal Transmission in Cell Signaling Networks). To this end, we have begun investigating the functional behavior of individual cardiac cells within multicellular syncytia (135). In the next section, we expand on these concepts and describe how the unraveling of network synchronization and organization may fundamentally drive the onset and progression of cellular dysfunction and cardiac disease.
The Pathogenic Consequences of Reduced Network Complexity
So far, we have described cardiac Ca2+ signaling in terms of complexity, plasticity, dynamic range, oscillatory behavior, and synchronization. These descriptive features are recognized hallmarks of a system with an intrinsic propensity for chaotic behavior (51, 133). Indeed cardiac function is dependent on inherently chaotic processes underpinning a homeostatic state that is characterized by a very high level of complexity and the perpetual approximation of stable equilibria over a wide dynamic range (43, 51, 53). Thus although it might be presumed that such a dynamic homeostatic state capable of adopting numerous functional configurations would be easy to perturb, the hierarchical organization of normal cell signaling networks in-builds a redundancy and robustness to create a scenario that is paradoxically both highly plastic and inherently stable.
However, if there were chronic alterations within the network, for example the long-term reduction in the abundances or activities of key signaling proteins (see section Dynamic Control of Signal Transmission in Cell Signaling Networks), the network would be forced to operate across a diminished number of functional states (i.e., restricted dynamic range). Thus it has been proposed that cardiac dysfunction is the product of a tractable loss of synchronization and a reduction in chaotic flexibility (51, 52, 113, 133, 166).
Over 30 years ago Mackey and Glass showed that destabilization of homeostatic physiological systems with an intrinsic propensity for chaotic behavior can generate sustained desynchronized oscillations (52, 90). They coined the term “dynamical diseases,” which are characterized by “the operation of a basically normal control system in a region of physiological parameters that produces pathological behavior” (90). This concept was advanced by Chialvo and colleagues (24), who described a chaotic disease-linked trajectory in which the speed of functional decline was predicted by the initial conditions. These studies beautifully describe the “out-of-gamut” consequences of normal physiological adaptation and galvanize the view that a system's susceptibility to perturbation (e.g., the response to a proarrhythmogenic trigger) is dependent on its basal characteristics and stability. These concepts also raise the issue of predictability and this is discussed in more detail in the section Towards an Understanding of Reduced Dynamic Range, Maladaptation, and the Disease-Linked Network State.
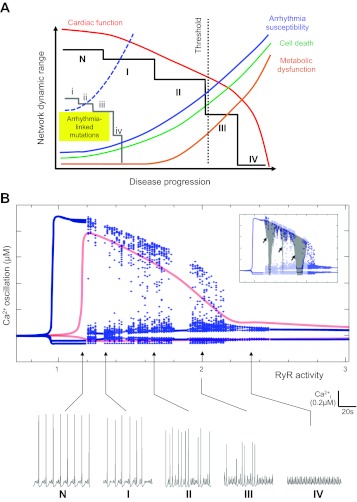
In Fig. 1A we describe a schematic model in which the progressive decline in cardiac function is linked to successive reductions in cellular network dynamic range. What are the factors that likely contribute to the progressive nature of diminished plasticity and complexity at the cellular level? Earlier in this section we considered the potential role of imbalanced protein levels or abundances in signaling pathways, and, intuitively, the gradual diminution of nodal protein abundance would be consistent with the progressive reduction in the dynamic range of the network.
Fig. 1.
Progressive and incremental reduction in system dynamic range is associated with dysfunction in coupled systems. A: incremental destabilization of a state defined as normal (N) through successive pseudo-stable states (I–V, solid black line) arises as a consequence of persistent (mal)adaptation that attempts to rebalance the network following perturbation. Intuitively, progressive network destabilization is expected to be associated with successively greater dysfunction (i.e., increasing step size) owing to the residual effect of successive malfunctions. In this scheme, the first two pseudo-stable states (I, II) are associated with small changes in interlinked systems [e.g., arrhythmia susceptibility (blue), cell death (green), and metabolic dysfunction (orange)], but a normal phenotype is maintained [cardiac function (red)]. However, the crossing of a threshold of system dysfunction, possibly as a consequence of failure to attenuate perturbation, accelerates these abnormalities and causes a steep decline in cardiac function. This scheme also illustrates that arrhythmia-linked genetic mutations may profoundly reduce basal network complexity (gray line) and introduce a heightened propensity to arrhythmia (dashed blue line) that is exacerbated by progression through successive pseudo-stable states (i–iv). B: the progressive instability and dysfunction described in A is reproduced in bifurcation diagrams generated by a model of the third-order system of differential equations describing cardiovascular dynamics developed by Parthimos and colleagues (111). This mathematical model of Ca2+ cycling incorporates terms that describe the activities of voltage- and receptor-operated Ca2+ channels (VOCC and ROC), Na+/Ca2+ exchanger (NCX), Ca2+ extrusion via plasma membrane ATPase (PMCA), sarcoplasmic (SR) reticulum Ca2+-ATPase (SERCA), and ryanodine receptor type 2 (RyR2) (111). Here we plotted the loci of maxima and minima of Ca2+ oscillatory activity for values of RyR2 activity (an index of the open state probability of RyRs or alternatively, proportional to the number of RyRs on the SR membrane) in a single cell (red lines) and two Ca2+-coupled cardiac cells (blue lines/points). In each scenario, continuous lines correspond to periodic solutions, whereas widely distributed points represent chaotic solutions or other hallmark types of nonlinear dynamics. Modeling of Ca2+ dynamics in single cells, where there is zero potential for intercellular desynchronization, results in entirely periodic solutions (red lines). Specific patterns of oscillatory behavior at various values of RyR2 activity (indicated by arrows) are shown in the series of panels N and I–IV. Inset, periodic windows of profoundly reduced complexity and low periodicity (gray shading) are superimposed over the bifurcation diagrams.
It is also plausible that a reduction in complexity of a graded output would restrict functional plasticity. Here we consider the example of β-adrenoceptor (β-AR) signaling that modulates cardiac contractility and rhythmicity through the synchronized activation of multiple downstream targets including RyR2, PLB, and LTCC (123) via modulation by PKA and CaMKII-dependent phosphorylation (159, 178, 179). If we borrow Lim's concept of protein kinases (on) and phosphatases (off) as digital “writers” and “erasers” (81) that modulate the approximately thirty phosphorylation substrates in the cascade, then the β-AR pathway could, in principle, support a complexity of approximately 1 billion permutations of phosphorylation states (230). So, even if we consider it very unlikely that every possible phosphorylation state could be achieved in situ, or that there would be a distinct functional outcome for each eventual phosphorylation configuration, it is easy to appreciate the enormous functional plasticity inherent in “normal” β-AR-dependent modulation of heart rate, rhythm, and contractility.
In contrast, chronic cardiac diseases are frequently associated with derangement in β-AR signaling that renders the modulation of heart rate and contractile function much less flexible. There is substantial evidence to support the idea that the graded output (or operational range) of the β-AR cascade becomes diminished by virtue of disease-linked alterations in the patterns of phosphorylation/dephosphorylation of downstream effectors. However, given the possible number of configurations that could be established in the β-AR cascade it is very unlikely that β-AR-linked phosphorylation goes wrong in an “all-or-none” manner. Rather, it is much more likely that successive (small) defects in phosphorylation, which arise as a consequence of altered expression or subcellular mislocalization of phosphatases or kinases or the accessibility of phosphorylation substrates, are linked to a progressive, incremental reduction in the dynamic range of the β-AR pathway. It is also conceivable that the graded loss of output could be the result of less directed (more randomly uniform) phosphorylation. Such scenarios could be experimentally tested through phosphoproteomic analysis using models of progressive cardiac dysfunction [e.g., transverse aortic constriction (TAC) induced heart failure].
In Fig. 1A, we describe a disease-linked trajectory involving the transition through discrete dysfunctional states (I–IV), with each state characterized by an incremental reduction in dynamic range and shifted further away from the initial “homeostatic” state (N). This model is consistent with the concepts drawn from chaotic behavior suggesting that dysfunctional phenotypic configurations arise via the inability of the network to appropriately reestablish stable steady states in response to (mal)adaptive changes that lie within the normal (physiologic) range (43, 52, 90). There is precedent for diminished physiologic function being attributed to the loss of complexity (54, 83). The depiction of four dysfunctional states (I–IV) in Fig. 1 represents an oversimplification of the process, and the progression from normal state to phenotypic dysfunction would likely occur via numerous discrete transitions, some of which (at least in the earlier stages) may be associated with an apparently normal phenotype. In the section Towards an Understanding of Reduced Dynamic Range, Maladaptation and the Disease-Linked Network State, we discuss how phenotypic deterioration may arise from an altered basal Ca2+ network “state” with reference to the pathogenic trajectory of cardiac dysfunction in the SERCA-knockout mouse.
In the section Synchronization of Cellular Processes and Uncoupling as a Pathogenic Event, we considered that attempting to describe the pathogenic unraveling of the cellular Ca2+ signaling network in terms of linear cause-and-effect-type thinking is of little use. Consequently, it is more useful to define the events that underpin these transitional reconfigurations between “healthy” and “diseased” states from a dynamical perspective. Specifically, transitions in a multidimensional parametric space can result in reductions in phase-space volume and/or system dimension. Pathological situations may arise owing to either of these transitions, and subtle experimental investigation on causality is required to distinguish between the two scenarios. Ultimately, however, the two properties are interrelated and sustained reduction in phase-space volume will result in lower dimension by the suppression of underlying physiological processes. From a practical or diagnostic point of view, quantification of a system's dimension is a potent way to establish a lower limit for the number of dominant elementary processes involved in a biological mechanism (148). Put more simply, the properties of maladapted transitional states are predicated on preexistent scenarios and depend on both a reduction in phase-space volume and a system dimension that ultimately results in a state of reduced complexity.
Importantly, our concept of a pathologic trajectory characterized by the progressive reduction in complexity illustrated in Fig. 1A is recapitulated by the mathematical modeling of cellular Ca2+ oscillations in response to the isolated perturbation of a single molecular component (RyR2) (Fig. 1B). The profile of the dynamic range associated with altered RyR2 activity in a single cell (red) and in two coupled cells (blue) is essentially the same, reflecting the fundamental similarities in Ca2+ handling dynamics in these two scenarios. However, intercellular coupling unmasks a new level of dynamic complexity and promotes the emergence of chaotic behavior (illustrated as the fracture of continuous blue lines into discrete points at RyR2 activity >1.2). Importantly, the same dynamical profile is maintained in numerical simulations of multicellular arrays. Although such configurations can exhibit emergent types of behavior, such as wave formation, the overall complexity of the system is ultimately limited by entrainment of its components.
In this coupled-cell scenario, panel N presents an example of apparently “regular” oscillatory behavior, which nevertheless resides on the border of chaotic dynamics (Fig. 1B). This positioning [akin to the normal state (N) in Fig. 1A] enables cardiac myocytes to operate with the requisite flexibility across a wide range of oscillatory responses through small variations of RyR2 activity (between values of 1 and 1.2, i.e., a 20% alteration in RyR2 function). Progressively larger (dysfunctional) augmentation of RyR activity (>1.4, Fig. 1B) results in irregular oscillatory activity, characterized by a successive reduction in amplitude (panels I through III). Eventually, oscillations in cytosolic Ca2+ are attenuated into stages of low complexity and amplitude (e.g., panel IV, RyR2 activity >2.2) that lack the inherent adaptability of healthy cardiac dynamics (cf. panel N). Note that the modeling data shown in Fig. 1B reproduce the accelerated functional decline at advanced stages of perturbation (RyR2 activity between values of 1.8 and 2.2) that were predicted to occur as a consequence of reduced dynamic range in Fig. 1A.
Figure 1A also depicts the progression from the normal state (N) to successively perturbed states (I–IV) occurring via sharp transitional points. Such sharp alteration in behavior (termed a phase-transition or crisis) is characteristic of chaotic systems (43), and this phenomenon is reproduced in our mathematical modeling (Fig. 1B, inset). Crises can be precipitated by increased noise (18), and thus the level of cellular noise may be an important determinant of the rapidity of pathologic decline i.e., transition between discrete maladapted states may be sensitive to noise in the network. Noise has been described as “unavoidable stochastic fluctuation” (36), and the functionality of a system has typically been considered dependent on its ability to attenuate (damp) and filter noise (128). Recent experimental evidence strengthens the argument that the failure to attenuate cellular noise via altered protein expression or mislocalization accelerates network dysfunction (96, 97, 175) (see also the section Dynamic Control of Signal Transmission in Cell Signaling Networks). Moreover, given the generalized abnormalities in Ca2+ signaling protein expression and activity in chronic heart disease (65), a causal link between the progressive alterations in protein and accelerated functional decline in the later stages of disease can be envisaged. Not all noise is bad though; noise may also be canalized for constructive purposes (36, 72, 116, 118, 119), enabling cellular processes to maintain a high sensitivity to signals over a wide dynamic range (128) or enhancing weak stimuli to cross biological thresholds and entrain large-scale fluctuations (stochastic resonance) (168). Thus a basal level of cellular noise may fundamentally contribute to the wide dynamic range of the “normal” signaling network.
Figure 1 also introduces the concept of genetic accelerants—mutations in signaling components that perturb the homeostatic state and establish a persistent network-level basal dysfunction. We propose that mutations create an intrinsically different basal homeostatic state that is characterized by reduced complexity and diminished plasticity. Consistent with this idea, there are several examples of genetic mutations that provoke malignant arrhythmias in response to normal physiological stimuli. For example, mutations in calsequestrin (CSQ) (74), PLB (132), LTCC (143), and ankyrin B (102) are associated with severe arrhythmias in response to moderate exercise or stress. Recent studies on the mechanisms of mutation-linked RyR2 dysfunction in stress-induced VT have shown that mutations perturb intramolecular interactions that stabilize RyR2 (48, 153). Subsequently, the resultant channel instability provokes an altered basal Ca2+ handling state (38) that reduces the threshold for severe stress-induced arrhythmia (47, 115, 149, 173).
Towards an Understanding of Reduced Dynamic Range, Maladaptation, and the Disease-Linked Network State
We have proposed a model in which the trajectory to ever greater phenotypic dysfunction is a consequence of the inability of an inherently chaotic system, restricted by diminished dynamic range, to reestablish the normal steady state. In this section we consider possible mechanisms that may initiate the transition from the normal plastic, adaptable state into a maladapted state characterized by desynchronization of cellular processes and reduced functional plasticity.
The remarkable plasticity and adaptability of the cardiac Ca2+ signaling machinery is exemplified by the cardiac phenotypes in mice following the cardiac-specific ablation of NCX (61) and the near-total elimination of SERCA (4). In NCX-null mice there is a profound adaptation of sarcolemmal ion fluxes that maintains functional output and apparently normal cardiac function (61, 114). In the SERCA2-deficient mouse, in which SERCA levels are reduced by ∼95% four weeks after excision of the serca2 locus, effectively normal heart function is preserved because of the concomitant augmentation of LTCC and NCX activities (4).
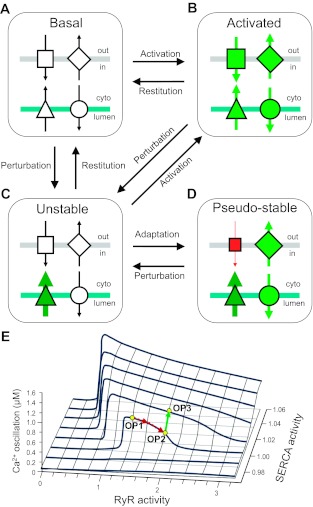
Indeed, central to the perspectives offered in this review is that within the framework of highly interconnected cellular pathways such extraordinary levels of functional adaptation in Ca2+ cycling can only be achieved by altering the behavior of other intimately linked processes. Accordingly, the normalization of steady state via negative and positive feedback loops and cross-talk can lead to “undershooting” and “overshooting” readjustment (dynamical hysteresis) that eventually settles into a new oscillatory steady state (functional compensation). This new state may be associated with a perceptibly normal phenotype, but it is fundamentally distinct from the “normal” basal state. Put more simply, functional (mal)adaption of the signaling network in an attempt to maintain normal steady state introduces a systems-level perturbation. We have termed this maladapted state the “pseudo-stable state” (44). This concept is further illustrated in Fig. 2 in which the functional rebalancing of ion fluxes in the (mal)adapted pseudo-stable state (Fig. 2D) results in a homeostatic scenario that is different from the normal basal state (Fig. 2A). Data from the SERCA knockout mouse serve to reinforce the idea that apparently normal cardiac function can be underscored by a different mode of Ca2+ signaling that predisposes the system to dysfunction [i.e., the “4 week” SERCA phenotype represents a pseudo-stable state (see Fig. 2D)]. There are equivalently low levels of SERCA abundance (<5% normal levels) between weeks 4 and 7 post-SERCA knockout, yet there is a steep decline in cardiac performance from near-normal function at 4 weeks to severe heart failure at 7 weeks. Clearly, this is an extreme example of the pathogenic decline described in the section The Pathogenic Consequences of Reduced Network Complexity and in Fig. 1, but it is intriguing to note that the deterioration of cardiac function between weeks 4 and 7 in this model is accompanied by an unsustainable increase in ATP consumption (80), altered cellular ultrastructure (146), and increased apoptosis (84). This phenotypic deterioration, which would suggest the profound uncoupling/de-synchronization of cellular processes from an already altered (pseudo-stable) homeostatic state (i.e., the “4-week” SERCA-knockout mouse) provides further experimental corroboration of the scheme described in Fig. 1. Although we are not aware of any reports that the cardiac NCX-knockout mouse is predisposed to overt cardiac dysfunction, the reduced L-type Ca2+ current and abbreviated action potential associated with the functional adaptation in this model (114) establishes a pseudo-stable state (cf. Fig. 2D) that would be anticipated to heighten arrhythmia susceptibility in some conditions (i.e., increased tendency to revert to the unstable arrhythmogenic state depicted by Fig. 2C).
Fig. 2.
System balance, homeostasis, and activation: the emergence of pseudo-stable states via functional (mal)adaptation. Using a simplified scheme of four components in the surface membrane (gray bar) and SR (blue bar) that mediate directional ion flux (arrows), we show that the proper transition from the basal state (A) to the fully activated state (B) depends on the synchronization of all components. The isolated perturbation of a single component in this system (triangle) normally leads to a transiently unstable state (C) that is either restored to the basal state (by subsequent modulation of this component) (A) or exists as an acute intermediate step en route to full activation (B). However, under some circumstances (e.g., persistent changes in abundance or activity of a component), functional adaptation renders the system functionally rebalanced such that it appears stable (D). This rebalanced state (illustrated here as a compensatory reconfiguration of ion fluxes) is fundamentally different from the basal (homeostatic) state (A). We term the state depicted in D as a pseudo-stable state. Black = basal activity, red = reduced activity, green = augmented activity. E: the emergence of pseudo-stable states through functional (mal)adaptation was tested by the mathematical model of cellular Ca2+ cycling described in Fig. 1B. We modeled the functional relationship between the SERCA pump and the ryanodine-sensitive Ca2+-induced Ca2+ release mechanism by plotting a family of bifurcation diagrams (as in Fig. 1B) for increasing values of SERCA pump activity. Increasing the activity of RyR2 shifts the oscillatory activity from an assumed “normal” operating point (OP1) to a suboptimal level of activity defined as OP2 (red arrow). Compensatory upregulation of the SERCA pump is potentially able to restore the amplitude of Ca2+ oscillations to its original level, thus reaching a new stable operating point (OP3, green arrow). The text section Towards an Understanding of Reduced Dynamic Range, Maladaptation, and the Disease-Linked Network State describes OP3-like scenarios that are characterized by apparently normal phenotype but are underscored by fundamentally different network properties (44).
There are other examples of the adverse consequences of functional (mal)adaptation. Michael and colleagues (100) described how the chronicity of homeostatic adaptation of multiple sarcolemmal K+ currents mediating the repolarization phase of the action potential (repolarization reserve) is arrhythmogenic. In the context of heart failure, Belevych and colleagues (9) recently demonstrated that reestablishing “normal” Ca2+ cycling through multicomponent adaptation involving CaMKII, RyR2, and L-type Ca2+ channels provoked cellular ionic instability. At a molecular level, the structural reconfiguration of RyR2 caused by the genetic deletion of exon 3 (85) lowers the threshold for channel activation that underlies malignant arrhythmias (17). At the cellular level, the compensatory mechanisms that normalize caffeine-evoked RyR2-dependent Ca2+ perturbation in isolated rat cardiomyocytes (150) augment the propensity for isoproterenol-induced Ca2+ dysfunction (154). Elsewhere, adaptive changes in Ca2+ cycling following prolonged exposure to low-dose caffeine resulted in an increased susceptibility of mouse HL-1 cardiac cells to apoptosis (50).
In Fig. 2B we present modeling data that reconcile the maladapted (pseudo-stable) state with apparently normal Ca2+ cycling. Recapitulating the experimental demonstration of an adaptive shift in SERCA activity as a result of increased cytosolic Ca2+ (169), our modeling shows that the right-shifted trajectory (red arrow) from a normal operating point (OP1) to another operating point 2 (OP2) in response to RyR2 activation can be reversed by very small alterations in SERCA activity (green arrow). However, although certain aspects of the new rebalanced operating point (OP3) may resemble the original configuration, the two states remain fundamentally apart in terms of the underlying dynamics and will therefore respond differently to further pathogenic perturbations, i.e., the network state represented by OP3 is a maladapted pseudo-stable configuration as described in Fig. 2D.
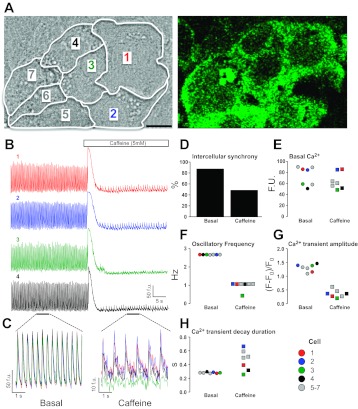
We further illustrate this concept experimentally using the spontaneous Ca2+ oscillatory behavior of an HL-1 cardiomyocyte syncytium (Fig. 3). Focusing on a seven-cell cluster (Fig. 3A), the basal state is characterized by a near-identical pattern of Ca2+ oscillations that exhibit a high level of intercellular synchronization (Fig. 3, B and C). Following the application of caffeine, an RyR2 agonist that triggers massive SR Ca2+ release (Fig. 3B and Supplemental Movie; Supplemental Material for this article is available online at the Journal website), a new steady state is established that is characterized by a reduced intercellular synchronization of Ca2+ oscillation (Fig. 3, C and D), a reduced oscillatory frequency (Fig. 3F), and a diminished Ca2+ release amplitude (Fig. 3G). Notably, this new oscillatory steady state exists against a background of unchanged basal Ca2+ levels (Fig. 3E). Moreover while some parameters exhibit unchanged intercellular variability in the post-caffeine state (e.g., Ca2+ transient amplitude, Fig. 3G), other parameters become desynchronized and exhibit much greater intercellular variability (e.g., decay phase of the Ca2+ transient, Fig. 3H). Intriguingly, one cell (cell no. 3, green) appears to become functionally isolated from its neighbors in terms of its oscillatory frequency (Fig. 3F). This experiment directly supports the idea that the post-caffeine state is a maladapted homeostatic state in which the intra- and intercellular signaling network has been rapidly readjusted.
Fig. 3.
New steady-state characteristics emerge from pharmacological perturbation of a functionally coupled cardiomyocyte syncytium. A: HL-1 cardiomyocytes (25) were cultured into a spontaneously oscillating monolayer, and Ca2+-dependent Fluo4 fluorescence was recorded using confocal laser scanning microscopy (46, 48, 50). The boundaries of seven cells are shown. Bar represents 10 μm. B: basal Ca2+ oscillations and the responses to a single bolus of caffeine (5 mM, added at 30 s) of four cells in this cluster (1, red; 2, blue; 3, green; 4, black) are shown. This experiment can be viewed in full via the Supplemental Movie. C: expanded sections of the basal and caffeine-stimulated states from cell nos. 1–4 (horizontal bars = 5 s; B) were overlaid. D: caffeine elicited a marked reduction in the intercellular synchronization of Ca2+ oscillations. E: basal Ca2+-dependent fluorescence was unchanged in cell nos. 1–7 following caffeine-stimulation [73.9 ± 6.5 vs. 64.4 ± 4.3 fluorescence units (FU)]. F: despite the near-identical oscillatory behavior of all cells in the basal state, consistent with a high-degree of intercellular synchronization, cell no. 3 (green square) is functionally isolated from its neighbors in the post-caffeine state and exhibited a reduced oscillatory frequency. Cell-to-cell contacts and cell boundaries remained unaltered following caffeine addition (see Supplemental Movie). G: caffeine reduced the steady-state Ca2+ transient amplitude in a uniform manner. The standard error expressed as a percentage of the mean value (SE % mean) was 6.0% post-caffeine compared with 4.0% in the basal nonstimulated state. H: the duration of Ca2+-transient decay following caffeine addition exhibited a level of cell-to-cell variability that was >16 times greater than that determined in the basal state [SE (% mean) = 6.0% (caffeine) vs. 0.36% (basal)].
Following on from the arguments presented in the section The Pathogenic Consequences of Reduced Network Complexity relating to the “predictability” of chaotic systems, we hypothesize that the response of each of these cells to a pharmacological stimulus (in this instance caffeine) could be predicted from a detailed understanding of their basal network state. To this end, we are using various mathematical techniques (i.e., modeling and numerical simulations, nonlinear statistical tools) (56, 57) to explore the overall complexity of the basal cellular state in functionally coupled cardiomyocyte syncytia (135, 136) to determine predictability of cardiac cellular response to pharmacologic modulation. We have preliminary evidence that suggests that the pro- and antiarrhythmic responses of cardiac cell networks to cardio-active compounds is modulated by basal Ca2+ signaling patterns (134). In other biological scenarios, there is substantial momentum behind harnessing emerging knowledge of the mechanistic basis of variability to be able to predict cellular and organ behavior (16, 73, 103, 104, 107, 120, 127, 137, 142).
In terms of identifying candidate mechanisms that promote or exacerbate network unraveling (albeit with the caveat that nonlinear systems do not strictly conform to the notion of cause and effect), it should be noted that it is also possible to decipher the signaling patterns to identify molecular culprits of cellular dysfunction. For example, the decay phase of the Ca2+ transient is the manifestation of the activities of SERCA, NCX, and mitochondrial uptake mechanisms and the sensitivity of RyR2 to the localized cytoplasmic Ca2+ environment. Consequently, in the example given in Fig. 3, the observed desynchronization of Ca2+ transient decay (Fig. 3, C and H) could be further investigated at a molecular level by focusing on relevant candidate proteins including those named above. In future, it is anticipated that the assignation of molecular culprits in cellular network dysfunction would be further augmented by new tools that enable the visualization of “global” cellular signaling events such as multiplexing novel probes and biosensors (e.g., reporters of PKA, cAMP and Ras activities) (37, 99, 117, 177) and the application of new imaging modalities (40, 60, 82, 98, 109, 131, 167).
Towards Therapeutic Re-Tuning of Cardiac Ca2+ Signaling
In this section we consider the possible implications of our model that describes the progressive deterioration of cardiac function occurring via successive maladapted (pseudo-stable) states for the development of new therapeutic approaches for cardiac disease.
The therapeutic “damping down” of multiple signaling processes via modulation of upstream hubs [e.g., β-blockers, angiotensin-converting enzyme (ACE) inhibitors, and modulators of the renin-angiotensin-aldosterone system (RAAS)] is a widely adopted regimen in the clinical management of cardiovascular disease but does not address the causal mechanisms of dysfunction (45, 49, 105). To this end, much research is focused on the search for discrete molecular “culprits” or druggable targets (45, 92, 105, 165). From the perspectives offered in this review, it is apparent that the targeting of discrete (protein) molecules has real limitations. As described in Pathogenic Consequences of Reduced Network Complexity and in Towards an Understanding of Reduced Dynamic Range, Maladaptation, and the Disease-Linked Network State, the interconnectivity of signaling components within the network means that modulating a single component or process in an effort to improve contractility or rhythmicity may directly contribute to cardiac dysfunction. Thus a system-level understanding of cardiovascular diseases may galvanize efforts towards pharmacologic pleiotropy [magic “shotguns” (125)] or adjunctive therapeutic strategies [e.g., metabolic regulators (1, 6, 151)] that could potentially modify other coupled components within the network.
Running counter to our arguments in the section Towards an Understanding of Reduced Dynamic Range, Maladaptation, and the Disease-Linked Network State that the altered configuration of signaling pathways would be anticipated to perturb signal transmission, it is important to note that in the setting of preexistent disease-linked states, further changes may actually normalize system function. For example, chronic β-blocker therapy evokes adaptive changes in the diseased heart that are beneficial (8, 70). Thus it may be feasible to manipulate disease-associated system parameters back into the normal range (129), an approach that has been described as “walking the system out of chaos” (43). Such a strategy is critically dependent on the reversibility of the process, and reports suggesting that such “reverse remodeling” may be feasible up until the very late stages of cardiac disease support this type of approach (10, 93).
However, there are potential hazards associated with “system-correction” strategies. Zhang and colleagues (176) showed that the targeted ablation of PLB in a model of CaMKII-dependent RyR2 hyperactivation actually provoked augmented SR Ca2+ leak, mitochondrial Ca2+ dysfunction, and increased cell death despite normalizing SR Ca2+ load. This study raises an important issue, namely that the anticipated rescue of one facet of cell dysfunction (Ca2+ handling) could accelerate phenotypic deterioration if other system defects persist (e.g., sustained activation of CaMKII or the physical loss of PLB from the network) (176). In another example, SERCA-mediated normalization of excitation-contraction coupling (ECC) is gathering momentum as a potentially viable chronotropic and antiarrhythmic approach in hypertrophy and heart failure (30, 86). However, SERCA-induced rescue of ECC is dependent on the myocardial energetic status (112) and indeed SERCA up-regulation in a “normal” scenario actually worsened cardiac dysfunction (112).
Conclusions
Normal cardiac function is dependent on the complex synchronization of ion fluxes, protein turnover, metabolism, cell death, and cell-to-cell connections that underpins the stability, plasticity, and robustness of normal signaling processes. In this review we have considered evidence that supports the view of cardiac disease as a phenotypic manifestation of progressive desynchronization and reduced plasticity of the Ca2+ signaling networks. Thus moving towards a network-level understanding of cardiac Ca2+ signaling, propelled by the synergy of computational and experimental approaches, will yield new insights into these pathogenic events. Such advances are essential to developing new and improved strategies for tackling the fundamental causes of cardiac disease.
GRANTS
The authors are supported by grants from British Heart Foundation (FS2000020, FS/04/088, BS/04/002, FS/06/082/21723, and FS/09/028/27602), Heart Research UK (RG2559), Royal Society, the Wellcome Trust (094210/Z/10/Z), and Cardiff University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.H.G. and D.P. prepared the figures; C.H.G. drafted the manuscript; C.H.G., D.P., and N.C.S. edited and revised the manuscript; C.H.G., D.P., and N.C.S. approved the final version of the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sarah Marsh for generating the experimental data used in Fig. 3. We are indebted to members of our laboratories, past and present, for valuable discussions that have informed the writing of this review.
Footnotes
Terms depicted in italics are defined in Table 1.
REFERENCES
- 1.Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, Frenneaux M. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 122: 1562–1569, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Alexeyenko A, Sonnhammer ELL. Global networks of functional coupling in eukaryotes from comprehensive data integration. Genome Res 19: 1107–1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral LAN, Scala A, Barthelemy M, Stanley HE. Classes of small-world networks. Proc Natl Acad Sci USA 97: 11149–11152, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson KB, Birkeland JAK, Finsen AV, Louch WE, Sjaastad I, Wang Y, Chen J, Molkentin JD, Chien K, Sejerstad OM, Christensen G. Moderate heart dysfunction in mice with inducible cardiomyocyte-specific excision of the Serca2 gene. J Mol Cell Cardiol 47: 180–187, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res 100: 474–488, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ Res 109: 86–96, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Atar D, Backx PH, Appel MM, Gao WD, Marban E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J Biol Chem 270: 2473–2477, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Barry WH, Gilbert EM. How do beta-blockers improve ventricular function in patients with congestive heart failure? Circulation 107: 2395–2397, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Gyorke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res 90: 493–502, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry JM, Le V, Rotter D, Battiprolu PK, Grinsfelder B, Tannous P, Burchfield JS, Czubryt M, Backs J, Olson EN, Rothermel BA, Hill JA. Reversibility of adverse, calcineurin-dependent cardiac remodeling. Circ Res 109: 407–417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Bers DM. Calcium handling, contraction, and ryanodine receptors: integration for cardiac “electricians”. Dialogues Cardiovasc Med 15: 247–252, 2010 [Google Scholar]
- 13.Bers DM. Macromolecular complex regulating cardiac ryanodine receptor function. J Mol Cell Cardiol 37: 417–429, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Bers DM. Ryanodine receptor S2808 phosphorylation in heart failure: smoking gun or red herring. Circ Res 110: 796–799, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science 283: 381–387, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Bhola PD, Simon SM. Determinism and divergence of apoptosis susceptibility in mammalian cells. J Cell Sci 122: 4296–4302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhuiyan ZA, van den Berg MP, van Tintelen P, Bink-Boelkens MT, Wiesfeld AC, Alders M, Postma AV, van Langen I, Mannens MM, Wilde AA. Expanding spectrum of human RYR2-related disease: new electrocardiographic, structural, and genetic features. Circulation 116: 1569–1576, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Bien H, Yin l, Entcheva E. Calcium instabilities in mammalian cardiomyocyte networks. Biophys J 90: 2628–2640, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boogerd FC, Bruggeman FJ, Hofmeyr JHS, Westerhoff HV. Afterthoughts as foundations for systems biology. In: Systems Biology: Philosophical Foundations, edited by Boogerd FC, Bruggeman FJ, Hofmeyr J-HS, Westerfhoff HV. Amsterdam: Elsevier, 2007, p. 321–336 [Google Scholar]
- 20.Breitling R. What is systems biology? Front Physiol 1: 9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DA, O'Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res 88: 241–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakir K, Daya SK, Tunin RS, Helm RH, Byrne MJ, Dinaano VL, Lardo AC, Abraham TP, Tomaselli GF, Kass DA. Reversal of global apoptosis and regional stress kinase activation by cardiac resynchronization. Circulation 117: 1369–1377, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Chapman J. System Failure (Online) London: Demos; 2004. [available at http://wwwdemos.co.uk/files/systemfailure2]. [Google Scholar]
- 24.Chialvo DR, Gilmour RF, Jr, Jalife J. Low dimensional chaos in cardiac tissue. Nature 343: 653–657, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Claycomb WC, Lanson NA, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979–2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook SA, Clerk A, Sugden PH. Are transgenic mice the ‘alkahest’ to understanding myocardial hypertrophy and failure? J Mol Cell Cardiol 46: 118–129, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Csete ME, Doyle JC. Reverse engineering of biological complexity. Science 295: 1664–1669, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev 80: 411–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dehmelt L, Bastiaens PI. Spatial organization of intracellular communication: insights from imaging. Nat Rev Mol Cell Biol 11: 440–452, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, Lewandowski ED, Hajjar RJ. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation 104: 1424–1429, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhein S. Pharmacology of gap junctions in the cardiovascular system. Cardiovasc Res 62: 287–298, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Dodge-Kafka KL, Kapiloff MS. The mAKAP signalling complex: integration of cAMP, calcium and MAP kinase signalling pathways. Eur J Cell Biol 85: 593–602, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci STKE 166: pe4, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Dorn GW, II, Scorrano L. Two close, too close: sarcoplasmic reticulum-mitochondrial crosstalk and cardiomyocyte fate. Circ Res 107: 689–699, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisner DA, Choi HS, Diaz ME, O'Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res 87: 1087–1094, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature 467: 167–173, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez-Suarez M, Ting AY. Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol 9: 929–943, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Velasco M, Reuda A, Rizzi N, Benitah JP, Colombi B, Napolitano C, Priori SG, Richard S, Gomez AM. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4996C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res 104: 201–209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figeys D. Mapping the human protein interactome. Cell Res 18: 716–724, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Fischer RS, Wu Y, Kanchanawong P, Shroff H, Waterman CM. Microscopy in 3D: a biologist's toolbox. Trends Cell Biol 21: 682–691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285: 14071–14077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gandhi TKB, Zhong J, Mathivanan S, Karthick L, Chandrika KN, Mohan SS, Sharma S, Pinkert S, Nagaraju S, Periaswamy B, Mishra G, Nandakumar K, Shen B, Deshpande N, Nayak R, Sarker M, Boeke JD, Parmigiani G, Schultz J, Bader JS, Pandey A. Analysis of the human protein interactome and comparison with yeast, worm and fly interaction datasets. Nat Genet 38: 285–293, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Garfinkel A, Spano ML, Ditto WL, Weiss JN. Controlling cardiac chaos. Science 257: 1230–1235, 1992 [DOI] [PubMed] [Google Scholar]
- 44.George CH. Sarcoplasmic reticulum Ca2+ leak in heart failure: mere observation or functional relevance? Cardiovasc Res 77: 302–314, 2008 [DOI] [PubMed] [Google Scholar]
- 45.George CH, Barberini-Jammaers SR, Muller CT. Refocussing therapeutic strategies for cardiac arrhythmias: defining viable molecular targets to restore cardiac ion flux. Expert Opin Ther Pat 18: 1–19, 2008 [Google Scholar]
- 46.George CH, Higgs GV, Lai FA. Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res 93: 531–540, 2003 [DOI] [PubMed] [Google Scholar]
- 47.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol 42: 34–50, 2007 [DOI] [PubMed] [Google Scholar]
- 48.George CH, Jundi H, Thomas NL, Walters N, West RR, Lai FA. Arrhythmogenic mutation-linked defects in ryanodine receptor autoregulation reveal a novel mechanism of Ca2+ release channel dysfunction. Circ Res 98: 88–97, 2006 [DOI] [PubMed] [Google Scholar]
- 49.George CH, Lai FA. Developing new anti-arrhythmics: clues from the molecular basis of cardiac ryanodine receptor (RyR2) Ca2+ release channel dysfunction. Curr Pharm Des 13: 3195–3211, 2007 [DOI] [PubMed] [Google Scholar]
- 50.George CH, Rogers SA, Bertrand BM, Tunwell RE, Thomas NL, Steele DS, Cox EV, Pepper C, Hazeel CJ, Claycomb WC, Lai FA. Alternative splicing of ryanodine receptors modulates cardiomyocyte Ca2+ signaling and susceptibility to apoptosis. Circ Res 100: 874–883, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Glass L. Synchronization and rhythmic processes in physiology. Nature 410: 277–284, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Glass L, Mackey MC. Pathological conditions resulting from instabilities in physiological control systems. Ann NY Acad Sci 316: 214–235, 1979 [DOI] [PubMed] [Google Scholar]
- 53.Glass L, Zeng WZ. Complex bifurcations and chaos in simple theoretical models of cardiac oscillations. Ann NY Acad Sci 591: 316–327, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Goldberger AL. Non-linear dynamics for clinicians: chaos theory, fractals, and complexity at the bedside. Lancet 347: 1312–1314, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Greenstein JL, Winslow RL. Integrative systems models of cardiac excitation-contraction coupling. Circ Res 108: 70–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffith TM, Parthimos D, Edwards DH. Non-linear analysis and modelling of the cellular mechanisms that regulate arterial vasomotion. Proc Inst Mech Eng, part C: J Mech Eng Sci 220: 367–381, 2006 [Google Scholar]
- 57.Griffith TM, Parthimos D, Edwards DH. Vasomotion: the case for chaos. Biorheology 23: 11–23, 2009 [Google Scholar]
- 58.Hartwell LH, Hopfield JJ, Leibler S, Murray AT. From molecular to modular cell biology. Nature 402: C47–C52, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Hashido M, Hayashi K, Hirose K, Iino M. Ca2+ lightning conveys cell-cell contact information inside the cells. EMBO Rep 7: 1117–1123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayashi T, Martone ME, Yu Z, Thor A, Doi M, Holst MJ, Ellisman MH, Hoshijima M. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J Cell Sci 122: 1005–1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henderson SA, Goldhaber JI, So JM, Han T, Motter C, Ngo A, Chantawansri C, Ritter MR, Friedlander M, Nicoll DA, Frank JS, Jordan MC, Roos KP, Ross RS, Philipson KD. Functional adult myocardium in the absence of Na+-Ca2+ exchange: cardiac specific knockout of NCX1. Circ Res 95: 604–611, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Heo M, Maslov S, Shakhnovich E. Topology of protein interaction network shapes protein abundances and strengths of their functional and non-specific interactions. Proc Natl Acad Sci USA 108: 4258–4263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science 306: 640–643, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Houser SR. Does protein kinase A-mediated phosphorylation of the cardiac ryanodine receptor play any role in adrenergic regulation of calcium handling in health and disease. Circ Res 106: 1672–1674, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Houser SR, Piacentino V, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32: 1595–1607, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res 81: 412–419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jordan JD, Landau EM, Iyengar R. Signaling networks: the origins of cellular multitasking. Cell 103: 193–200, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jorgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, Larsen B, Wilkinson DG, Linding R, Pawson T. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science 326: 1502–1509, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Kitano H. Grand challenges in systems physiology. Front Physiol 1: 3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kubon C, Mistry NB, Grundvold I, Halvorsen S, Kjeldsen SE, Westheim AS. The role of beta-blockers in the treatment of chronic heart failure. Trends Pharmacol Sci 32: 206–212, 2011 [DOI] [PubMed] [Google Scholar]
- 71.Kurz FT, Aon MA, O'Rourke B, Armoundas AA. Spatio-temporal oscillations of individual mitochondria in cardiac myocytes reveal modulation of synchronized mitochondrial clusters. Proc Natl Acad Sci USA 107: 14315–14320, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ladbury JE, Arold ST. Noise in cellular signaling pathways: causes and effects. Trends Biochem Sci 37: 173–178, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lage K, Mollgard K, Greenway S, Wakimoto H, Gorham JM, Workman CT, Bendsen E, Hansen NT, Rigina O, Roque FS, Wiese C, Christoffels VM, Roberts AE, Smoot LB, Pu WT, Donahoe PK, Tommerup N, Brunak S, Seidman CE, Seidman JG, Larsen LA. Dissecting spatio-temporal protein networks driving human heart development and related disorders. Mol Syst Biol 6: 381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lahat H, Pras E, Olender T, Avadan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M. A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine- induced polymorphic ventricular tachycardia in Bedouin families from Israel. Am J Hum Genet 69: 1378–1384, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lakatta EG. Intracellular Ca2+ cycling is a general regulator of how fast and how strong the heart beats. Dialogues Cardiovasc Med 15: 253–278, 2010 [Google Scholar]
- 76.Lakatta EG, DiFrancesco D. What keeps us ticking: a funny current, a calcium clock, or both? J Mol Cell Cardiol 47: 157–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lakatta EG, Maltsev V, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 106: 659–673, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lakatta EG, Maltsev VA, Bogdanov KY, Stern MD, Vinogradova TM. Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ Res 92: E45–E50, 2003 [DOI] [PubMed] [Google Scholar]
- 79.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SL, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123: 25–35, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li L, Louch WE, Niederer SA, Aronsen JM, Christensen G, Sejerstad OM, Smith NP. Sodium accumulation in SERCA knockout-induced heart failure. Biophys J 102: 2039–2048, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim WA. Designing customized cell signalling circuits. Nat Rev Mol Cell Biol 11: 393–403, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lippincott-Schwartz J, Patterson GH. Photoactivatable fluorescent proteins for diffraction-limited and super-resolution imaging. Trends Cell Biol 19: 555–565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA 267: 1806–1809, 1992 [PubMed] [Google Scholar]
- 84.Liu XH, Zhang ZY, Andersson KB, Husberg C, Enger UH, Raeder MG, Christensen G, Louch WE. Cardiomyocyte-specific disruption of Serca2 in adult mice causes sarco(endo) plasmic reticulum stress and apoptosis. Cell Calcium 49: 201–207, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Lobo PA, Kimlicka L, Tung CC, Van Petegem F. The deletion of exon 3 in the cardiac ryanodine receptor is rescued by β strand switching. Structure 19: 790–798, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O'Gara P, Liang L, Kohlbrenner E, Hajjar R, Peters NS, Poole-Wilson PA, MacLeod KT, Harding SE. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol 4: 362–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Ma'ayan A, Blitzer RD, Iyengar R. Toward predictive models of mammalian cells. Annu Rev Biophys Biomol Struct 34: 319–349, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma'ayan A, Jenkins SL, Neves S, Hasseldine A, Grace E, Dubin-Thaler B, Eungdamrong NJ, Weng G, Ram PT, Rice JJ, Kershenbaum A, Stolovitzky GA, Blitzer RD, Iyengar R. Formation of regulatory patterns during signal propagation in a mammalian cellular network. Science 309: 1078–1083, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mackey MC, Glass L. Oscillation and chaos in physiological control systems. Science 197: 287–289, 1977 [DOI] [PubMed] [Google Scholar]
- 91.Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470: 404–408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu Pp Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 331: 1439–1443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Margulies KB, Matiwala S, Cornejo C, Olsen H, Craven WA, Bednarik D. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ Res 96: 592–599, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine-isoleucine zippers. J Cell Biol 154: 699–708, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000 [DOI] [PubMed] [Google Scholar]
- 96.Maslov S, Ispolatov I. Propagation of large concentration changes in reversible protein-binding networks. Proc Natl Acad Sci USA 104: 13655–13660, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maslov S, Sneppen K, Ispolatov I. Spreading out of perturbations in reversible reaction networks. New J Phys 9: 273, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mattheyses AL, Simon SM, Rappoport JZ. Imaging with total internal reflection fluorescence microscopy for the cell biologist. J Cell Sci 123: 3621–3628, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mehta S, Zhang J. Reporting from the field: genetically encoded fluorescent reporters uncover signaling dynamics in living biological systems. Annu Rev Biochem 80: 375–401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michael G, Xiao L, Qi XY, Dobrev D, Nattel S. Remodelling of cardiac repolarization: how homeostatic responses can lead to arrhythmogenesis. Cardiovasc Res 81: 491–499, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science 298: 824–827, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421: 634–639, 2003 [DOI] [PubMed] [Google Scholar]
- 103.Moreno JD, Zhu ZI, Yang PC, Bankston JR, Jeng MT, Kang C, Wang L, Bayer JD, Christini DJ, Trayanova NA, Ripplinger CM, Kass RS, Clancy CE. Computational model to predict the effects of class I anti-arrhythmic drugs on ventricular rhythms. Sci Transl Med 3: 98ra83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakamura K, Yamazawa T, Okubo Y, Iino M. Temporal switching and cell-to-cell variability in Ca2+ release activity in mammalian cells. Mol Syst Biol 5: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov 5: 1034–1049, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Neubauer S. Mechanisms of disease: the failing heart–an engine out of fuel. N Engl J Med 356: 1140–1151, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Noujaim SF, Berenfeld O, Kalifa J, Cerrone M, Nanthakumar K, Atienza F, Moreno J, Mironov S, Jalife J. Universal scaling law of electrical turbulence in the mammalian heart. Proc Natl Acad Sci USA 104: 20985–20989, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Novak B, Tyson JJ. Design principles of biochemical oscillators. Nat Rev Mol Cell Biol 9: 981–991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Novak P, Li C, Shevchuk AI, Stepanyan R, Caldwell M, Hughes S, Smart TG, Gorelik J, Ostanin VP, Lab MJ, Moss GWJ, Frolenkov GI, Klenerman D, Korchev YE. Nanoscale live-cell imaging using hopping probe ion conductance microscopy. Nat Methods 6: 279–281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park JM, Kim TY, Lee SY. Prediction of metabolic fluxes by incorporating genomic context and flux-converging pattern analyses. Proc Natl Acad Sci USA 107: 14931–14936, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parthimos D, Edwards DH, Griffith TM. Minimal model of arterial chaos generated by coupled intracellular and membrane Ca2+ oscillators. Am J Physiol Heart Circ Physiol 277: H1119–H1144, 1999 [DOI] [PubMed] [Google Scholar]
- 112.Pinz I, Tian R, Swanson E, Dillmann W, Ingwall JS. Compromised myocardial energetics in hypertrophied mouse hearts diminish the beneficial effect of overexpressing SERCA2a. J Biol Chem 286: 10163–10168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pool R. Is it healthy to be chaotic? Science 243: 604–607, 1989 [DOI] [PubMed] [Google Scholar]
- 114.Pott C, Philipson KD, Goldhaber JI. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: reduced transsarcolemmal Ca2+ flux. Circ Res 97: 1288–1295, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res 108: 871–883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quaranta V, Garbett S. Not all noise is waste. Nat Methods 7: 269–272, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods 5: 877–879, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rao CV, Wolf DM, Arkin AP. Control, exploitation and tolerance of intracellular noise. Nature 420: 231–237, 2002 [DOI] [PubMed] [Google Scholar]
- 119.Raser JM, O'Shea EK. Noise in gene expression: origins, consequences, and control. Science 309: 2010–2013, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raychaudhuri S, Willgohs E, Nguyen TN, Kahn EM, Goldkorn T. Monte Carlo simulation of cell death signaling predicts large cell-to-cell stochastic fluctuations through the type 2 pathway of apoptosis. Biophys J 95: 3559–3562, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts: role of phosphatases and response to isoproterenol. J Biol Chem 278: 444–453, 2003 [DOI] [PubMed] [Google Scholar]
- 122.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766, 1998 [DOI] [PubMed] [Google Scholar]
- 123.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature 415: 206–212, 2002 [DOI] [PubMed] [Google Scholar]
- 124.Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovasc Res 88: 40–50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for moods disorders and schizophrenia. Nat Rev Drug Discov 3: 353–359, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Ruiz-Meana M, Fernandez-Sanz C, Garcia-Dorado D. The SR-mitochondria interaction: a new player in cardiac pathophysiology. Cardiovasc Res 88: 30–39, 2010 [DOI] [PubMed] [Google Scholar]
- 127.Sarkar AX, Christini DJ, Sobie EA. Exploiting mathematical models to illuminate electrophysiological variability between individuals. J Physiol 590: 2555–2567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sartori P, Tu Y. Noise filtering strategies in adaptive biochemical signaling networks. J Stat Phys 142: 1206–1217, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sato D, Xie LH, Nguyen TN, Weiss JN, Qu Z. Irregularly appearing early after-depolarizations in cardiac myocytes: random flucuatations or dynamical chaos? Biophys J 99: 765–773, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Satoh M, Matter CM, Ogita H, Takeshita K, Wang CY, Dorn GW, 2nd, Liao JK. Inhibition of apoptosis-regulated signaling kinase-1 and prevention of congestive heart failure by estrogen. Circulation 115: 3197–3204, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schermellah L, Heintzmann R, Leonhardt H. A guide to super-resolution fluorescence microscopy. J Cell Biol 190: 165–175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299: 1410–1413, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Sharma V. Deterministic chaos and fractal complexity in the dynamics of cardiovascular behavior: perspectives on a new frontier. Open Cardiovasc Med J 3: 110–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Silvester NC, Ashton PM, Barberini-Jammaers SR, Lai FA, George CH. Adaptive retuning of small Ca2+ fluxes in cardiomyocyte syncitia predicts the response to pro-arrhythmic stimuli. Biophys J 98: 105a, 2010 [Google Scholar]
- 135.Silvester NC, Barberini-Jammaers SR, Ashton PM, Jayanthi A, Yeung WY, Jundi H, Kotecha A, Lai FA, George CH. Investigating the Ca2+-cycling basis of rhythmicity and synchronicity in coupled cardiomyocyte monolayers. Biophys J Suppl 96: 273a–274a, 2009 [Google Scholar]
- 136.Silvester NC, George CH. Searching for new cardiovascular drugs: towards improved systems for drug screening? Expert Opin Drug Discov 6: 1155–1170, 2011 [DOI] [PubMed] [Google Scholar]
- 137.Snijder B, Pelkmans L. Origins of regulated cell-to-cell variability. Nat Rev Mol Cell Biol 12: 119–125, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Song DW, Lee JG, Youn HS, Eom SH, Kim DH. Ryanodine receptor assembly: a novel systems biology approach to 3D mapping. Prog Biophys Mol Biol 105: 145–161, 2011 [DOI] [PubMed] [Google Scholar]
- 139.Song Y, Altarejos J, Goodarzi MO, Inoue H, Guo X, Berdeaux R, Kim JH, Goode J, Igata M, Paz JC, Hogan MF, Singh PK, Goebel N, Vera L, Miller N, Ciu J, Jones MR, CHARGE consortium, GIANT consortium Chen YD, Taylor KD, Hseuh WA, Rotter JI, Montminy M. CRTC3 links catecholamine signalling to energy balance. Nature 468: 933–939, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sosnovick DE, Nahrendorf M, Panizzi P, Matsui T, Aikawa E, Dai G, Li L, Reynolds F, Dorn GW, II, Weissleder R, Josephson L, Roesenzweig A. Molecular MRI detects low levels of cardiomyocyte apoptosis in a transgenic model of chronic heart failure. Circ Cardiovasc Imaging 2: 468–475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sperling SR. Systems biology approaches to heart development and congenital heart disease. Cardiovasc Res 91: 269–278, 2011 [DOI] [PubMed] [Google Scholar]
- 142.Spiller DG, Wood CD, Rand DA, White MR. Measurement of single-cell dynamics. Nature 465: 736–745, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Cav1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119: 19–31, 2004 [DOI] [PubMed] [Google Scholar]
- 144.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85: 1093–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 145.Strogatz SH. Exploring complex networks. Nature 410: 268–276, 2001 [DOI] [PubMed] [Google Scholar]
- 146.Swift F, Franzini-Armstrong C, Oyehaug L, Enger UH, Andersson KB, Christensen G, Sejerstad OM, Louch WE. Extreme sarcoplasmic reticulum volume loss and compensatory T-tubule remodeling after Serca2 knockout. Proc Natl Acad Sci USA 109: 3997–4001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem 275: 15305–15513, 2000 [DOI] [PubMed] [Google Scholar]
- 148.Takens F. Detecting strange attractors in turbulence. In: Dynamical Systems and Turbulence: Lecture Notes in Mathematics, vol. 898. New York: Springer-Verlag, 1981, pp. 366–381 [Google Scholar]
- 149.Thomas NL, George CH, Williams AJ, Lai FA. Ryanodine receptor mutations in arrhythmias: advances in understanding the mechanisms of channel dysfunction. Biochem Soc Trans 35: 946–951, 2007 [DOI] [PubMed] [Google Scholar]
- 150.Trafford AW, Diaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol 522: 259–270, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tuunanen H, Knuuti J. Metabolic remodelling in human heart failure. Cardiovasc Res 90: 251–257, 2011 [DOI] [PubMed] [Google Scholar]
- 152.Tyson JJ, Chen KC, Novak B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr Opin Cell Biol 15: 221–231, 2003 [DOI] [PubMed] [Google Scholar]
- 153.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto M, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res 106: 1413–1424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves- threshold sarcoplasmic reticulum calcium content is required. Circ Res 100: 105–111, 2007 [DOI] [PubMed] [Google Scholar]
- 155.Ventura-Clapier R, Garnier A, Veksler V. Energy metabolism in heart failure. J Physiol 555: 1–13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vest JA, Wehrens XHT, Reiken SR, Lehnart SE, Dobrev DD, Chandra P, Danilo P, Ravens U, Rosen M, Marks AR. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111: 2025–2032, 2005 [DOI] [PubMed] [Google Scholar]
- 157.Vinayagam A, Stezl U, Foulle R, Plassmann S, Zenkner M, Timm J, Assmus HE, Andrada-Navarro MA, Wanker EE. A directed protein interaction network for investigating intracellular signal transduction. Sci Signal 4: rs8, 2011 [DOI] [PubMed] [Google Scholar]
- 158.Vinogradova TM, Zhou YY, Maltsev V, Lyashkov A, Stern M, Lakatta EG. Rhythmic ryanodine receptor Ca2+ release during diastolic depolarization of sinoatrial pacemaker cells do not require membrane depolarization. Circ Res 94: 802–809, 2004 [DOI] [PubMed] [Google Scholar]
- 159.Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained beta1-adrenergic receptor stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res 95: 798–806, 2004 [DOI] [PubMed] [Google Scholar]
- 160.Wang X, Wei X, Thijssen B, Das J, Lipkin SM, Yu H. Three-dimensional reconstruction of protein networks provides insight into human genetic disease. Nat Biotechnol 30: 159–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wasserstrom JA, Sharma R, Kapur S, Kelly JE, Kadish AH, Balke CW, Aistrup GL. Multiple defects in intracellular calcium cycling defects in whole failing rat heart. Circ Heart Fail 2: 223–232, 2009 [DOI] [PubMed] [Google Scholar]
- 162.Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature 393: 440–442, 1998 [DOI] [PubMed] [Google Scholar]
- 163.Wehrens XHT, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840, 2003 [DOI] [PubMed] [Google Scholar]
- 164.Wehrens XHT, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci USA 103: 511–518, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wehrens XHT, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin 2. Science 304: 292–296, 2004 [DOI] [PubMed] [Google Scholar]
- 166.Weiss JN. How does falling out of phase of intracellular calcium and action potentials across the heart's wall spell the beginning of chaos for the heart? Dialogues Cardiovasc Med 15: 302–310, 2010 [Google Scholar]
- 167.Welch CM, Elliot H, Danuser G, Hahn KM. Imaging the coordination of multiple signalling activities in living cells. Nat Rev Mol Cell Biol 12: 749–756, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wiesenfeld K, Moss F. Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature 373: 33–36, 1995 [DOI] [PubMed] [Google Scholar]
- 169.Wu KD, Bungard D, Lytton J. Regulation of SERCA Ca2+ pump expression by cytoplasmic [Ca2+] in vascular smooth muscle cells. Am J Physiol Cell Physiol 280: C843–C851, 2001 [DOI] [PubMed] [Google Scholar]
- 170.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Heller Brown J, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116: 675–682, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xiang YK. Compartmentalization of beta-adrenergic signals in cardiomyocytes. Circ Res 109: 231–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, Bogdanov K, Allen PD, Stern MD, Lakatta EG, Boheler KR. The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci USA 99: 9225–9230, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yano M, Yamamoto T, Ikemoto N, Matsuzaki M. Abnormal ryanodine receptor function in heart failure. Pharmacol Ther 107: 377–391, 2005 [DOI] [PubMed] [Google Scholar]
- 174.Yosef N, Zalckvar E, Rubinstein AD, Homilius M, Atias N, Vardi L, Berman I, Zur H, Kimchi A, Ruppin E, Sharan R. ANAT: a tool for constructing and analyzing functional protein networks. Sci Signal 4: pl1, 2011 [DOI] [PubMed] [Google Scholar]
- 175.Zhang J, Maslov S, Shakhnovich EI. Constraints imposed by non-functional protein-protein interactions on gene expression and proteome size. Mol Syst Biol 4: 210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang T, Guo T, Mishra S, Dalton ND, Kranias EG, Peterson KL, Bers DM, Brown JH. Phospholamban ablation rescues sarcoplasmic reticulum Ca2+ handling but exacerbates cardiac dysfunction in CaMKIIδc transgenic mice. Circ Res 106: 354–362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhao Y, Araki S, Wu JC, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, Campbell RE. An expanded palette of genetically encoded Ca2+ indicators. Science 333: 1888–1891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zheng M, Zhu W, Han Q, Xiao RP. Emerging concepts and therapeutic implications of β-adrenergic receptor subtype signaling. Pharmacol Ther 108: 257–268, 2005 [DOI] [PubMed] [Google Scholar]
- 179.Zhu W, Zeng X, Zheng M, Xiao RP. The enigma of β2-adrenergic receptor Gi signaling in the heart: the good, the bad, and the ugly. Circ Res 97: 507–509, 2005 [DOI] [PubMed] [Google Scholar]
- 180.Ziviani E, Lippi G, Bano D, Munarriz E, Guiducci S, Zoli M, Young KW, Nicotera P. Ryanodine receptor-2 upregulation and nicotine-mediated plasticity. EMBO J 30: 194–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.