Abstract
Clinical trials of bone marrow mesenchymal stem cell (MSC) therapy have thus far demonstrated moderate and inconsistent benefits, indicating an urgent need to improve therapeutic efficacy. Although administration of sufficient cells is necessary to achieve maximal therapeutic benefits, documented MSC clinical trials have largely relied on injections of ∼1 × 106 cells/kg, which appears too low to elicit a robust therapeutic response according to published preclinical studies. However, repeated cell passaging necessary for large-scale expansion of MSC causes cellular senescence and reduces stem cell potency. Using the RNA mimetic polyinosinic-polycytidylic acid [poly(I:C)] to engage MSC Toll-like receptor 3 (TLR3), we found that poly(I:C), signaling through multiple mitogen-activated protein kinase pathways, induced therapeutically relevant trophic factors such as interleukin-6-type cytokines, stromal-derived factor 1, hepatocyte growth factor, and vascular endothelial growth factor while slightly inhibiting the proliferation and migration potentials of MSC. At the suboptimal injection dose of 1 × 106 cells/kg, poly(I:C)-treated MSC, but not untreated MSC, effectively stimulated regeneration of the failing hamster heart 1 mo after cell administration. The regenerating heart exhibited increased CD34+/Ki67+ and CD34+/GATA4+ progenitor cells in the presence of decreased inflammatory cells and cytokines. Cardiac functional improvement was associated with a ∼50% reduction in fibrosis, a ∼40% reduction in apoptosis, and a ∼55% increase in angiogenesis, culminating in prominent cardiomyogenesis evidenced by abundant distribution of small myocytes and a ∼90% increase in wall thickening. These functional, histological, and molecular characterizations thus establish the utility of TLR3 engagement for enabling the low-dose MSC therapy that may be translated to more efficacious clinical applications.
Keywords: mesenchymal stem cell; poly(I:C), Toll-like receptor 3; IL-6-type cytokines; cardiac repair
recent interest in regenerative medicine has prompted preclinical and clinical studies on the feasibility and safety of bone marrow mesenchymal stem cells (MSC) for cell therapy (29, 34, 80). The clinical utility of MSC stems in part from the recognition that MSC possess immunomodulatory properties that can be explored for nonautologous stem cell therapy (64, 74). Notably, although MSC exhibit prominent multilineage potential, this cellular feature is now believed to contribute little to their therapeutic effects. Instead, the secretion of multiple trophic factors by MSC provides the underlying tissue healing mechanism (4, 12, 25, 40, 61). These findings broach a novel concept of stem cell trophic factor-mediated tissue repair.
Despite encouraging results from preclinical studies, however, clinical trials of stem cell therapy have thus far demonstrated moderate and inconsistent benefits (2, 48, 73), indicating an urgent need to optimize the therapeutic platform and enhance stem cell potency. Lessons learned from hematopoietic stem cell (HSC) therapy following myeloablation reveal that administration of sufficient HSC promotes faster cell recovery and reduces hospitalizations (52). Cell dose-related therapeutic benefits have also been demonstrated in our preclinical MSC trial for heart failure (66) and noted in a recent clinical trial of MSC therapy for refractory angina (24). However, unlike HSC transplantation, it is necessary to extensively amplify MSC in culture to generate at least tens of millions of cells required for clinical applications because MSC represent a minor fraction of the total nucleated bone marrow cells. This ex vivo cell amplification step unavoidably creates several issues that can confound MSC therapeutics, among which cellular senescence caused by repeated passaging of MSC (6, 10, 76) can suppress expression of MSC trophic factors (75) and potentially compromise clinical trials of MSC therapy. Thus, strategies aimed at enhancing the expression of MSC trophic factors may be explored to overcome this therapeutic hurdle.
Although the heart possesses a limited ability for regeneration, the skeletal muscle is endowed with an impressive ability to regenerate, which is coupled to its autocrine and paracrine release of many trophic factors beneficial for heart health (59, 77). Contracting muscle during exercise produces trophic factors possessing cardioprotective effects (13, 69, 82), which may underlie the finding that exercise is associated with low cardiovascular risk (72). This notion of distal organ protection of the heart has been explored in a strategy referred to as remote ischemic preconditioning, which is based on brief controlled episodes of intermittent ischemia of limb muscle to elicit protection against myocardial ischemia and reperfusion injury (3, 28). Not surprisingly, cardioprotection offered by remote ischemia preconditioning has been found to be mediated by trophic factors (44, 67). Along this line, we previously demonstrated a remote MSC therapeutic regimen for the failing hamster heart based on intramuscular injection of MSC (65, 66, 90). This extra-cardiac therapeutic regimen uncovered a central role of trophic factors such as VEGF, hepatocyte growth factor (HGF), stromal-derived factor 1 (SDF1), and insulin-like growth factor (IGF) in myocardial regeneration.
In our MSC trials, we had evaluated the relationship between the injected cell doses (2–40 × 106 cells/kg animal body wt) and therapeutic benefits, and found most effective cardiac repair with the highest cell dose (40 × 106 cells/kg) (66). However, published clinical trials of MSC therapy have largely relied on injections of ∼1 × 106 cells/kg patient body weight (17, 27, 32, 38, 71), which appears suboptimal based on our cell dose study, assuming a linear cell dose translation from the rodent model (∼0.1 kg) to the clinical setting (∼70 kg). However, scaling up the production of MSC for human applications can present major logistical challenges associated with cellular senescence and high treatment costs. The present work was initiated to identify an MSC-boosting strategy for enhancing therapeutic efficacy at a suboptimal cell dose. We took advantage of the Toll-like receptor (TLR) pathway, which has been shown to stimulate production of many trophic factors, including IL-6-type cytokines, from immune cells and non-immune cells (20, 54), and tested the hypothesis that cardiac therapeutic potency of MSC can be significantly enhanced by engagement of the MSC TLR3 pathway.1
METHODS AND MATERIALS
Animals.
The TO2 strain cardiomyopathic hamsters (4-mo-old males) were obtained from Bio Breeders (Watertown, MA). All procedures and protocols conformed to institutional guidelines for the care and use of animals in research and were approved by the University of Buffalo Institutional Animal Care and Use Committee.
Echocardiography.
Animals were anesthetized by intraperitoneal (ip) injection of xylazine (2 mg/kg) and ketamine (30 mg/kg) and remained semiconscious throughout the protocol. Multiple M-mode images were obtained from the short axis view of the left ventricle at the level of the papillary muscles with a GE Vingmed echo machine using a 10-MHz transducer. Left ventricular end-systolic dimension (LVDs), LV end-diastolic dimension (LVDd), end-systolic wall thickness (ESWT), and end-diastolic wall thickness (EDWT) were measured in an operator-blinded manner and averaged from at least two consecutive cardiac cycles. Left ventricular ejection fraction (LVEF) = (LV end-diastolic volume − LV end-systolic volume) ÷ LV end-diastolic volume × 100. Fractional shortening (FS) = (LVDd − LVDs) ÷ LVDd × 100. ΔWall thickness (WT) = ESWT − EDWT. %WT = ΔWT ÷ EDWT × 100.
Cell culture, poly(I:C) treatment, and cell implantation.
Porcine bone marrow MSC were isolated and maintained in DMEM/F-12 supplemented with 10% fetal bovine serum (FBS) as described previously (45, 75). The MSC express multiple surface markers such as CD29, CD49c, CD49e, CD51, CD73, CD90, CD105, and CD106 and were negative for CD31, CD34, CD45, CD133, and VEGF receptor 2 (VEGF-R2). The concentration of polyinosinic-polycytidylic acid [poly(I:C)] (no. P-9582, Sigma) was calibrated by absorbance at 260 nm. MSC were treated with 0.8–20 μg/ml poly(I:C) for 24 h (abbreviated as MSC-IC), following which cells and conditioned medium were harvested for in vitro and in vivo studies. Intramuscular (im) MSC injection was described previously (65, 66). In brief, each TO2 hamster received untreated or poly(I:C)-treated MSC resuspended in 0.8 ml Hanks' balanced salt solution (HBSS) equally divided in the left and right hamstrings. Control hamsters received the same volume of HBSS. Animals were killed after 1 mo to evaluate therapeutic effects. The established mouse cardiac muscle cell line HL-1 (15) was maintained in DMEM/F-12 supplemented with 10% FBS. HL-1 myocytes were used to assess the mitogenic activity of MSC-conditioned medium (MSC CM).
Cell proliferation assay.
Cell proliferation was analyzed using MTT assay as described previously (45). MSC were plated on 24-well plates (105 cells/well). Poly(I:C) was added at 0.8, 4, and 20 μg/ml after overnight attachment. MTT assay was performed 24 h after poly(I:C) addition. For MTT assay after removal of poly(I:C), MSC were first plated on 35-mm plates (5 × 105 cells/plate). After overnight attachment, cells were treated with 4 μg/ml poly(I:C) for 24 h, following which cells were trypsinized, washed with HBSS twice to remove poly(I:C), and plated on 24-well plates (1 × 105 cells/well). MTT assay was performed 1–3 days after plating.
Transwell migration assays.
MSC pretreated with 4 μg/ml poly(I:C) for 24 h as described above were plated in serum-free medium on fibronectin-coated 24-well inserts (BD Falcon PET membrane with 8 μm pores; 1,000 cells per insert). The lower compartment of the chamber was filled with 0.3 ml of DMEM/F-12 supplemented with 10% FBS. After overnight incubation, inserts were taken off the plate, and cells on the upper side of the insert were erased with a cotton swab. Cells on the lower side of the insert were fixed with 4% paraformaldehyde and stained with DAPI. Images of the entire insert were taken using Zeiss Axioimager fluorescence microscope at ×100 magnification, and nuclei were counted using ImageJ (National Institutes of Health, Bethesda, MD). Migration index derived from quantification of the migrated cells is presented as the ratio of migrated poly(I:C)-treated MSC to migrated untreated MSC.
qRT-PCR.
RNA extraction was performed using Qiagen's RNA isolation kits. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to analyze gene expression as described (45). PCR was performed using the MyIQ machine with the SYBR green kit (Bio-Rad). Melting curve analysis was performed to check for a single amplicon. β2-Microglobulin (B2M) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the reference gene for calculations. Data were analyzed by the 2ΔΔCT method. Oligonucleotides were synthesized by Midland Oligo. Primer sequences are listed in Table 1.
Table 1.
Primer sequences for qRT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Porcine | ||
| GAPDH | TGCCCAGAACATCATCCC | GGATGACCTTGCCCACAG |
| FGF1 | AGAGGCTGGAGGAAAACCAT | GGCTTTCTGGCCATAGTGAG |
| FGF2 | GGAGTGTGTGCAAACCGTTA | TCGTTTCAGTGCCACATACC |
| HGF | AGAGGTCCCATGGATCACAC | AGCCCTTGTCGGGATATCTT |
| IL-1α | AACCCGTGTTGCTGAAGGAGCT | AAACAACTTTGGATGGGCGGCTG |
| IL-6 | TTCACCTCTCCGGACAAAAC | TCTGCCAGTACCTCCTTGCT |
| IL-10 | GCGGCGCTGTCATCAATTTCTGC | ACACCCCTCTCTTGGAGCTTGCT |
| IL-11 | TGCGTGCTAGCTGGACATAC | CCGTGATCACTTCCTCCAAT |
| LIF | GTCACCCATGTCACAGCAAC | CCCCTGGGCTGTGTAGTAGA |
| SDF-1 | CCTTGCCGATTCTTTGAGAG | CAATTTTGGGTCAATGCACA |
| TLR3 | ACAAGAGTTCTCTCCAGGGTGT | TTCTCTGTCAGACTGGGGCT |
| TNF-α | TGGCCCCTTGAGCATCAACCCT | CAAGGGGCCAGCTGGAAACTCT |
| VEGF | CTACCTCCACCATGCCAAGT | ACACAGGACGGCTTGAAGAT |
| Hamster | ||
| β2-Microglobulin | TCTCTTGGCTCACAGGGAGT | ATGTCTCGTTCCCAGGTGAC |
| GATA4 | CTGTGCCAACTGCCAGACTA | CTGGTTTGAATCCCCTCCTT |
| IL-1β | CCAGGATGAGGACCTGAGAA | CGAGGCATTTCTGTTGTTCA |
| IL-10 | AAGACCCTCAGGAGGCAACT | CGCCTTTCTCTTGGAGCTTA |
| Mef2c | CCTGACTCCTCTTACGCACTC | GGTGGAACAGCAGGAATTTT |
| Nkx2.5 | CCAACAGCAACTTCGTGAAC | GTGGAGACGCCCGAATTG |
| TNF-α | AACTCCAGCCGGTGCCTAT | GTTCAGCAGGCAGAAGAGGATT |
Western blot analysis.
MSC were washed and treated with 4 μg/ml poly(I:C) in DMEM/F-12 supplemented with 10% FBS. Cells were harvested in a buffer containing 50 mM Tris (pH 7.6), 2% SDS, 5 mM EDTA, 5 mM EGTA, 25 mM NaF, 1 mM Na3VO4, 100 nM okadaic acid, and 1 × protease inhibitor cocktail (no. P-8340, Sigma). Proteins were resolved by SDS-PAGE and electrotransferred to Immobilon-P membrane, which was incubated with a 1,000-fold diluted primary antibody solution overnight at 4°C. Washed membrane was probed with a horseradish peroxidase-conjugated secondary antibody diluted to ∼10 ng/ml. Protein signals were developed using the SuperSignal chemiluminescent substrate from Pierce Biotechnology and quantified by Quantity One. Primary antibodies were purchased from Cell Signaling (phospho-p38, no. 9215; total p38, no. 9212; phospho-JNK, no. 9251; total JNK, no. 9258; phospho-ERK, no. 9101; total ERK, no. 9102; integrin αV, no. 4711), BD Biosciences (integrin α5, no. 610633; integrin β1, no. 610467), Thermo Scientific (CXCR4, no. PA1–12542), and Santa Cruz (GAPDH, no. 25778).
Quantification of fibrosis, apoptosis, and capillary density.
Quantification of capillary density is as described previously (88). OCT-embedded cryosections were fixed in acetone:ethanol mix (3:1 ratio) for 5 min. Fluorescein-labeled Griffonia Simplicifolia Lectin I Isolectin B4 (GSL-IB4; no. FL-1201, Vectors Laboratories) diluted 1:100 was incubated with the tissue sections overnight at 4°C. Cardiomyocytes were stained with a cardiac Troponin T antibody (no. MS295, Thermo Scientific). Images were taken in 20 random fields at ×200 magnification. The number of capillaries was counted and the area was quantified using ImageJ. Capillary density was normalized to total area in mm2. Paraformaldehyde-fixed, paraffin-embedded heart sections were processed for TUNEL assay and Masson trichrome staining. Analysis of apoptosis was performed as documented previously (88) using the ApopTag kit (Millipore) per manufacturer's instructions. Apoptotic nuclei were counted and normalized to total nuclei in 20 random fields at ×200 magnification. Masson trichrome-stained sections were used for fibrosis analysis, which was performed by ImageJ-aided quantification of image pixels as described (66). At least 15 random fields at ×200 magnification were assessed for each slide. The ratio of fibrotic area to total tissue area was calculated as percentage of the fibrotic area.
Morphometric analysis.
Hematoxylin and eosin (H&E)-stained paraffin sections were used to determine cross-sectional myocyte area using a modified measurement protocol (66). At least 15 random fields at ×200 magnification were assessed for each slide in an operator-blinded fashion. Cross-sectional area was measured manually by tracing along the perimeter of every myocyte with a central nucleus in full view. At least 350 random myocytes were measured for each animal using the AxioVision LE software measurement tool.
ELISA analysis.
Concentrations of IL-6-type cytokines present in MSC-conditioned medium were measured using ELISA kits from R&D Systems (no. DY686 for IL-6 and no. DY218 for IL-11). Cytokine concentrations are expressed as pg/ml culture medium.
In situ immunostaining.
The immunostaining protocol has been documented (90). Paraformaldehyde-fixed, paraffin-embedded heart sections were processed for CD34, CD45, GATA4, and Ki67 immunostaining. Antigen retrieval was done in 10 mM citrate buffer (pH 6) in a 95°C water bath for 20 min. Primary antibodies were purchased from BD Pharmingen (CD34, no. 553731; CD45, no. 550539), Santa Cruz (GATA4, no. 9053), and Thermo Scientific (Ki67, no. RM9106). A homemade myosin heavy chain (MHC) antibody was used to stain myocytes. The sections were then incubated with Alexa-647-, Alexa-555-, or Alexa-488- conjugated secondary antibodies. Sections were analyzed using Zeiss Axioimager fluorescence microscope at ×200 and ×630 magnification. Images were taken in 20 random fields. The number of positive cells and the total number of nuclei were counted.
Statistical analysis.
Comparisons between two and multiple experimental groups were made with Student's t-test and one-way ANOVA, respectively. A value of P < 0.05 was considered significant. Data (n ≥ 3 in each experiment) are expressed as means ± SE.
RESULTS
Amplification of MSC trophic factors through poly(I:C) engagement of TLR3.
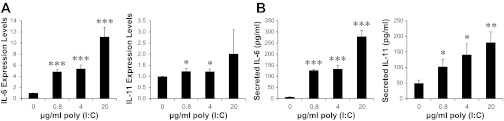
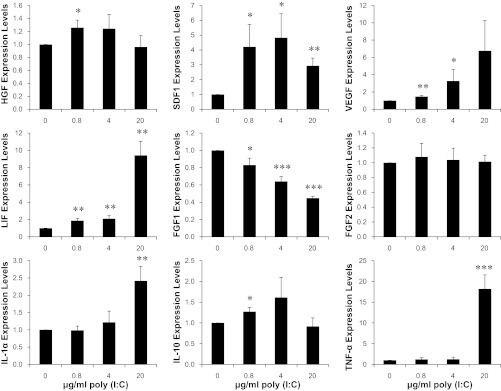
Our previous MSC therapy showed that injections of 40 × 106 cells/kg were necessary to achieve efficacious cardiac repair (66). Since current clinical trials of MSC therapy largely rely on injections of suboptimal doses of MSC (∼1 × 106 cells/kg) (17, 27, 32, 38, 71), we sought to identify an MSC-boosting strategy that might enhance the low-dose MSC therapy. Engagement of the TLR pathway has been shown to stimulate production of many trophic factors from immune cells and non-immune cells (20, 54). MSC are known to express many TLRs, including TLR3, which is the receptor for double-stranded RNA, either of viral or synthetic origin (1, 49). Noting that the RNA mimetic poly(I:C) interacts with TLR3 and is often used as a TLR3 agonist, we treated MSC with three different concentrations of poly(I:C) for 24 h to examine the downstream effect on expression of trophic factors. Since we previously demonstrated the central role of IL-6-type cytokines in MSC-mediated cardiac repair (65), we initially characterized expression of IL-6 and IL-11 by qRT-PCR (Fig. 1A) and ELISA (Fig. 1B). These assays revealed a prominent induction of IL-6 by treatment with 0.8–20 μg/ml poly(I:C) (up to ∼10-fold increase in IL-6 mRNA and ∼40-fold increase in secreted IL-6). A less than ∼2-fold induction of IL-11 mRNA and ∼4-fold induction of secreted IL-11 were also observed. We further used the PCR assay to identify additional growth factor/cytokine genes that might be affected (Fig. 2). Leukemia inhibitory factor (LIF), another member of the IL-6-type cytokines, was also induced. SDF1, VEGF, and HGF, all of which are activated by IL-6/JAK/STAT3 signaling (65), were significantly induced by poly(I:C). The fibroblast growth factors FGF1 and FGF2 were either slightly downregulated or unaffected. Interestingly, the antiinflammatory cytokine IL-10 was significantly induced. The inflammatory cytokines interleukin-1α (IL-1α) and tumor necrosis factor-α (TNF-α) were only induced by the highest poly(I:C) concentration (20 μg/ml). This finding prompted us to adopt an MSC-boosting protocol using 4 μg/ml poly(I:C) for 24 h, which induced IL-6, IL-10, IL-11, LIF, VEGF, SDF1, and HGF without induction of the inflammatory cytokines.
Fig. 1.
Polyinosinic-polycytidylic acid [poly(I:C)] increases expression of mesenchymal stem cell (MSC) IL-6-type cytokines. MSC (5 × 105 cells per 35-mm dish) maintained in DMEM/F-12 supplemented with 10% FBS were treated with an equal volume of saline, which served as no poly(I:C) control, or 0.8–20 μg/ml poly(I:C). Cells and culture medium were harvested after 24 h, and expression of IL-6 and IL-11 was analyzed by qRT-PCR (A) and ELISA (B). GAPDH was used as the reference gene in PCR analysis. Concentrations of IL-6 and IL-11 present in the conditioned-medium are expressed as pg/ml. n = 3–4; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. no poly(I:C) control.
Fig. 2.
Poly(I:C) differentially affects expression of MSC trophic factors. MSC (5 × 105 cells per 35-mm dish) maintained in DMEM/F-12 supplemented with 10% FBS were treated with an equal volume of saline, which served as no poly(I:C) control, or 0.8–20 μg/ml poly(I:C). Cells were harvested after 24 h for qRT-PCR analysis of trophic factor gene expression using GAPDH as the reference gene. LIF, leukemia inhibitory factor; HGF, hepatocyte growth factor; SDF1, stromal-derived factor 1. n = 3–4; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. no poly(I:C) control.
Activation of major MAPK pathways after poly(I:C) conditioning of MSC.
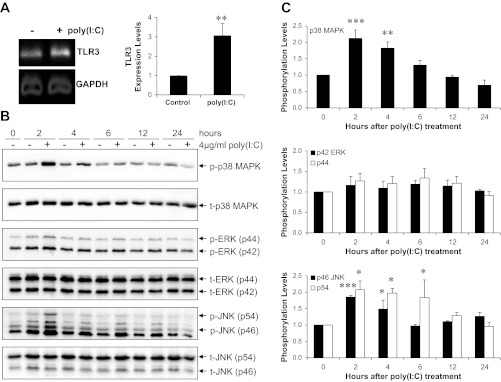
Since TLR3 is the receptor for poly(I:C) (1, 49), we confirmed by qRT-PCR that the porcine bone marrow MSC used here indeed express TLR3. Figure 3A shows TLR3 expression, which was further increased ∼3-fold after poly(I:C) treatment. TLR3 activation of the immune system is known to result in phosphorylation of JNK/SAPK, which are members of the mitogen-activated protein kinases (MAPKs) activated by a broad spectrum of cytokines and environmental stresses (33, 58). We examined the kinetic response of the three major MAPK pathways (p38 MAPK, JNK/SAPK, and ERK1/2) after 4 μg/ml poly(I:C) treatment. Western blotting (Fig. 3B) revealed a ∼2-fold increase in phosphorylation of p38 MAPK (Thr180/Tyr182) and JNK/SAPK (Thr183/Tyr185), peaking at 2–4 h after treatment. A mild activation of ERK1/2 through phosphorylation of Thr202/Tyr204 is also evident (Fig. 3C). Activation of the major MAPK signaling pathways is thus consistent with the pleiotropic effects of the poly(I:C)/TLR3 axis on expression of MSC trophic factors.
Fig. 3.
MSC Toll-like receptor 3 (TLR3) activation is mediated through MAPK signaling pathways. A: qRT-PCR analysis of MSC TLR3 expression in response to poly(I:C) treatment. Representative qRT-PCR products were fractionated by agarose gel electrophoresis (left) and relative expression levels derived from CT are illustrated (right, n = 4). B: MSC were treated with 4 μg/ml poly(I:C) for 2–24 h, following which cells were harvested and processed for SDS-PAGE and Western blot analysis. Proteins (45 μg) were loaded in each lane. Representative Western blots of three independent experiments examining p38 MAPK, JNK/SAPK, and ERK1/2 are illustrated. C: digital quantification of the three signaling pathways. Phosphorylation levels represent the ratios of each phosphorylated kinase to the respective total kinase at the time points illustrated. n = 3; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. no poly(I:C) control.
Marginal effects of TLR3 activation on MSC proliferation and migration.
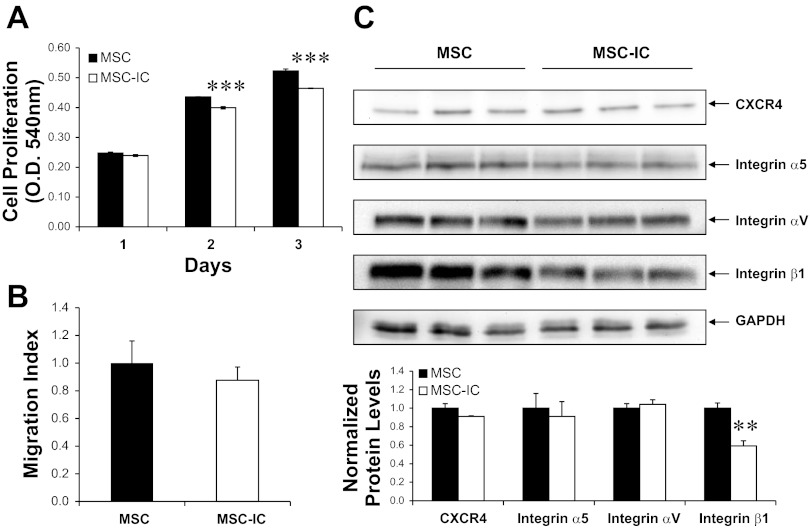
TLRs are known to regulate embryonic development, cell growth/differentiation, and apoptosis, in addition to their well-established function in the innate immune system (58). Relevant to stem cell therapy is the ability of poly(I:C)-treated MSC to proliferate and migrate in response to extracellular cues. The MTT cell proliferation assays presented in Fig. 4A show that MSC exposed to 4 μg/ml poly(I:C) for 24 h exhibited a marginal but statistically significant reduction in proliferation potential even after removal of poly(I:C). The transwell migration assay presented in Fig. 4B indicates that the poly(I:C) treatment had a statistically insignificant effect on MSC migration in vitro. Since previous studies of MSC trafficking implicated the chemokine receptor CXCR4 and the adhesion receptor integrin β1 in MSC migration (30, 68), we characterized their protein levels by Western blotting. Figure 4C shows that integrin β1 was significantly downregulated by poly(I:C) while CXCR4, integrin α5, and integrin αV were not significantly affected after normalization by GAPDH. These studies together indicate that the 4 μg/ml poly(I:C) treatment of MSC elicits pleiotropic effects on MSC.
Fig. 4.
MSC proliferation and migration are marginally affected by poly(I:C) treatment. MSC were treated with saline (MSC) or 4 μg/ml poly(I:C) (MSC-IC) for 24 h and washed to remove poly(I:C). Poly(I:C)-treated MSC are designated as MSC-IC. Cells were then analyzed by MTT assay, migration assay, and Western blotting. A: cells were plated in 24-well plates (105 cells/well). MTT assays were performed 1, 2, and 3 days after plating. OD, optical density. B: transwell migration assay for MSC and MSC-IC. Migration index is defined as the ratio of migrated MSC-IC to migrated MSC. C: cells were harvested 2 days after plating and processed for SDS-PAGE and Western blot analysis. Proteins (45 μg) were loaded in each lane. The ratios of chemokine receptor CXCR4, integrin β1, integrin α5, and integrin αV to GAPDH are graphed. n = 3; **P < 0.01 and ***P < 0.001 vs. untreated MSC.
Enhancement of low-dose MSC therapy (1 × 106 cells/kg) for heart failure.
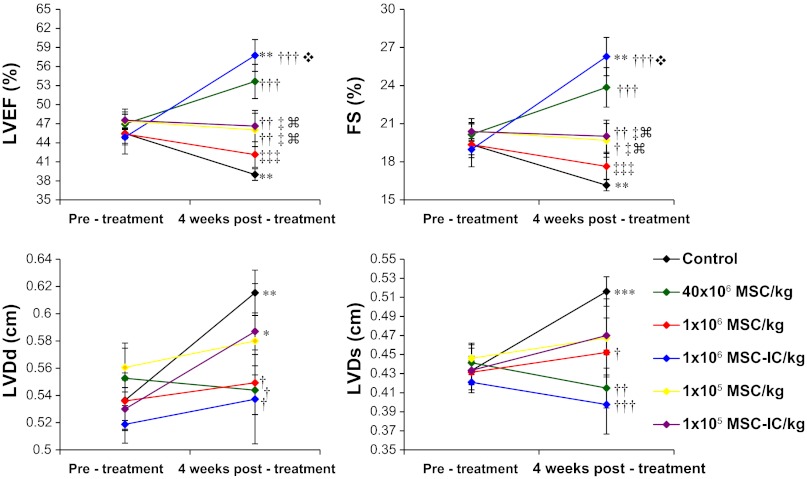
Increased expression of MSC trophic factors after poly(I:C) treatment prompted us to determine whether this strategy might lower the effective therapeutic cell dose. Since our previous MSC trials revealed increased therapeutic benefits conferred by increased MSC doses with 40 × 106 MSC/kg achieving maximal therapeutic potency, we lowered the injection dose to 1 × 106 cells/kg and 1 × 105 cells/kg for the study. Echocardiography performed 1 mo after MSC administration (Fig. 5) shows that poly(I:C)-treated MSC at 1 × 106 cells/kg improved function (LVEF 57.7 ± 2.5%) of the failing hamster heart significantly better than untreated MSC (LVEF 42.2 ± 2.0%) at the same injection dose, and achieved the improvement comparable to or better than that mediated by 40 × 106 untreated MSC/kg (LVEF 53.7 ± 2.7%). Contractile dysfunction of the failing TO2 hamster heart is associated with progressive ventricular dilation, which was prominently attenuated by poly(I:C)-treated MSC at 1 × 106 cells/kg (LVDs 0.40 ± 0.03 cm vs. 0.52 ± 0.02 cm in the saline control). This improvement is again better than that mediated by untreated MSC (0.45 ± 0.002 cm). Thus, the 24-h poly(I:C) conditioning strategy reduced the effective therapeutic dose by ∼40-fold. The MSC dose of 1 × 105 cells/kg appeared to have fallen below the critical cell dosage threshold because the poly(I:C) treatment failed to exert a stimulatory effect on this low-cell dose group (Fig. 5). Since the 1 × 106 cells/kg injection dose is widely used in current clinical trials, we further focused on this cell dose group in the following studies to characterize the mechanisms through which poly(I:C) enhances the low-dose MSC therapy.
Fig. 5.
Echocardiography shows poly(I:C) treatment enhances low-dose MSC therapy for heart failure. Echocardiography was performed in an operator-blinded manner before and 1 mo after MSC treatment. Poly(I:C)-treated MSC are designated as MSC-IC. Left ventricular ejection fraction (LVEF), fractional shortening (FS), LV end-diastolic dimension (LVDd), and LV end-systolic dimension (LVDs) are presented in the context of three cell dose groups: 40 × 106/kg, 1 × 106/kg, and 1 × 105/kg, which represent 4 × 106, 1 × 105, and 1 × 104 MSC per animal, respectively. Effect of 4 μg/ml poly(I:C) treatment was tested for the 1 × 106/kg and 1 × 105/kg groups only. n = 4–11 per group; *P < 0.05 vs. pretreatment; **P < 0.01 vs. pretreatment; ***P < 0.001 vs. pretreatment; †P < 0.05 vs. saline control; ††P < 0.01 vs. saline control; †††P < 0.001 vs. saline control; ‡P < 0.05 vs. 40 × 106 MSC/kg; ‡‡‡P < 0.001 vs. 40 × 106 MSC/kg;  P < 0.001 vs. 1 × 106 MSC/kg;
P < 0.001 vs. 1 × 106 MSC/kg; 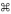 P < 0.001 vs. 1 × 106 MSC-IC/kg.
P < 0.001 vs. 1 × 106 MSC-IC/kg.
Amplification of cardiac progenitor cells by poly(I:C)-treated MSC.
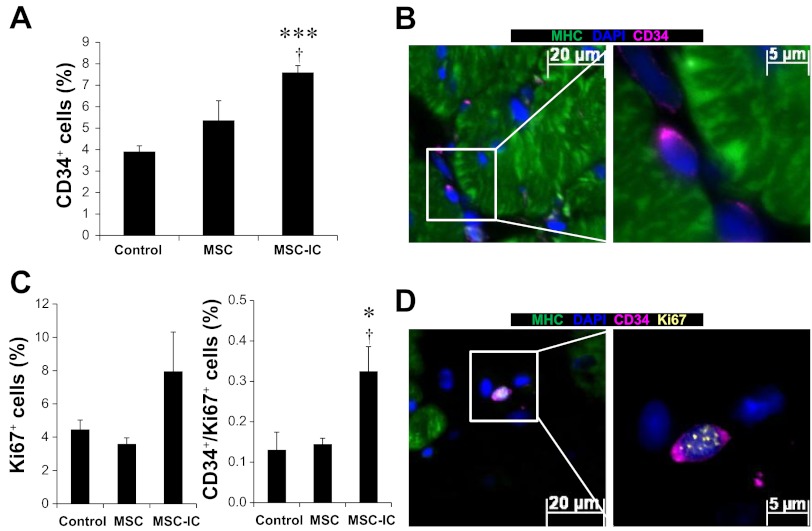
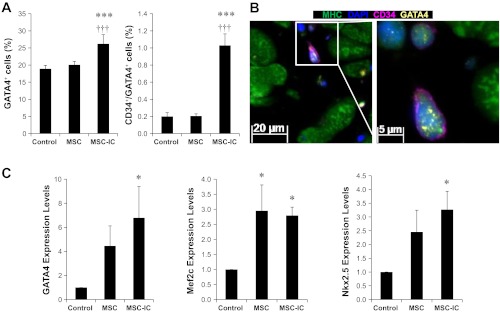
Emerging evidence reveals that cardiac progenitor/stem cells play a central role in myocardial regeneration (7, 22, 42). In particular, CD34+ progenitor cells, which can secrete trophic factors and differentiate into cardiomyocytes (84, 85), have been used in clinical trials for cardiac repair (47, 57). We used in situ immunostaining to quantify CD34+ cardiac progenitor cells after therapy. The identified CD34+ cells are small (∼5 μm in size), displaying perinuclear staining with little cytoplasmic volume (Fig. 6B), which resembles the documented CD34+ progenitor cells (37). While untreated MSC slightly increased the CD34+ cells, only poly(I:C)-treated MSC significantly increased this cell population above the control level, reaching ∼7% of total myocardial cells (Fig. 6A). Using Ki67 staining to identify cells capable of DNA replication, we found that only poly(I:C)-treated MSC significantly increased CD34+/Ki67+ progenitor cells (Fig. 6C). The representative CD34+/Ki67+ progenitor cell exhibits punctate Ki67 staining in the nucleus (Fig. 6D), accounting for ∼0.3% of total myocardial cells. GATA4 immunostaining was further used to define these CD34+ cells. Figure 7, A and B, shows that only poly(I:C)-treated MSC significantly increased GATA4+ cells (∼25%) and CD34+/GATA4+ progenitor cells (∼1%), which are more abundant than the CD34+/Ki67+ progenitor cells. Our previous therapeutic studies also reveal the involvement of c-kit+ progenitor cells in cardiac repair (66, 88). Similar to CD34 immunostaining, c-kit immunostaining reveals a ∼30% increase in c-kit+ progenitor cells by poly(I:C)-treated MSC compared with untreated MSC (data not shown). qPCR analysis further confirms that myocardial expression of the cardiogenic transcription factors GATA4, Nkx2.5, and Mef2c were all significantly increased in poly(I:C)-treated MSC (Fig. 7C). These studies suggest that poly(I:C) treatment of MSC enhances cardiac therapeutic potency at least in part through activation of cardiac progenitor cells.
Fig. 6.
Amplification of CD34+/Ki67+ progenitor cells by poly(I:C)-conditioned MSC. Paraffin sections were prepared from ventricular tissue 1 mo after the low-dose (1 × 106 cells/kg) MSC therapy. CD34 antibody (pink), myosin heavy chain (MHC) antibody (green), Ki67 antibody (yellow), and DAPI (blue) were used. A: quantification of CD34+ cells. B: a representative MHC−/CD34+ cell from the TO2 heart is illustrated at two magnifications (×200 and ×630). The highlighted area was imaged at ×630 magnification. C: quantification of total Ki67+ and CD34+/Ki67+ progenitor cells. D: a representative image of a MHC−/CD34+/Ki67+ progenitor cell. n = 3–4 per group; †P < 0.05 vs. MSC, *P < 0.05 and ***P < 0.001 vs. saline control.
Fig. 7.
Amplification of CD34+/GATA4+ progenitor cells by poly(I:C)-conditioned MSC. A: paraffin sections were prepared from ventricular tissue 1 mo after the low-dose (1 × 106 cells/kg) MSC therapy. CD34 antibody (pink), MHC antibody (green), GATA4 antibody (yellow), and DAPI (blue) were used. Quantification of GATA4+ and CD34+/GATA4+ progenitor cells is presented. B: a representative MHC−/CD34+/GATA4+ progenitor cell is illustrated at two magnifications (×200 and ×630). The highlighted area was imaged at ×630 magnification. C: qRT-PCR analysis of the cardiac transcription factors GATA4, Nkx2.5, and Mef2c. n = 3–4 per group; *P < 0.05 vs. saline control; ***P < 0.001 vs. saline control; †††P < 0.001 vs. untreated MSC.
Attenuation of myocardial inflammation by MSC independent of poly(I:C) treatment.
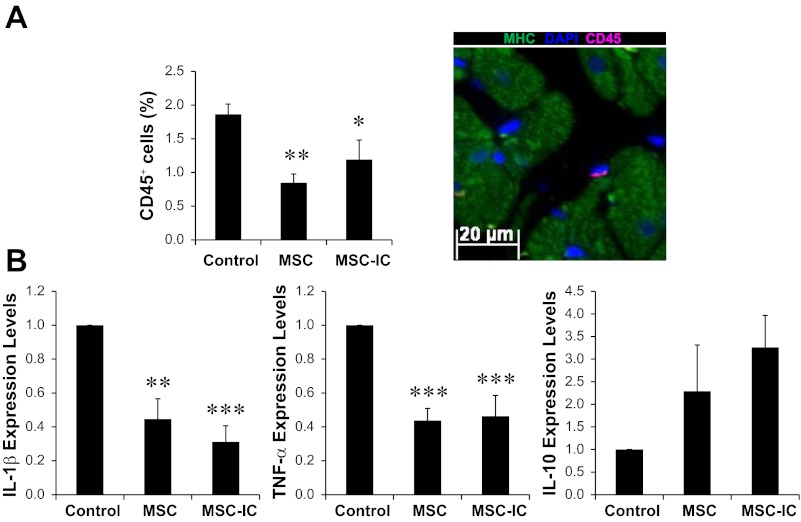
The δ-sarcoglycan protein deficiency of the TO2 hamster heart causes cell membrane fragility and permeability to Ca2+, which induce damage to myocytes, smooth muscle cells, and endothelial cells (36, 46, 62). Since the ongoing tissue injury causes myocardial inflammation (23, 51), we further addressed the impact of poly(I:C) conditioning and MSC therapy on abundance of myocardial inflammatory cells. Immunostaining of CD45 (leukocyte common antigen) showed that both untreated and poly(I:C)-treated MSC similarly reduced these immune cells by ∼50% compared with the saline injection group (Fig. 8A). Consistent with the immunostaining analysis, both untreated and poly(I:C)-treated MSC similarly decreased myocardial expression of the inflammatory cytokines IL-1β and TNF-α by ∼50%, and modestly increased expression of the antiinflammatory cytokine IL-10 (Fig. 8B). These findings thus indicate that poly(I:C) conditioning of MSC enhances cardiac therapeutic potency independent of the immunomodulatory function of MSC and that antiinflammation therapy alone is insufficient for cardiac repair.
Fig. 8.
MSC therapy attenuates myocardial inflammation independent of poly(I:C) treatment. A: paraffin sections were prepared from ventricular tissue 1 mo after the low-dose (1 × 106 cells/kg) MSC therapy. CD45 antibody (pink) was used to identify and quantify myocardial immune cells. MHC antibody (green) was used to stain cardiomyocytes. DAPI (blue) was used to stain nuclei. Quantification of CD45+ cells is presented and a representative CD45+ cell is illustrated (×200). B: RNA was isolated from the heart and analyzed by qRT-PCR to determine expression of the inflammatory cytokines IL-1β, TNF-α, and the antiinflammatory cytokine IL-10. n = 3–4 per group; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. saline control.
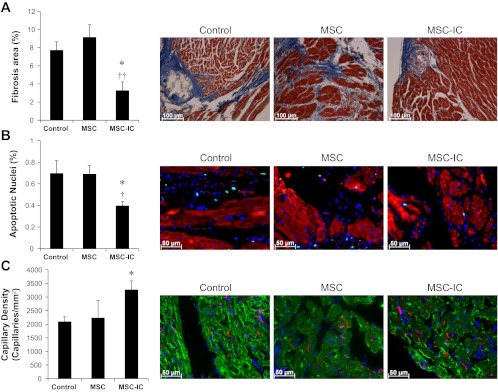
Improved function correlates with attenuated myocardial fibrosis and apoptosis as well as enhanced angiogenesis.
Histological analyses were further performed to ascertain cardiac repair after MSC therapy. The failing TO2 hamster heart exhibits progressive fibrosis, culminating in 7–10% fibrotic areas at 5 mo of age (66). Poly(I:C)-treated MSC reduced myocardial fibrosis by ∼50%, which was not observed with untreated MSC (Fig. 9A). The cardiomyopathic heart at 5 mo of age exhibits 0.7–1% apoptotic myocyte nuclei (88). Poly(I:C)-treated MSC, but not untreated MSC, reduced myocardial apoptosis by ∼40% (Fig. 9B). Poly(I:C)-treated MSC, but not untreated MSC, also significantly promoted angiogenesis by ∼55% as revealed by increased capillary staining (Fig. 9C). Thus, at the low injection dose of 1 × 106 cells/kg, only poly(I:C)-treated MSC reduced fibrosis and apoptosis as well as increased angiogenesis.
Fig. 9.
Antifibrotic, antiapoptotic, and proangiogenic activities are promoted by poly(I:C)-treated MSC. Heart tissue sections were prepared 1 mo after the low-dose (1 × 106 cells/kg) MSC therapy and processed for analysis of fibrosis, apoptosis, and angiogenesis. A: analysis of myocardial fibrosis by trichrome staining. B: analysis of myocardial apoptosis by TUNEL staining. Myocytes were stained by a troponin T antibody (red). Total nuclei were stained by DAPI (blue). Apoptotic nuclei are cyan colored. C: analysis of capillary density by GSL-IB4 staining. Myocytes were stained by a troponin T antibody (green). Total nuclei were stained by DAPI (blue). Capillaries are red/pink colored. Representative images of fibrosis, apoptosis, and stained capillaries for each group are illustrated (×200). n = 3–4 per group; *P < 0.05 vs. saline control; †P < 0.05 and ††P < 0.01 vs. untreated MSC.
Improved function correlates with enhanced cardiomyogenesis.
Elevated levels of trophic factors produced by poly(I:C)-treated MSC are also expected to promote cardiomyogenesis. Using the cultured HL-1 cardiomyocyte cell system (15), we found that the conditioned medium prepared from poly(I:C)-treated MSC (MSC-IC CM) caused a ∼17% increase in cardiomyocyte proliferation after 3 days compared with control MSC CM (Fig. 10A). Echocardiography confirmed that the robust cardiomyogenic events after administration of 1 × 106 poly(I:C)-treated MSC/kg resulted in significantly increased myocardial anterior wall thickening (Fig. 10, B and C). Morphometric analysis of cardiomyocyte size provides a histological indicator of the extent of cardiomyocyte regeneration (66, 70). This study reveals a mean cross-sectional myocyte area of ∼250 μm2 for the control and untreated MSC groups, which was decreased to ∼200 μm2 in the poly(I:C)-treated MSC group (data not shown). Myocyte size distribution analysis reveals that this shift was caused by a significant increase in the smaller (new) myocytes of 50–100 μm2 in size after poly(I:C) conditioning, which correlated with significantly reduced frequency of larger myocytes in the 250–600 μm2 range (blue line in Fig. 10D). These in vitro and in vivo studies together establish the utility of TLR3 engagement for effective low-dose MSC therapy.
Fig. 10.
Poly(I:C)-treated MSC effectively promotes formation of new cardiomyocytes. A: HL-1 cardiomyocytes preplated in a 24-well plate were treated with 50% MSC-conditioned medium (MSC CM or MSC-IC CM). Cell proliferation was monitored by MTT assays for 3 days, comparing the difference between MSC CM and MSC-IC CM at the time points indicated; n = 3. B and C: echocardiography was used to measure % anterior wall thickening (B) and anterior wall thickness (Δanterior WT) (C). Heart tissue sections were prepared 1 mo after the low-dose (1 × 106 cells/kg) MSC therapy. H&E-stained paraffin sections were used for morphometric analysis. D: myocyte size distribution analysis derived from the morphometric analysis. n = 4 per group; *P < 0.05 and ***P < 0.001 saline control vs. MSC-IC; †P < 0.01 and †††P < 0.001 untreated MSC vs. MSC-IC; ††P < 0.01 untreated MSC vs. MSC-IC; ‡P < 0.05; ‡‡P < 0.01 vs. pretreatment; #P < 0.01 vs. MSC CM.
DISCUSSION
In our remote MSC therapy for hamster heart failure, an injection of up to 40 × 106 MSC/kg was necessary to achieve maximal and consistent therapeutic benefits (65, 66). Therapeutic importance of the injected cell dose has also been demonstrated by others previously (31, 52). However, given the large body weight difference between hamsters and humans (0.1 kg vs. 70 kg), obtaining the large quantity of stem cells necessary for mounting a prominent therapeutic response in humans can present a daunting challenge. MSC senescence caused by ex vivo cell amplification and host aging-associated tissue impairment can further compromise regenerative therapy (9, 41, 75, 76). The present work suggests that the poly(I:C)-based TLR3 engagement protocol may be adopted to address these challenges. In particular, we show that poly(I:C) treatment of MSC enables the low-dose MSC (1 × 106 cells/kg) therapy for cardiac repair. This finding appears clinically relevant because current clinical trials of MSC therapy largely rely on injections of ∼1 × 106 MSC/kg (17, 27, 32, 38, 71).
Although TLRs and their ligands are known to control MSC function and multilineage phenotype, contradictory results regarding the effects of TLR activation are evident in the literature (20, 60, 78). Since TLRs sense exogenous and endogenous stress signals, the intensity and duration of these signals may differentially affect the phenotype of MSC as recently recognized (78). Indeed, we show here that exposure of MSC to 4 and 20 μg/ml poly(I:C) might trigger differential trophic responses. For instance, TNF-α was dramatically induced by 20 but not 4 μg/ml poly(I:C), prompting us to adopt the 4 μg/ml MSC conditioning regimen. Similar to the effects observed here, baculoviral transduction of MSC also triggered robust production of IL-6 without induction of the inflammatory cytokines such as TNF-α and IL-1 (14). This is in contrast to the effects of TLR3 activation in the liver, which causes increased expression of TNF-α (39).
Whereas TLR activation of the immune system is associated with chronic inflammation, an unexpected protective role for TLR3 in the arterial wall upon systemic administration of poly(I:C) has been documented (16). Similarly, poly(I:C) administration alone has been found to protect against cerebral and renal ischemia-reperfusion injury (56). Given that MSC are widely present in vivo and their perivascular origin in multiple human organs has been demonstrated (12, 18, 19), it is possible that these prophylactic benefits of poly(I:C) may be mediated through its trophic stimulatory effect on the endogenous MSC niches as illustrated in the current study. Notably, despite the finding that poly(I:C) treatment of MSC slightly inhibited the proliferation and migration potentials, therapeutic potency is enhanced through amplified expression of MSC trophic factors. This demonstration is consistent with the emerging recognition that the secretion of multiple trophic factors by MSC provides the major underlying tissue healing mechanism (4, 12, 25, 40, 61). Indeed, using the hamster heart failure model we have demonstrated similar therapeutic benefits mediated by cell-free treatment regimens, such as intramuscular injections of MSC CM (66) and VEGF (88, 89). This therapeutic mechanism appears to explain why systemic delivery of MSC is also effective for cardiac repair (5, 53). Notably, ongoing clinical trials of MSC for heart therapy by Osiris Therapeutics are based on intravenous infusion of allogeneic MSC (27), which although largely trapped in the lungs upon infusion are capable of cardioprotection, presumably through MSC trophic actions as elaborated here. Furthermore, clinical trials of MSC for multiple sclerosis patients demonstrated similar benefits mediated by either intrathecal or intravenous cell administration route (35). Along this line, we found that the intramuscular MSC therapeutic approach could raise some growth factor levels in extra-cardiac organs such as the brain (65), suggesting potential application for neurodegenerative repair.
The δ-sarcoglycan null strain of TO2 hamster exhibits cardiac dysfunction and develops dilated cardiomyopathy, leading to congestive heart failure resembling that seen in many patients (26, 50, 63). Deficiency of δ-sarcoglycan protein causes cell membrane fragility and permeability to Ca2+, inducing damages to myocytes, smooth muscle cells, and endothelial cells (36, 46, 62). Since cardiac dysfunction in TO2 hamsters exhibits prominent myocardial inflammation (51), the immunomodulatory property of MSC is ideally suited for antiinflammatory therapy (74). Although the current study employed porcine MSC for hamster heart therapy, we observed similar cardiac therapeutic benefits with MSC prepared from hamster bone marrow (data not shown). Indeed, we demonstrated that the xenogeneic MSC approach attenuated tissue injury without inflaming the host immune system (64).
The trophic actions of MSC can decrease host production of inflammatory cytokines while inducing the release of antiinflammatory cytokines (55, 64, 79). Our demonstration that both untreated and poly(I:C)-treated MSC similarly attenuated myocardial immune cells (CD45+) and downregulated inflammatory cytokines is consistent with the conclusion that poly(I:C)-mediated TLR3 activation does not appreciably affect the immunomodulatory function of MSC (20). We recently demonstrated that blocking JAK/STAT3 signaling and VEGF/SDF1 signaling could each abrogate the therapeutic effects on the failing hamster heart (65, 89). The 4 μg/ml MSC conditioning regimen likely activates the JAK/STAT3 axis via elevated IL-6-type cytokines and improves therapeutic efficacy through synergistic actions of the induced trophic factors such as VEGF, SDF1, and HGF. Along this line, it has been proposed that MSC are released from their perivascular location during local injury and activated by TLR ligands, thus establishing a regenerative milieu through secretion of multifunctional trophic factors (12, 20).
Our previous SDF1 blockade study shows that mobilization of multilineage bone marrow progenitor cells is critically involved in myocardial regeneration (89). The newly approved drug plerixafor (AMD3100) potently induces mobilization of bone marrow CD34+ progenitor cells, and plerixafor-mobilized CD34+ cells are now used in HSC therapy following myeloablation (43, 52). CD34+ progenitor cells are known to express markers of several other lineages such as those of smooth muscle cells, endothelial cells, skeletal myoblasts, and cardiomyocytes (31, 81). Isolated CD34+ progenitor cells actively secrete trophic factors such as VEGF (84). Cultured CD34+ progenitor cells are able to repair infarcted myocardium through trans-differentiation (83, 85). Encouraging results from administration of autologous CD34+ progenitor cells for cardiac repair have been documented in recent clinical trials (47, 57). Their potential as functional cardiac progenitor cells is demonstrated here by robust expression of GATA4, Nkx2.5, and Mef2c as also demonstrated for c-kit+ cardiac progenitor cells (7). However, we found that the c-kit+ cardiac progenitor cells are stably maintained in the failing hamster heart in contrast to the CD34+ cardiac progenitor cells, which are further downregulated after 5 mo of age in accordance with the progressive age-related decline in ventricular function (data not shown), suggesting that restoration of the CD34+ cardiac progenitor pool may be explored for rescuing the failing heart. Since both the CD34 and c-kit markers are also expressed by mast cells, which are often prevalent at the site of tissue injury (21, 86), these surface epitopes would need to be characterized in the context of cardiomyogenesis. Accordingly, the current study reached a calculated abundance of ∼0.3% and ∼1% for CD34+/Ki67+ and CD34+/GATA4+, respectively, after poly(I:C) conditioning of MSC. These progenitor cells are expected to gradually lose their proliferation potential and stem cell surface marker upon cardiomyogenic differentiation, as demonstrated for CD34+ muscle stem cells after the early stages of injury-mediated activation (8, 11).
In summary, the current work demonstrates that poly(I:C) conditioning of MSC significantly lowers the effective therapeutic cell dose for heart repair, providing a feasible strategy for eliciting prominent therapeutic response in humans. Poly(I:C)-treated MSC express higher levels of therapeutically important trophic factors, which synergistically enhance therapeutic efficacy through activation of cardiac progenitor cells and cardiomyogenesis. Poly(I:C) conditioning of MSC through its engagement of TLR3 thus represents a clinically attractive and testable therapeutic strategy that can be performed at a reduced cell dose and lower the treatment costs.
GRANTS
The work was supported in part by National Institutes of Health (National Heart, Lung, and Blood Institute Grant HL-84590) and Biomedical Research Service Center of University at Buffalo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.M. and T.L. conception and design of the research; M.M., Z.S., T.M., C.J.G., L.B., and G.S. performed the experiments; M.M., Z.S., C.J.G., G.S., and T.L. analyzed the data; M.M. and T.L. interpreted the results of the experiments; M.M. prepared the figures; T.L. drafted the manuscript; T.L. edited and revised the manuscript; T.L. approved the final version of the manuscript.
Footnotes
This article is the topic of an Editorial Focus by Oliver Zimmermann (87).
REFERENCES
- 1. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732– 738, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Allison M. Genzyme backs Osiris, despite Prochymal flop. Nat Biotechnol 27: 966– 967, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta-analysis of randomized controlled trials. J Invasive Cardiol 24: 42– 48, 2012 [PubMed] [Google Scholar]
- 4. Bai L, Lennon DP, Caplan AI, Dechant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 108: 863– 868, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22: 675– 682, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci USA 104: 14068– 14073, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151: 1221– 1234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102: 131– 135, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol 7: 14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells 26: 3194– 3204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 9: 11– 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chae CH, Jung SL, An SH, Park BY, Wang SW, Cho IH, Cho JY, Kim HT. Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience 164: 1665– 1673, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Chen GY, Shiah HC, Su HJ, Chen CY, Chuang YJ, Lo WH, Huang JL, Chuang CK, Hwang SM, Hu YC. Baculovirus transduction of mesenchymal stem cells triggers the toll-like receptor 3 pathway. J Virol 83: 10548– 10556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA 95: 2979– 2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, Davies AH, Flavell RA, Feldmann M, Monaco C. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci USA 108: 2372– 2377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 11: 150– 156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev 21: 1299– 1308, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301– 313, 2008 [DOI] [PubMed] [Google Scholar]
- 20. DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm 2010: 865601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Drew E, Huettner CS, Tenen DG, McNagny KM. CD34 expression by mast cells: of mice and men. Blood 106: 1885– 1887, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, Purushothaman S, Galuppo V, Iaconetti C, Waring CD, Smith A, Torella M, Cuellas Ramon C, Gonzalo-Orden JM, Agosti V, Indolfi C, Galinanes M, Fernandez-Vazquez F, Nadal-Ginard B. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol 58: 977– 986, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Fiaccavento R, Carotenuto F, Minieri M, Fantini C, Forte G, Carbone A, Carosella L, Bei R, Masuelli L, Palumbo C, Modesti A, Prat M, Di Nardo P. Stem cell activation sustains hereditary hypertrophy in hamster cardiomyopathy. J Pathol 205: 397– 407, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Friis T, Haack-Sorensen M, Mathiasen AB, Ripa RS, Kristoffersen US, Jorgensen E, Hansen L, Bindslev L, Kjaer A, Hesse B, Dickmeiss E, Kastrup J. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand Cardiovasc J 45: 161– 168, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204– 1219, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goineau S, Pape D, Guillo P, Ramee MP, Bellissant E. Hemodynamic and histomorphometric characteristics of dilated cardiomyopathy of Syrian hamsters (Bio TO-2 strain). Can J Physiol Pharmacol 79: 329– 337, 2001 [PubMed] [Google Scholar]
- 27. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54: 2277– 2286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol 8: 619– 629, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 134: 1790– 1807, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell 18: 2873– 2882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, Sadamoto K, Horii M, Matsumoto T, Murasawa S, Shibata T, Suehiro S, Asahara T. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation 113: 1311– 1325, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Jiang R, Han Z, Zhuo G, Qu X, Li X, Wang X, Shao Y, Yang S, Han ZC. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med 5: 94– 100, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem 283: 3988– 3996, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67: 1187– 1194, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci 265: 131– 135, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Kato Y, Iwase M, Takagi K, Nishizawa T, Kanazawa H, Matsushita A, Umeda H, Izawa H, Noda A, Koike Y, Nagata K, Yokota M. Differential myolysis of myocardium and skeletal muscle in hamsters with dilated cardiomyopathy. Circ J 70: 1497– 1502, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2: 22– 31, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE, Svinareva DA, Drize NJ, Savchenko VG. Multipotent Mesenchymal Stromal Cells for the Prophylaxis of Acute Graft-versus-Host Disease-A Phase II Study. Stem Cells Int 2012: 968213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lang KS, Georgiev P, Recher M, Navarini AA, Bergthaler A, Heikenwalder M, Harris NL, Junt T, Odermatt B, Clavien PA, Pircher H, Akira S, Hengartner H, Zinkernagel RM. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest 116: 2456– 2463, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee T. Host tissue response in stem cell therapy. World J Stem Cells 2: 61– 66, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehrke S, Mazhari R, Durand DJ, Zheng M, Bedja D, Zimmet JM, Schuleri KH, Chi AS, Gabrielson KL, Hare JM. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res 99: 553– 560, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Li TS, Suzuki R, Ueda K, Murata T, Hamano K. Analysis of the origin and population dynamics of cardiac progenitor cells in a donor heart model. Stem Cells 25: 911– 917, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, Dale DC. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 102: 2728– 2730, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 105: 651– 655, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Lin H, Shabbir A, Molnar M, Yang J, Marion S, Canty JMJ, Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J Cell Physiol 216: 458– 468, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Lipskaia L, Pinet C, Fromes Y, Hatem S, Cantaloube I, Coulombe A, Lompre AM. Mutation of delta-sarcoglycan is associated with Ca2+-dependent vascular remodeling in the Syrian hamster. Am J Pathol 171: 162– 171, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res 109: 428– 436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther 90: 532– 541, 2011 [DOI] [PubMed] [Google Scholar]
- 49. Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem Biophys Res Commun 293: 1364– 1369, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Minieri M, Fiaccavento R, Carosella L, Peruzzi G, Di Nardo P. The cardiomyopathic hamster as model of early myocardial aging. Mol Cell Biochem 198: 1– 6, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Missihoun C, Zisa D, Shabbir A, Lin H, Lee T. Myocardial oxidative stress, osteogenic phenotype, and energy metabolism are differentially involved in the initiation and early progression of delta-sarcoglycan-null cardiomyopathy. Mol Cell Biochem 321: 45– 52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohty M, Duarte RF, Croockewit S, Hubel K, Kvalheim G, Russell N. The role of plerixafor in optimizing peripheral blood stem cell mobilization for autologous stem cell transplantation. Leukemia 25: 1– 6, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 287: H2670– H2676, 2004 [DOI] [PubMed] [Google Scholar]
- 54. O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 7: 353– 364, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA 104: 11002– 11007, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Packard AE, Hedges JC, Bahjat FR, Stevens SL, Conlin MJ, Salazar AM, Stenzel-Poore MP. Poly-IC preconditioning protects against cerebral and renal ischemia-reperfusion injury. J Cereb Blood Flow Metab 32: 242– 247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pasquet S, Sovalat H, Henon P, Bischoff N, Arkam Y, Ojeda-Uribe M, Bouar R, Rimelen V, Brink I, Dallemand R, Monassier JP. Long-term benefit of intracardiac delivery of autologous granulocyte-colony-stimulating factor-mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy 11: 1002– 1015, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153– 183, 2001 [DOI] [PubMed] [Google Scholar]
- 59. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379– 1406, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, Cohen IR, Zipori D. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 109: 1422– 1432, 2007 [DOI] [PubMed] [Google Scholar]
- 61. Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther 82: 241– 243, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Ryoke T, Gu Y, Ikeda Y, Martone ME, Oh SS, Jeon ES, Knowlton KU, Ross J., Jr Apoptosis and oncosis in the early progression of left ventricular dysfunction in the cardiomyopathic hamster. Basic Res Cardiol 97: 65– 75, 2002 [DOI] [PubMed] [Google Scholar]
- 63. Sakamoto A, Ono K, Abe M, Jasmin G, Eki T, Murakami Y, Masaki T, Toyo-Oka T, Hanaoka F. Both hypertrophic and dilated cardiomyopathies are caused by mutation of the same gene, delta-sarcoglycan, in hamster: an animal model of disrupted dystrophin-associated glycoprotein complex. Proc Natl Acad Sci USA 94: 13873– 13878, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by non-autologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation 87: 1275– 1282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shabbir A, Zisa D, Lin H, Mastri M, Roloff G, Suzuki G, Lee T. Activation of host tissue trophic factors through JAK/STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am J Physiol Heart Circ Physiol 299: H1428– H1438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a non-invasive therapeutic regimen. Am J Physiol Heart Circ Physiol 296: H1888– H1897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 117: 191– 200, 2009 [DOI] [PubMed] [Google Scholar]
- 68. Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells 24: 1254– 1264, 2006 [DOI] [PubMed] [Google Scholar]
- 69. Suetta C, Clemmensen C, Andersen JL, Magnusson SP, Schjerling P, Kjaer M. Coordinated increase in skeletal muscle fiber area and expression of IGF-I with resistance exercise in elderly post-operative patients. Growth Horm IGF Res 20: 134– 140, 2010 [DOI] [PubMed] [Google Scholar]
- 70. Suzuki G, Lee T, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res 96: 767– 775, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 307: 1169– 1177, 2012 [DOI] [PubMed] [Google Scholar]
- 72. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, Fletcher GF, Gordon NF, Pate RR, Rodriguez BL, Yancey AK, Wenger NK. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 107: 3109– 3116, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program 2011: 280– 284, 2011 [DOI] [PubMed] [Google Scholar]
- 74. Tyndall A, Walker UA, Cope A, Dazzi F, De Bari C, Fibbe W, Guiducci S, Jones S, Jorgensen C, Le Blanc K, Luyten F, McGonagle D, Martin I, Bocelli-Tyndall C, Pennesi G, Pistoia V, Pitzalis C, Uccelli A, Wulffraat N, Feldmann M. Immunomodulatory properties of mesenchymal stem cells: a review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther 9: 301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Jr, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol 205: 194– 201, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLos One 3: e2213, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Walsh K. Adipokines, myokines and cardiovascular disease. Circ J 73: 13– 18, 2009 [DOI] [PubMed] [Google Scholar]
- 78. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLos One 5: e10088, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wehner R, Wehrum D, Bornhauser M, Zhao S, Schakel K, Bachmann MP, Platzbecker U, Ehninger G, Rieber EP, Schmitz M. Mesenchymal stem cells efficiently inhibit the proinflammatory properties of 6-sulfo LacNAc dendritic cells. Haematologica 94: 1151– 1156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 109: 923– 940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation 110: 3213– 3220, 2004 [DOI] [PubMed] [Google Scholar]
- 82. Wu G, Rana JS, Wykrzykowska J, Du Z, Ke Q, Kang P, Li J, Laham RJ. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am J Physiol Heart Circ Physiol 296: H389– H395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yeh ET, Zhang S, Wu HD, Korbling M, Willerson JT, Estrov Z. Transdifferentiation of human peripheral blood CD34+-enriched cell population into cardiomyocytes, endothelial cells, and smooth muscle cells in vivo. Circulation 108: 2070– 2073, 2003 [DOI] [PubMed] [Google Scholar]
- 84. Yoshioka T, Ageyama N, Shibata H, Yasu T, Misawa Y, Takeuchi K, Matsui K, Yamamoto K, Terao K, Shimada K, Ikeda U, Ozawa K, Hanazono Y. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells 23: 355– 364, 2005 [DOI] [PubMed] [Google Scholar]
- 85. Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation 110: 3803– 3807, 2004 [DOI] [PubMed] [Google Scholar]
- 86. Zhou Y, Pan P, Yao L, Su M, He P, Niu N, McNutt MA, Gu J. CD117-positive cells of the heart: progenitor cells or mast cells? J Histochem Cytochem 58: 309– 316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zimmermann O. Mesenchymal stem cells and cardiac regeneration: a sophisticated approach depends on trophic effects—what's left over? Focus on “Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency.” Am J Physiol Cell Physiol (September 12, 2012) doi10.1152/ajpcell.00295.2012 [DOI] [PubMed] [Google Scholar]
- 88. Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol 297: R1503– R1515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zisa D, Shabbir A, Mastri M, Taylor T, Aleksic I, McDaniel M, Suzuki G, Lee T. Intramuscular VEGF activates an SDF1-dependent progenitor cell cascade and an SDF1-independent muscle paracrine cascade for cardiac repair. Am J Physiol Heart Circ Physiol 301: H2422– H2432, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zisa D, Shabbir A, Suzuki G, Lee T. Vascular endothelial growth factor (VEGF) as a key therapeutic trophic factor in bone marrow mesenchymal stem cell-mediated cardiac repair. Biochem Biophys Res Commun 390: 834– 838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]