Abstract
Diabetes mellitus is characterized by hyperglycemia and excessive production of intermediary metabolites including methylglyoxal (MGO), a reactive carbonyl species that can lead to cell injuries. Interacting with proteins, lipids, and DNA, excessive MGO can cause dysfunction of various tissues, especially the vascular walls where diabetic complications often take place. However, the potential vascular targets of excessive MGO remain to be fully understood. Here we show that the vascular Kir6.1/SUR2B isoform of ATP-sensitive K+ (KATP) channels is likely to be disrupted with an exposure to submillimolar MGO. Up to 90% of the Kir6.1/SUR2B currents were suppressed by 1 mM MGO with a time constant of ∼2 h. Consistently, MGO treatment caused a vast reduction of both Kir6.1 and SUR2B mRNAs endogenously expressed in the A10 vascular smooth muscle cells. In the presence of the transcriptional inhibitor actinomycin-D, MGO remained to lower the Kir6.1 and SUR2B mRNAs to the same degree as MGO alone, suggesting that the MGO effect is likely to compromise the mRNA stability. Luciferase reporter assays indicated that the 3′-untranslated regions (UTRs) of the Kir6.1 but not SUR2 mRNA were targeted by MGO. In contrast, the SUR2B mRNAs obtained with in vitro transcription were disrupted by MGO directly, while the Kir6.1 transcripts were unaffected. Consistent with these results, the constriction of mesenteric arterial rings was markedly augmented with an exposure to 1 mM MGO for 2 h, and such an MGO effect was totally eliminated in the presence of glibenclamide. These results therefore suggest that acting on the 3′-UTR of Kir6.1 and the coding region of SUR2B, MGO causes instability of Kir6.1 and SUR2B mRNAs, disruption of vascular KATP channels, and impairment of arterial function.
Keywords: ATP-sensitive potassium channel, metabolites, vascular smooth muscles, diabetes, vascular complications
diabetes mellitus is a systemic disorder that affects 8.3% of the population in the United States (14). Persistent hyperglycemia in diabetic patients causes metabolic alternations, leading to overproduction of a variety of intermediary metabolites. One of the metabolites is methylglyoxal (MGO), a reactive carbonyl species (RCS). MGO is a transient triosephosphate intermediate produced mainly via the glycolytic pathway. MGO is membrane permeable and can readily cross the cell membranes without any cofactors or transporters (46). Under physiological conditions, MGO is retained at a rather low level by metabolic enzymes (17). The MGO concentration rises above the permissive levels in pathological conditions owing to the buildup of its precursor molecules and a disruption of the cellular antioxidant detoxification systems (8). Indeed, the plasma levels of MGO are markedly elevated in patients with diabetes (19). The presence of excessive MGO in interstitial fluids is attributable to the progression of diabetic complications (5, 8, 54) and diabetes-related vascular disorders including hypertension (34, 50). Despite the accumulating evidence for the adverse vascular effects of MGO, the mechanisms and the molecular targets by which MGO acts on the vascular walls remain to be demonstrated.
The vascular smooth muscle (VSM) isoform of the ATP-sensitive K+ (KATP) channels is a critical player in vascular tone regulation (4, 22, 36, 60). This channel consisting of Kir6.1 and SUR2B subunit (59) is activated by vasodilators and inhibited by vasoconstrictors, contributing to the control of regional blood flows in response to circulating hormones and cellular metabolites (30, 33, 39, 43). Disruptions of the KATP channel have severe consequences as knockout of the Kir6.1 gene in mice leads to coronary vasospasm and sudden death (11, 28), as well as lethal sensitivity to bacterial endotoxins (13, 21).
The vascular KATP channel is susceptible to several pathophysiological conditions (25). In diabetic patients, the vascular responses to KATP channel openers are impaired, resulting in a defect in vasodilation (15, 29). Our previous studies (55, 56) have shown that the vascular KATP channel activity is inhibited via protein S-glutathionylation in oxidative stress. The oxidative stress, known to contribute to diabetic vascular complications, is produced by several reactive species including reactive oxygen species (ROS), reactive nitrogen species, reactive lipid species, and RCS. Accumulating evidence suggests that the RCS consisting of MGO, glyoxal, and 3-deoxyglucosone may play a prominent role in the development of diabetic complications (3, 15).
The vascular KATP channel could be a potential target of MGO, as disruption of functional KATP channels can lead to abnormalities in cellular excitability, impairment of VSM contraction, as well as vascular responses to vasoconstrictors and vasodilators. Therefore, we performed these studies to test the hypothesis that MGO affects the function of vascular KATP channels. Our results showed that MGO treatment caused disruption of both Kir6.1 and SUR2B mRNAs through two distinct mechanisms. Such an effect is likely to impair membrane potentials and K+ homeostasis of VSMs, exacerbating the vascular complications of diabetes.
METHODS
Reagents.
Chemicals and reagents were purchased from Sigma unless stated otherwise. Reagents were prepared in high-concentration stocks in double-distilled water or DMSO. The final concentration of DMSO in the solutions used for experiments was less than 0.1% (vol/vol), which did not have any detectable effect on the channel activity.
Cell culture.
The HEK293 cells (CRL-1573; ATCC, Manassas, VA) and the rat VSM cells (the A10 cell line, CRL-1476; ATCC) were cultured in DMEM/F-12 (with additions of 10% FBS and penicillin/streptomycin) as a monolayer in a 5% CO2 atmosphere at 37°C.
Heterologous expression of KATP channel. KATP channels were expressed in HEK293 cells as previously described (42, 56, 62). The HEK293 cells cultured in a 35-mm petri dish were transfected with 1 μg of pcDNA3.1 (a eukaryotic expression vector) containing rat Kir6.1 (GenBank No. D42145) and SUR2B cDNA (GenBank No. D86038, mRNA isoform NM_011511) using Lipofectamine2000 (Invitrogen, Carlsbad, CA). Green fluorescent protein cDNA (0.4 μg, pEGFP-N2; Clontech, Palo Alto, CA) was included in the cDNA transfection mixture to facilitate the identification of positively transfected cells. One day after transfection, the cells were dissociated with trypsin (0.25%) and then transferred to coverslips. Electrophysiological experiments were performed on the cells present on the coverslips for 2 continuous days.
Electrophysiology.
Patch-clamp experiments were performed at room temperature as described previously (39–41, 57). In brief, patch pipettes made of 1.2-mm borosilicate glass capillaries were fire polished to have 2–5 MΩ resistance. Whole cell currents were recorded in voltage clamp with a holding potential 0 mV and stepped to −80 mV every 3 s. The bath solution contained the following (in mM): 10 KCl, 135 potassium gluconate, 5 EGTA, 5 glucose, and 10 HEPES (pH 7.4). The pipette was filled with a solution containing the following: 10 KCl, 133 potassium gluconate, 5 EGTA, 5 glucose, 1 K2ATP, 0.5 NaADP, 1 MgCl2, and 10 HEPES (pH 7.4). All solutions containing ATP and/or ADP were freshly made and used within 4 h to avoid nucleotide degradation. The recordings were obtained from the Axopatch 200B amplifier (Molecular Devices, Union City, CA), low-pass filtered (2 kHz, Bessel 4-pole filter, −3 dB), and digitized (10 kHz, 16-bit resolution) with Clampex 9 (Molecular Devices.). Symmetric K+ (145 mM in total) was used in both bath and pipette solution to make the reverse potential of K+ close to 0 mV. K2ATP (1 mM) and NaADP (0.5 mM) were also included in the bath solution for maintaining the channel activity.
RT-PCR.
Total RNAs were extracted from A10 smooth muscle cell line with an RNeasy Mini Kit (Qiagen). cDNA was reverse transcribed from 0.5 μg total RNAs in a 20-μl reaction containing 200 U Superscript III Reverse Transcriptase (Invitrogen), 300 ng random hexamers, 0.5 mM dNTPs, 40 U RNaseOut, and 10 mM DTT. The RT product was treated with 5 U RNase H for 20 min. Regular PCR was performed in the Mastercycler (Eppendorf Pro S) in a final volume of 50 μl containing 1 μl of the RT product, 1.25 U of GoTaq DNA polymerase (Promega, Madison, WI), 250 μM dNTP, 2.5 μl DMSO, and 0.5 μM primers. The cycling conditions included an initial denaturation at 95°C for 5 min followed by 30 cycles of denaturation at 95°C for 45 s, annealing at 52°C for 45 s, elongation at 72°C for 75 s, and a final elongation at 72°C for 10 min. PCR products were separated by electrophoresis on a 1% agarose gel for visualization.
Real-time quantitative RT-PCR.
Quantitative RT-PCR (qPCR) was performed with a Fast Real-Time PCR system (Applied Biosystems 7500). Applied Biosystems Primer Express 3.0 software was used for the primer design. The resulting primers confirmed with BLAST for specificity were synthesized by Sigma Genesis (Sigma). Each reaction (20 μl) contained 1× Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen), 0.2 μM mixture of forward and reverse primers, 0.04 μl ROX reference dye, and 0.4 μg cDNA. Thermal cycling conditions were set according to the manufacturer's instructions, which included an initial UNG incubation at 50°C for 2 min, Platinum Taq DNA polymerase activation at 95°C for 2 min, 40 cycles of denaturing at 95°C for 3 s, annealing and extension at 60°C for 30 s, followed by routine melting curve analysis. Relative quantitation of target gene expression was calculated by a standard 2−ΔΔCt method (27). The first step in the relative quantitation analysis was to obtain ΔCt by normalizing target gene expression level to the internal control GAPDH. The second step (ΔΔCt) was to compare the difference between normalized target gene expression in treated and control samples. Each sample was tested in triplicates or quadruplicates under the same reaction conditions in each experiment, and the experiments were repeated at least three times.
Genomic DNA isolation and 3′-untranslated regions.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Georgia State University following protocols. Sprague-Dawley rat (Jackson Laboratory) tail tissue was used for isolating the genomic DNA using the DNeasy blood and tissue kit (Qiagen) following manufacturer's protocol. Primer pairs with a 5′-XhoI overhang for the 3′-untranslated regions (UTRs) of Kir6.1/SUR2 genes were designed based on the conserved sequences from rat genomic sequence (accession no. AC_000072.1). PCR was performed in a 20-μl reaction containing 1 μg isolated genomic DNA, 0.75 mM dNTPs, 2.5 μl DMSO, 1× Pfu ultra buffer, 25 nM primer mixture, and 1 μl of Pfu ultra DNA polymerase using Mastercycler (Eppendorf Pro S) with an initial denaturation period of 95°C for 2 min, followed by 36 cycles of denaturation at 95°C for 50 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min, with a final elongation at 72°C for 10 min. The PCR products were analyzed on a 2% agarose gel.
Cloning of the 3′-UTR.
The pmirGLO luciferase expression vector (Promega) was digested with Xho I restriction enzyme (restriction site located downstream of the firefly-luc2 gene) and gel purified with QIAquick gel extraction kit (Qiagen). The appropriate PCR products corresponding to the 3′-UTR of Kir6.1 and SUR2 were purified (QIAquick PCR purification kit) and digested with Xho I enzyme followed by purification of the digested fragments. Ligation was performed in a 10-μl vol. Digested vector (50 ng) and digested fragment (300 ng) were incubated with 400 U of T4 DNA ligase (NEB) at 16°C for 16 h. The ligated products were transformed into XL1-blue competent cells (Agilent) for amplification. The amplified DNA was obtained through plasmid Mini-preparation (Qiagen). The correct constructs were confirmed by DNA sequencing.
Luciferase assay.
Luciferase assay was performed on A10 cells. The cells were grown to ∼90% confluence in a 35-mm petri dish and transfected with 1 μg of either Kir6.1–3′-UTR or SUR2B-3′-UTR containing pmirGLO vector using the Fugene-6 transfection reagent (Roche Applied Science, Indianapolis, IN). The cells were dissociated with 0.25% trypsin 8–12 h after transfection and then seeded into a 96-well plate for further growth. MGO treatment (in triplicates) was performed after 18–24 h.
Transfected A10 cells were incubated with freshly prepared MGO diluted to the required concentration in DMEM medium for 5 h, following which Dual-Glo luciferase assay was performed according to the manufacturer's instructions (Promega). Briefly, a volume of Dual-Glo substrate equal to the volume of the culture medium was added to each well and the firefly luminescence was measured using a luminometer (Model 1420 Victor3V; Perkin Elmer, Waltham, MA) after a 15- to 20-min incubation period. After the firefly luciferase reading was obtained, a volume of Dual Glo Stop & Glo reagent equal to the volume of the original culture medium was added to each well and the renilla luminescence was measured after 15–20 min. The ratio of firefly to renilla luciferase was calculated for each well. The well ratio of each sample was normalized to the control well ratio.
In vitro transcription.
cDNA sequences of rat Kir6.1 (GenBank No. D42145) and mouse SUR2B (GenBank No. D86038, mRNA isoform NM_011511) were cloned into the pcDNA 3.1 cloning vector. The linear DNA template was obtained by using a restriction site downstream to the 3′-end of the cDNA insert in the vector for in vitro transcription reactions. The in vitro transcription reactions were performed in RNase free environment with MEGAscript T7 high yield transcription kit (Ambion) according to the manufacturer's instructions. The RNA pellet obtained was dissolved in appropriate volume of RNase free H2O.
For the MGO treatment, 2 μg of RNA were treated with MGO (100, 300, or 600 μM) at 37°C for 6-, 18-, or 24-h time periods. After MGO treatment, 95% formamide containing gel loading buffer was added to the MGO/RNA mixture and heat denatured at 65°C for 45 min followed by chilling on ice. The denatured RNAs were separated on a 1.5% agarose gel for visualization with ultraviolet fluorescence. The density of the RNA bands on the gel was determined by densitometry analysis using ImageJ (1).
Mesenteric artery preparation and tension measurement.
After the Sprague-Dawley rats (200–250 g body wt) were euthanized, mesenteric arteries were dissected free and placed in physiological salt solution containing the following (in mM): 140 NaCl, 4.6 KCl, 1.5 CaCl2, 1 MgCl2, 10 glucose, and 5 HEPES, pH 7.3. The arteries were cut into small rings (2 mm in length) and transferred to ice-cold Krebs solution containing the following: 118.0 NaCl, 25.0 NaHCO3, 3.6 KCl, 1.2 MgSO4, 1.2 KH2PO4, 11.0 glucose, and 2.5 CaCl2 and bubbled with 95% O2-5% CO2. The arterial ring was mounted on a force-electricity transducer (i-FOT2, GlobalTown Sarasota, Florida) for measurements of isometric force contraction in a 2-ml tissue bath filled with the air bubbled Krebs solution.
Data analysis.
Data are presented as the means ± SE. Differences in means tested with Student's t-test or the ANOVA were accepted as significant if P < 0.05.
RESULTS
Decreases in vascular KATP channel mRNAs with prolonged MGO treatment.
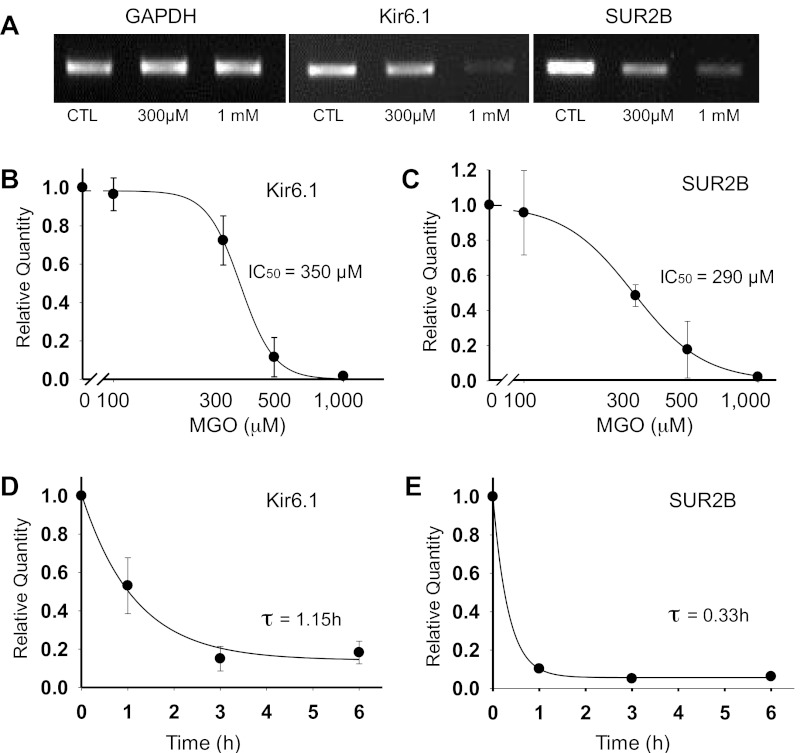
To test the hypothesis that prolonged MGO treatment affects the expression of vascular KATP channels, we studied the mRNAs of Kir6.1 and SUR2B subunits using regular and qPCR in A10 smooth muscle cell line, as they endogenously express the vascular Kir6.1/SUR2B isoform of KATP channels (40, 44). The concentration of MGO has been determined to be ∼400 μM (29.3 μg/ml) in diabetic patients with poor glycemic control (24, 32). Therefore, we used MGO with concentrations ranging from 100 μM to 1 mM in our experiment to better mimic the pathological condition. After the cells were treated with varying concentrations of MGO for 12 h, the total RNAs were extracted followed by PCR and qPCR analyses. Regular PCR showed evident decrease in the levels of Kir6.1 and SUR2B mRNAs with a 300 μM MGO treatment (Fig. 1A). A higher concentration of MGO (1 mM) caused nearly a total loss of Kir6.1 and SUR2B mRNAs (Fig. 1A). The mRNA levels of the internal control GAPDH did not show a significant change in response to similar MGO treatments (Fig. 1A), suggesting that MGO does not induce a general disruption of RNA molecules. Consistent with these findings, qPCR analysis showed that MGO treatments resulted in concentration-dependent reductions in the levels of Kir6.1 and SUR2B transcripts with IC50 of 350 μM for Kir6.1 and 290 μM for SUR2B, respectively (Fig. 1, B and C). The effect of MGO on Kir6.1 and SUR2B mRNAs also showed a clear time dependence. With a 500-μM MGO treatment, the time constant was 1.15 h and 0.33 h for Kir6.1 and SUR2B mRNAs, respectively (Fig. 1, D and E).
Fig. 1.
Prolonged exposure to methylglyoxal (MGO) causes a decrease in the levels of Kir6.1 and SUR2B mRNAs. A: A10 cells were treated with MGO for 12 h. Regular PCR analysis following MGO treatment showed no detectable change in the levels of GAPDH mRNA with increasing concentrations of MGO, whereas Kir6.1 and SUR2B mRNA levels decreased with a prior exposure to 300 μM MGO. An increase in MGO concentration to 1 mM caused nearly a total loss of the Kir6.1 and SUR2B mRNAs. CTL, control. B-E: quantitative (q)PCR analysis of Kir6.1 and SUR2B mRNA following MGO treatment. B: levels of Kir6.1 mRNA dropped after a 12-h treatment with 300 μM MGO, while with a 1-mM MGO treatment the mRNA levels were barely detectable. Data were fitted by the Hill's equation with IC50 of 350 μM for MGO mediated decrease in Kir6.1 mRNA. C: similar experiments showed a similar decrease in the levels of SUR2B mRNAs with increasing concentration of MGO. IC50 for MGO-mediated SUR2B mRNA decrease was 290 μM. D: MGO-mediated decrease in the levels of Kir6.1 mRNAs was a function of time that was described by a single exponential equation with the time constant τ, 1.15 h. E: SUR2B mRNA levels also decreased in a time-dependent manner in response to MGO with τ, 0.33 h.
Suppression of the Kir6.1/SUR2B currents with MGO treatment.
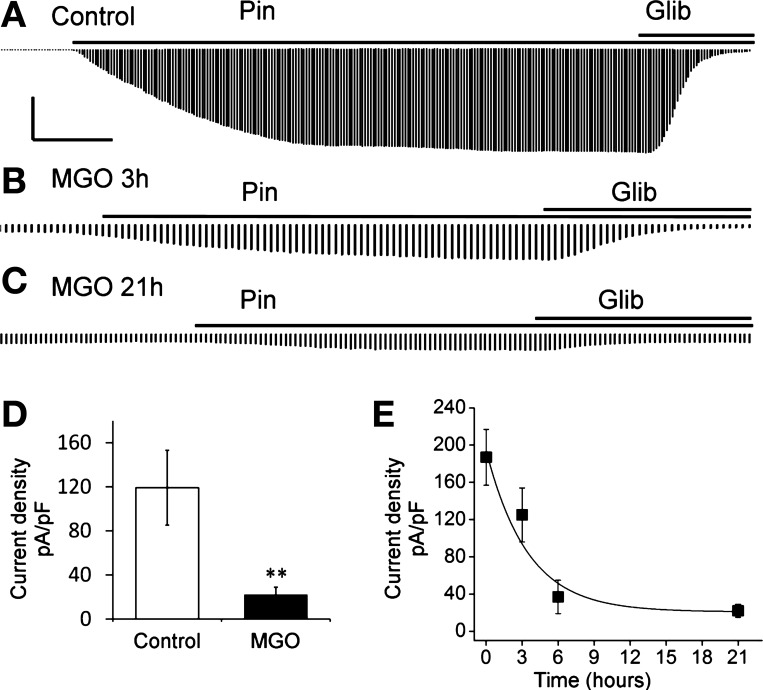
The decrease in the levels of Kir6.1 and SUR2B mRNA should have a functional impact on the vascular KATP channel. To demonstrate this effect, we studied heterologously expressed Kir6.1/SUR2B channel in HEK293 cells, as the resulting KATP currents are relatively large allowing long-term analysis. Whole cell currents were recorded in voltage clamp. Equal concentrations of K+ (145 mM) were applied to both sides of the patch membranes. Standard protocol was used in measuring KATP current: membrane potential of HEK293 cells expressing Kir6.1/SUR2B channel was held at 0 mV and stepped to −80 mV every 3–4 s. Kir6.1 containing KATP channel has low open probability under physiological conditions, showing small basal currents in the HEK293 cells (57). An exposure to 10 μM pinacidil, a specific KATP channel opener, led to the activation of Kir6.1/SUR2B currents that were sequentially suppressed by 10 μM glibenclamide (Glib), a KATP channel inhibitor (Fig. 2A). A significant decrease in the KATP currents occurred after the cells were exposed to 1 mM MGO for ∼3 h (Fig. 2B). After a 21-h treatment with 1 mM MGO, the KATP currents were barely detectable (Fig. 2C). Quantitatively, a 21-h treatment with 1 mM MGO led to a reduction of the Kir6.1/SUR2B current density by ∼90% with a time constant of 3.46 h (Fig. 2, D and E). Therefore, the MGO-mediated suppression in the mRNAs of the Kir6.1/SUR2B channel has functional consequences on the channel activity.
Fig. 2.
Prolonged treatments with MGO led to channel inhibition. A: currents were induced by pinacidil (Pin) from a cell treated with the sham solution overnight. Glib, glibenclamide. B: pinacidil-induced currents became much smaller in another cell treated with 1 mM MGO for 3 h. C: overnight MGO treatment further reduced the pinacidil-induced currents. D: summary of the current density changes in control and MGO treatment (21 h, 1 mM). **P < 0.01. E: currents were recorded from cells treated with 1 mM MGO for different time periods and described as a function of time with a single exponential equation. Current density was calculated by dividing the current amplitude by the whole cell capacitance in each cell. Calculated time constant is 3.46 h. Calibrations are 5 nA/2 min for A, 3 nA/1 min for B, and 3 nA/1.2 min for C.
Transcriptional inhibition vs. mRNA degradation.
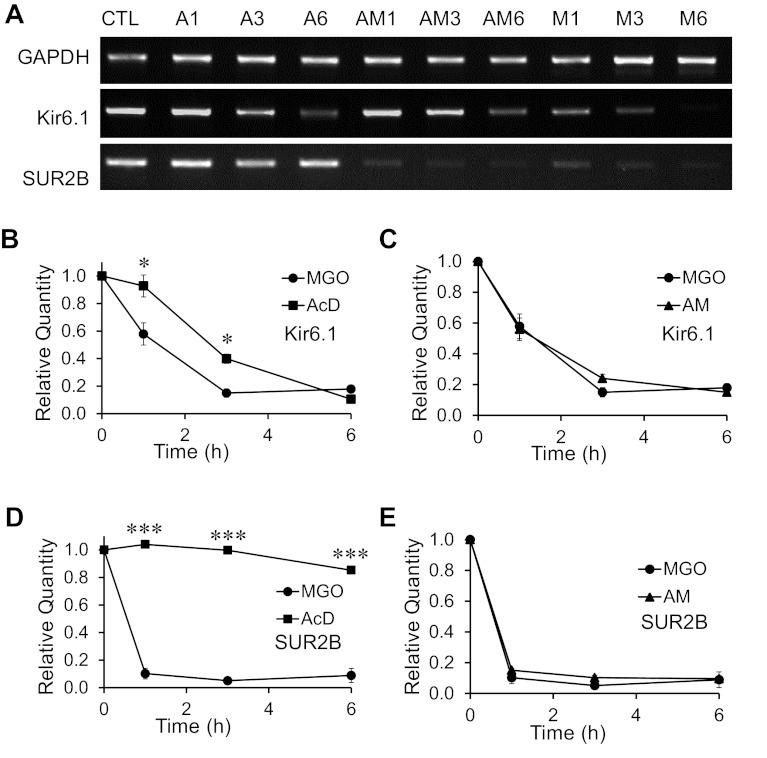
The decrease in the mRNA levels of vascular KATP channels could be a result of transcriptional inhibition, mRNA instability, or both. To test these possibilities, we treated A10 cells for 1–6 h with actinomycin D (AcD; 5 μg/ml), a transcriptional inhibitor. Subsequent PCR analysis showed that the AcD treatment alone without MGO caused a decrease in the mRNA levels of Kir6.1 in a time-dependent manner (Fig. 3A). In contrast, the mRNA levels of SUR2B and GAPDH did not display such a time-dependent reduction in response to the same AcD treatment (Fig. 3A), suggesting that continuous transcription is necessary to maintain the Kir6.1 mRNA level, while SUR2B and GAPDH mRNAs are relatively stable within this time period. When MGO (300 μM) and AcD (5 μg/ml) were applied together, marked decreases in the mRNA levels of both Kir6.1 and SUR2B (but not GAPDH) were clearly seen (Fig. 3A).
Fig. 3.
Transcriptional inhibition vs. mRNA instability. Mechanisms (transcriptional inhibition or mRNA instability) that may underlie the decrease in the levels of Kir6.1 and SUR2B mRNAs in response to prolonged MGO exposure, were studied by treating A10 cells with actinomycin-D (AcD), MGO, or both at different time points. A: gel images of the PCR (reverse transcribed cDNA as template) products of GAPDH, Kir6.1, and SUR2B mRNAs from A10 cells treated with AcD (A), AcD and MGO (AM), and MGO alone (M) for different time periods (1, 3 and 6 h, respectively) denoted as a numerical following each treatment condition. B–E: qPCR analysis of Kir6.1 and SUR2B mRNAs following AcD, MGO and combined treatment of AcD and MGO (AM) at different time points. B and C: comparisons of the time-dependent decrease in the levels of Kir6.1 mRNA in response to AcD, MGO, and AM treatments. D and E: comparisons of the time-dependent decrease in the levels of SUR2B mRNA in response to AcD, MGO, and AM treatments. *P < 0.05; ***P < 0.001 (n = 11 to 23 samples from 3 to 6 experiments).
We further analyzed the mRNA reduction kinetics by qPCR and found that the AcD treatment caused an obvious reduction in the Kir6.1 mRNA levels in 3 and 6 h (Fig. 3B). A more drastic reduction in Kir6.1 mRNA was found with MGO treatment alone. These two effects were not additive, as a joint treatment with AcD and MGO produced a drop in Kir6.1 mRNA to almost the same degree as MGO alone (Fig. 3C). Because the Kir6.1 mRNA was less stable as shown with AcD treatment alone (Fig. 3B), the additional effect of MGO was too small to show evidence for transcription inhibition by MGO.
The AcD treatment caused a marginal decrease in SUR2B mRNA levels over a 6-h period, while MGO almost totally eliminated the SUR2B mRNAs in 1 h (Fig. 3D). A combined treatment of MGO and AcD did not lead to a further reduction in the levels of SUR2B mRNA (Fig. 3E). Therefore, MGO caused a reduction in the levels of SUR2B mRNA as well, likely through mRNA instability.
3′-UTR of the Kir6.1 gene.
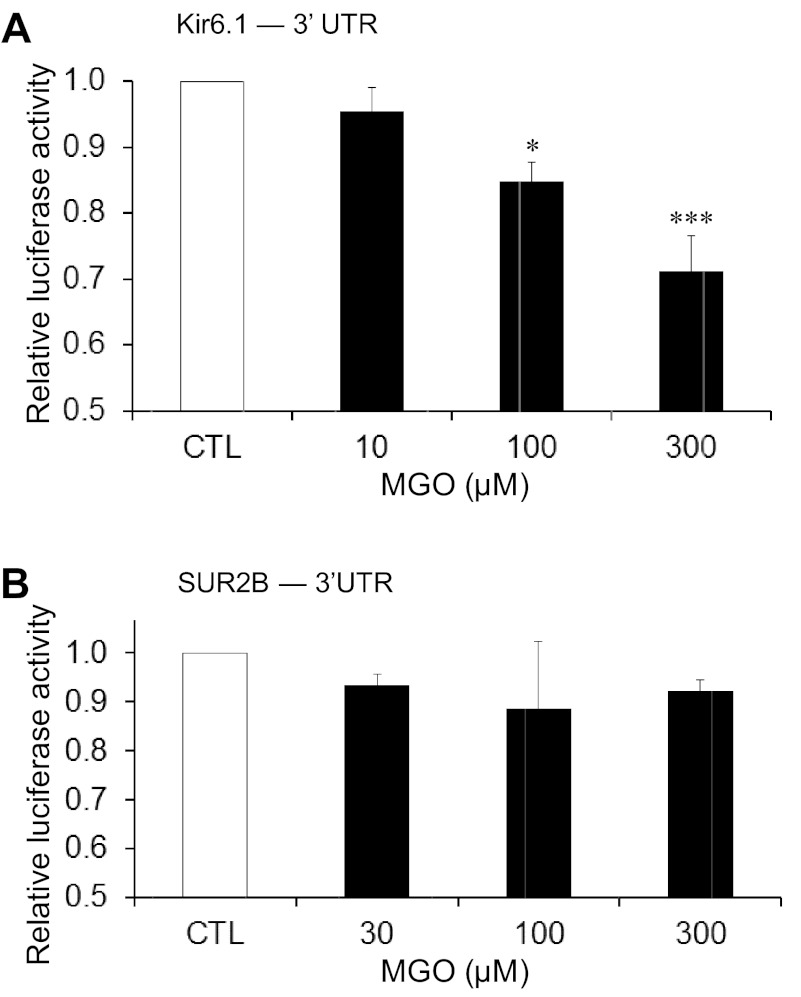
The mRNA stability largely relies on its 3′-UTR sequence (37). Thus we cloned a 1,000- and a 2,600-bp fragment at the 3′-end of Kir6.1 and SUR2 genes, respectively, to a luciferase reporter vector that was then transfected to the A10 smooth muscle cells. These regions cover the suggested 3′-UTR region shown in NCBI genomic database. Presuming that the fragment contained the 3′-UTR, we used the term 3′-UTR in the rest of the statements. The effect of MGO on the luciferase expression was determined after the transfected cells were treated with MGO (10, 100, and 300 μM, respectively) for 5 h. If the 3′-UTR of Kir6.1 was targeted by MGO, the mRNA of firefly luciferase reporter with the 3′-UTR of Kir6.1 should be less stable in the presence of MGO, resulting in a net reduction in the firefly luciferase activity. Renilla luciferase with its own 3′-UTR was designed as an internal control, which supposedly should not be targeted by MGO. Indeed, we found that the renilla luciferase signal did not show any significant changes with or without MGO treatments (P > 0.05; n = 5 for each experiment). In the cells transfected with Kir6.1−3′-UTR containing vector, a concentration-dependent reduction in the firefly luciferase reporter activity was found (Fig. 4A). A treatment with 300 μM MGO led to a decrease in the luciferase activity by 28.8 ± 5.3% (P < 0.001; n = 5). The luciferase activity decreased by 15.2 ± 2.9% (P < 0.05; n = 5) in cells treated with 100 μM MGO. Both of the drops were significantly different from the control (Fig. 4A). A slight and insignificant reduction in luciferase activity (4.5 ± 3.5%; n = 4) was seen in cells treated with 10 μM MGO.
Fig. 4.
Action of MGO on the 3′-untranslated regions (UTRs) of KATP channel mRNAs. A10 cells transfected with Kir6.1–3′-UTR or SUR2B-3′-UTR containing luciferase reporter vector (pmirGLO) were treated with different concentrations of MGO for 5 h, followed by the measurement of the luciferase activity. The ratio of firefly luciferase activity (experimental reporter): renilla luciferase activity (control reporter) was calculated for each well. The well ratio of each sample was normalized to the control well ratio and is represented on the y-axis as the relative luciferase activity in response to different MGO treatments along with the control. A: summary of the effect of different concentrations (10, 100, and 300 μM) of MGO on the luciferase activity of Kir6.1–3′-UTR containing luciferase reporter vector. B: summary of the effect of different concentrations of MGO (30, 100, and 300 μM) on the luciferase activity of SUR2B-3′-UTR containing luciferase reporter vector. *P < 0.05; ***P < 0.001 (n = 5 experiments).
In contrast, the same MGO treatments did not produce any significant decreases in luciferase activity in the cells transfected with the SUR2–3′-UTR containing vector (Fig. 4B). Therefore, it is likely that MGO acts on the 3′-UTR of Kir6.1 mRNA causing its instability, while MGO may affect SUR2B mRNA stability via a different mechanism.
Direct targeting at the SUR2B coding region.
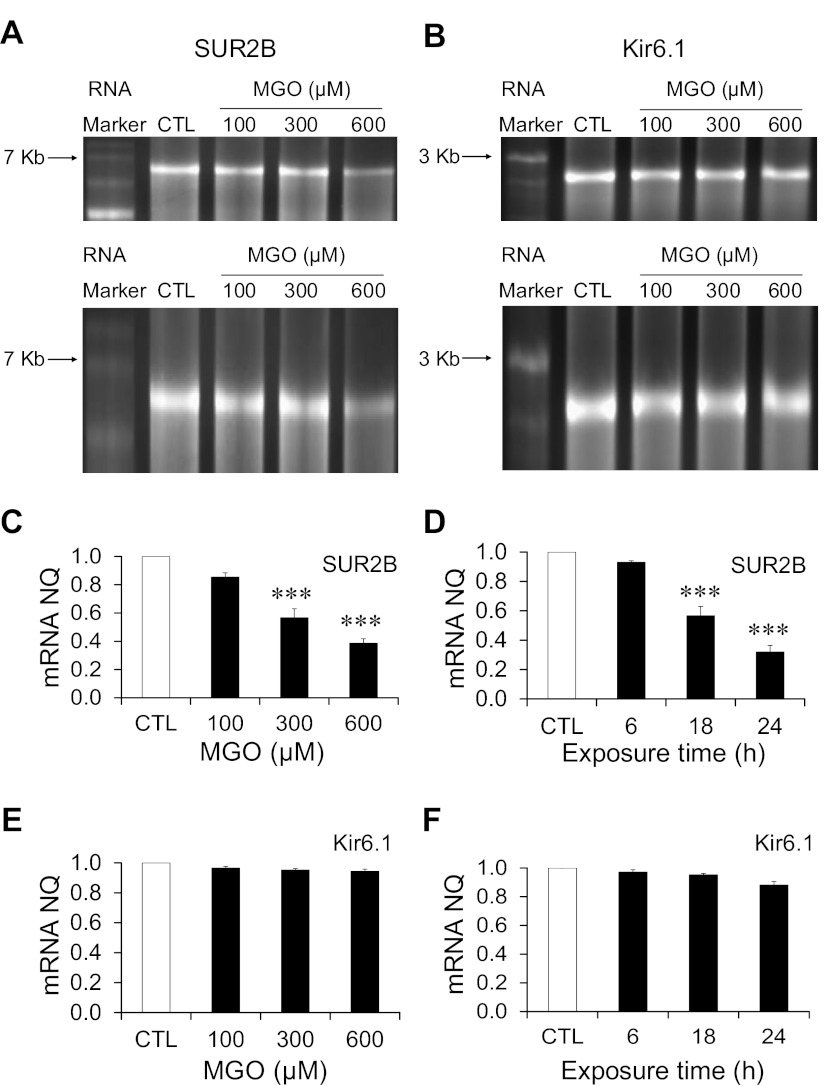
Since MGO has been shown to interact directly with nucleic acids, especially single stranded nucleotides (23), we tested the possibility that the coding sequences of the Kir6.1/SUR2B mRNA may be targeted by MGO, leading to a reduction in the levels of the mRNAs. The Kir6.1 and SUR2B mRNAs were synthesized by in vitro transcription. These mRNAs were then treated with MGO at different concentrations for a given time period. The change in the mRNA levels upon MGO treatment was visualized by gel electrophoresis and quantified. Obvious reduction in the band density of SUR2B mRNA was seen with 300-μM MGO treatments, and the decrease in the SUR2B mRNA band was more prominent with 600-μM MGO treatment (Fig. 5A). The mRNA degradation may also cause a reduction in the size of the RNA. Thus we elongated the image obtained from ultraviolet luminescence in a vertical direction to determine if there is any difference in the migration distance of MGO-treated mRNA samples compared with the control. This manipulation showed that SUR2B mRNA treated with 600 μM MGO migrated a slightly longer distance than that of control mRNA (Fig. 5A, bottom), indicating a decrease in SUR2B mRNA size with the MGO treatment. Therefore, our data suggest that SUR2B mRNA may be degraded upon MGO treatment.
Fig. 5.
Effects of MGO on the coding region of the Kir6.1 and SUR2B mRNAs. Kir6.1 and SUR2B mRNAs were synthesized by in vitro transcription and treated with different concentrations of MGO. A and B, top: gel image of SUR2B and Kir6.1 mRNAs treated with 100, 300, and 600 μM MGO, respectively, along with sham-treated mRNA as control for 18 h. A and B, bottom: vertical elongation images of SUR2B and Kir6.1 gel images from the corresponding top. RNA band sizes on the RNA marker are labeled at left. C: summary of normalized quantity (NQ) of SUR2B mRNA following MGO treatment (100, 300, and 600 μM) for 18 h. D: summary of NQ of SUR2B mRNA treated with 300 μM MGO for different time periods (6, 18 and 24 h). E: summary of NQ of Kir6.1 mRNA following MGO treatment (100, 300, and 600 μM) for 18 h. F: summary of NQ of Kir6.1 mRNA treated with 300 μM MGO for different time points (6, 18, and 24 h). ***P < 0.001 (n = 4 experiments).
The levels of the SUR2B mRNA decrease were further analyzed quantitatively with increasing concentrations of MGO in an 18-h incubation period (Fig. 5C). A 600-μM MGO treatment led to a decrease in the SUR2B mRNA band density by 61.4 ± 3.2% (P < 0.001; n = 4) compared with the control value. Treatment with lower concentrations of MGO produced a smaller, but significant, decrease: 43.4 ± 6.4% (P < 0.001; n = 4) with 300-μM MGO and 14.4 ± 2.9% (n = 4) with 100-μM MGO treatment.
To determine the time dependence for the effect of MGO on SUR2B mRNA levels, the SUR2B mRNA was treated with 300 μM MGO for 6, 18, and 24 h (Fig. 5D). The SUR2B mRNA levels decreased modestly by 6.9 ± 1.0% (n = 4) after a 6-h MGO exposure, while the exposure to MGO for 18 and 24 h caused graded decreases in the mRNA levels by 43.4 ± 6.4% (P < 0.001; n = 4) and 68.0 ± 4.4% (P < 0.001; n = 4), respectively.
Similar experiments were also performed on the in vitro transcribed Kir6.1 mRNA, and no evident decrease in the mRNA levels was observed (Fig. 5, B, E, and F). Taken together, these results suggest that MGO is likely to cause SUR2B mRNA instability by targeting at the coding region.
KATP channel-dependent augmentation of vasoconstriction by MGO.
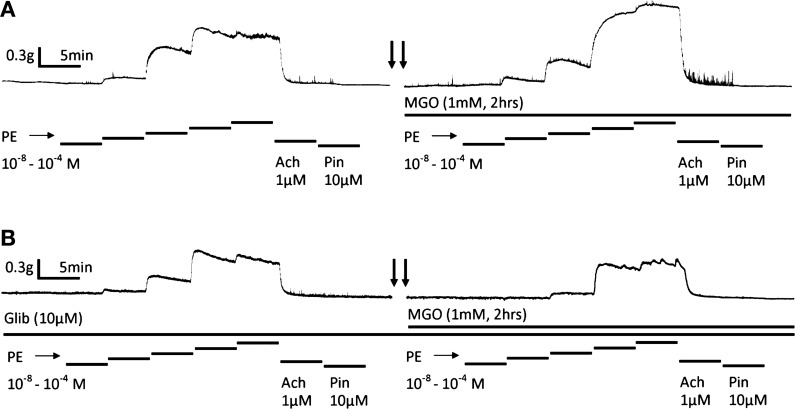
To further understand whether the modulation of vascular KATP channel by MGO could affect vascular tone, vasoconstriction was studied in endothelium-intact mesenteric arterial rings with electricity-force transducers. With a 0.4 g preload, phenylephrine (PE) produced concentration-dependent constrictions in the arterial rings, which was subsequently relaxed by 1 μM acetylcholine and pinacidil (Pin 10 μM; Fig. 6A). After three times of washout, the ring was exposed to 1 mM MGO for 2 hrs. Stronger vasoconstriction was observed with the same concentrations of PE (Figs. 6A and 7A). In the presence of Glib, such an MGO-mediated PE-induced vasoconstriction was completely abolished (Figs. 6B and 7B). These data thus suggest that the presence of functional KATP channels is necessary for MGO to augment the PE-induced vasoconstriction.
Fig. 6.
KATP channel-dependent augmentation of vasoconstriction by MGO. A: vasoconstriction was studied in an endothelium-intact mesenteric arterial ring at 36°C in vitro with an electricity-force transducer. With a preload of 0.4 g, phenylephrine (PE) produced concentration-dependent constrictions. The ring was then relaxed by the vasodilators Ach (1 μM) and pinacidil (10 μM), followed by three times of washout. Such vasoconstriction was markedly increased after the ring was exposed to 1 mM MGO for 2 hr. Arrows indicate a 2-h interval. B: same experiment was done in another mesenteric ring in the presence of Glib (10 μM). Under this condition, the MGO-mediated augmentation of PE-induced vasoconstriction was eliminated.
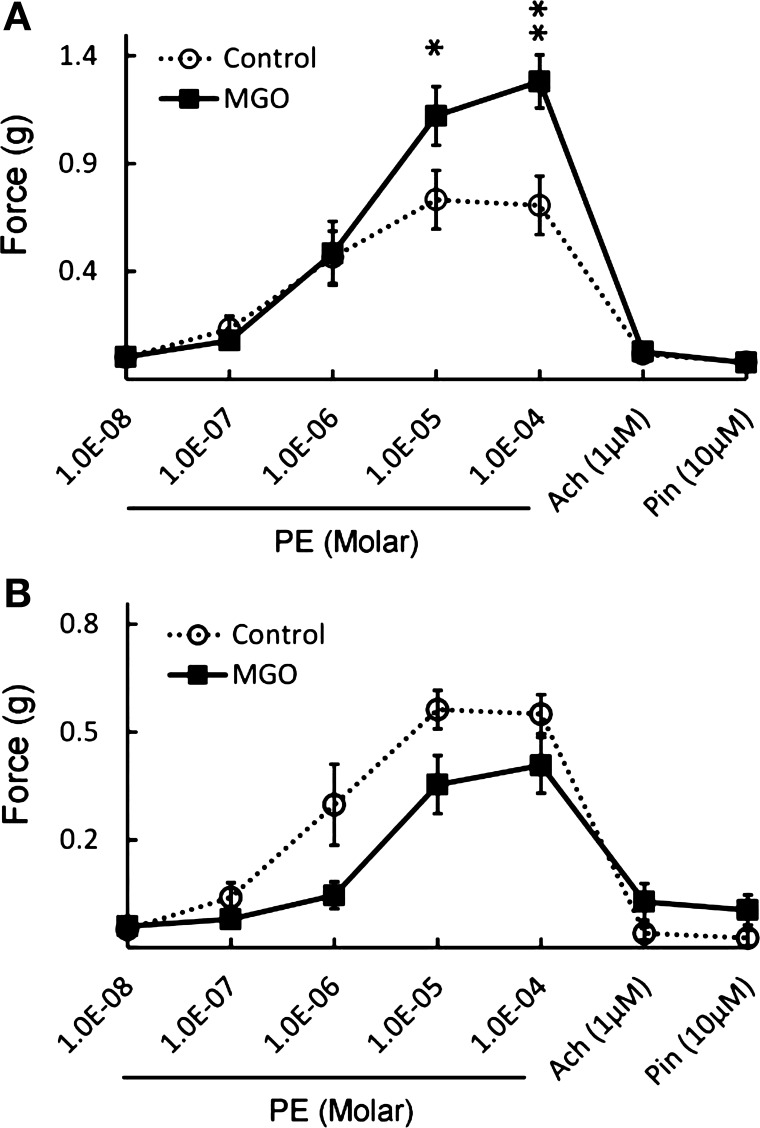
Fig. 7.
Summary of the KATP channel-dependent augmentation of vasoconstriction by MGO. A: exposure to 1 mM MGO for 2 h led to augmentation of PE-induced vasoconstriction by 30–40% at the peak levels (*P < 0.05; **P < 0.01). B: such an effect disappeared after a pretreatment of the rings with 10 μM Glib. Under this condition, 1 mM MGO resulted in weaker PE-induced vasoconstriction instead. Data were obtained from 6 rings in each group in A and B.
DISCUSSION
In the present study, we have found evidence for the disruption of vascular KATP channels with an MGO exposure and shown the underlying mechanisms. MGO treatment causes a major reduction in the levels of Kir6.1 and SUR2B mRNAs and impairs activity of the KATP channels. Remarkably, MGO appears to act on the 3′-UTR of the Kir6.1 gene and the coding region of the SUR2B gene, leading to mRNA instability and a loss of functional KATP channels in plasma membranes.
Emerging evidence suggests that RCS including MGO play a major role in the development of diabetic complications in multiple organs and systems (5). Like ROS, RCS are highly reactive and can modify proteins, DNAs, and lipids via Maillard covalent links, producing advanced glycation end products (AGEs; Ref. 47). AGEs can cause direct damages to intracellular cellular structures and act on extracellular AGE receptors, producing more reactive species in the cytoplasm through intracellular signaling systems (8). When the cellular mechanisms for the detoxification of the RCS are overwhelmed by excessive RCS production, the so-called carbonyl stress takes place. The carbonyl stress has been suggested playing even more important role than oxidative stress in the development of diabetic vascular complications (15, 17, 50).
Although the production of RCS is normally controlled by antioxidants, scavengers, and their degrading enzymes, there are circumstances when key molecules for the maintenance of homeostatic states become defective or dysfunctional, allowing these reactive species to be overly produced. Membrane potential appears to be one of such mechanisms (26). Depolarization can open the voltage-activated Ca2+ channels leading to Ca2+ influx (2, 52). Because K+ channels are the primary regulators of membrane potentials, inhibition of the K+ channels results in depolarization and an elevation in intracellular Ca2+ (12). Since several NADPH oxidases for ROS production are stimulated by elevated intracellular Ca2+ (6, 18, 20, 61), and since RCS and ROS share the antioxidant detoxification systems, sustained depolarization may augment the production of these reactive species and aggravate oxidative/carbonyl stress.
The adverse effects of MGO on the vasculature have been studied previously. Most of these studies, however, are focused on the molecules of endothelium cells. For example, MGO causes impairment of endothelium-dependent vasorelaxation of rat mesenteric arteries in hyperglycemic conditions by causing dysfunction of the endothelium (7). MGO stimulates the expression of angiopoietin-2 protein through the covalent modification of the transcriptional repressor mSin3A, causing endothelial cell death in mice (58). Recently, the effect of MGO on VSM contraction has been reported. Acting on unidentified targets, MGO impairs norepinephrine and KCl-induced smooth muscle contractions (31). Our studies indicate that MGO suppresses K+ channel activity in VSM cells. This effect, however, is not mediated by the well-known modulation of the lysine and arginine residues of the channel protein. Rather, MGO affects KATP channels by acting on the mRNAs of Kir6.1 and SUR2B.
Previous studies (48) suggest that MGO can react with the NH2 groups present on the guanine nucleotides to form tricyclic compounds. Because the double-stranded structure of DNAs protects the guanine nucleotides from the electrophilic attack (35, 38), MGO preferentially reacts with single stranded nucleotides, including mRNA and denatured DNA (23). Therefore, our data are consistent with the existing literature regarding the action of MGO on mRNAs. Remarkably, we have found that two completely different mechanisms are in action in the process. MGO causes the instability of KATP channel mRNAs by targeting at the 3′-UTR of Kir6.1 gene but not that of SUR2B. On the other hand, the coding region of SUR2B but not Kir6.1 transcripts may be targeted by MGO. The mechanism for the mRNA instability is still unclear. We speculate MGO exposure might cause cell stress and trigger the expression of microRNAs. Depending on the targeting sites, these microRNAs might act on the 3′-UTR of the Kir6.1 mRNA. It is worth noting that these two distinct mechanisms revealed by different experiments serve mutually as negative controls and demonstrate the specificity of the MGO modification on Kir6.1 and SUR2B, respectively. It is also worth noting that our data did not rule out the possibility that MGO may direct modulate KATP channel protein, which warrants further investigation.
Although MGO can activate the AGE receptor-NF-κB signaling pathway, we believe that this pathway may not be the underlying mechanism for KATP channel inhibition by MGO for two reasons: 1) our in vitro assay, in which all the signaling components are missing, shows that MGO can cause mRNA degradation, indicating a direct action rather than an indirect action via signaling pathways; and 2) in our previous studies (40), we have shown that by activating the NF-κB signaling pathway, lipopolysaccharides augment, rather than suppress, the expression of vascular KATP channel mRNAs (40).
Previous studies have shown that MGO levels are ∼300 μM when measured in cultured mammalian cells (10) and the plasma MGO levels is in the order of tens of micromolar range in healthy rats (51). Several orders of elevation in the MGO levels are expected to take place in patients with persistent hyperglycemia (9). Indeed, it is reported that MGO concentration is ∼400 μM in diabetic patients with poorly controlled hyperglycemia (24, 32). The MGO levels may be even higher in local tissues including the vasculature before it enters the circulation. Our results have shown that the IC50 levels of MGO for a suppression of Kir6.1 and SUR2B mRNAs are ∼300 μM, a level that is likely to occur in diabetes. Therefore, our findings are relevant to the conditions seen in diabetic patients.
The dysfunction of VSM is remarkable as the VSM cells regulate vascular tones. The disruption of the key proteins in the VSM cells for vascular tone regulation tends to impair not only membrane potentials and cellular contractility but also the VSM responses to circulating hormones, neurotransmitters, as well as the local factors released by endothelial cells. Consistently, our results have shown that the constriction of mesenteric arterial rings is augmented with the exposed to 1 mM MGO for 2 h, and such an MGO effect was totally eliminated in the presence of a KATP channel specific inhibitor Glib, indicating the functional impact of the vascular KATP disruption by MGO.
Oxidative stress and carbonyl stress have an intimate relationship. Firstly, previous studies have shown that the elevated levels of MGO can lead to oxidative stress by inactivation of antioxidant enzymes like superoxide dismutase (16) and glyoxalase (45). Secondly, MGO can cause mitochondrial dysfunction, leading to uncontrolled production of ROS in VSMs (49), which may play a role in the progression of diabetic vascular complications. Thirdly, MGO can promote oxidative stress through AGE receptor (8) and the NF-κB signaling pathway to produce ROS (53). Moreover, several antioxidant detoxification systems are shared by RCS and ROS. An overproduction of one of the reactive species can compromise the detoxification of the other.
Because of the intertwining relationship of ROS and RCS, it is necessary to elucidate whether the cellular dysfunction seen in MGO treatment is produced by MGO itself or mediated via the associated oxidative stress, as both are seen in diabetes mellitus. Our current studies support that the mRNAs of Kir6.1 and SUR2B are directly targeted by MGO, while our previous studies (55, 56) have shown that vascular KATP channel protein is modulated in oxidative stress conditions through the posttranslational regulation S-glutathionylation. The present studies indicate that MGO has a unique effect on the vascular KATP channels, although its adverse consequence on channel inhibition is similar to that of oxidative stress. Therefore, carbonyl stress and oxidative stress seem to work synergistically on the vascular KATP channels. It is likely that the vascular KATP channels are disrupted by oxidative/carbonyl stress at both protein and mRNA levels, resulting in abnormalities in VSM contractility, dysfunction in vascular responses to circulating/local vasoactive regulators, and exacerbation of oxidative/carbonyl stress.
GRANTS
This study is supported by the National Institutes of Health Grants 1R21-HD-060959 and 1R01-NS-073875 and American Heart Association Grant 09GRNT2010037.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.Y. and C.J. conception and design of research; Y.Y., S.L., A.S.K., S.Z., T.C.T., W.S., N.C., L.Y., and Y.W. performed experiments; Y.Y., S.L., A.S.K., S.Z., T.C.T., W.S., N.C., L.Y., Y.W., and C.J. analyzed data; Y.Y., D.Z., and C.J. interpreted results of experiments; Y.Y., S.L., A.S.K., and C.J. prepared figures; Y.Y. drafted manuscript; Y.Y., A.S.K., and C.J. edited and revised manuscript; Y.Y., S.L., A.S.K., S.Z., T.C.T., W.S., N.C., L.Y., Y.W., D.Z., and C.J. approved final version of manuscript.
ACKNOWLEDGMENTS
Y. Yang was a Brains and Behavior Fellow of Georgia State University. We thank Dr. Jason Matthews for help and suggestions for RNA assay.
Present addresses: Y. Yang: Dept. of Neurology, Yale University School of Medicine, New Haven, CT 06510; W. Shi: Dept. of Cardiothoracic Surgery, Carlyle Fraser Heart Center, Emory University Hospital Midtown, Emory University School of Medicine, Atlanta, GA 30308.
REFERENCES
- 1. Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International 11: 36–42, 2004 [Google Scholar]
- 2. Anzai K, Ogawa K, Ozawa T, Yamamoto H. Oxidative modification of ion channel activity of ryanodine receptor. Antioxid Redox Signal 2: 35–40, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol 108: 315–323, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bourajjaj M, Stehouwer CD, van Hinsbergh VW, Schalkwijk CG. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem Soc Trans 31: 1400–1402, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med 49: 687–706, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia 53: 989–1000, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Chang T, Wu L. Methylglyoxal, oxidative stress, hypertension. Can J Physiol Pharmacol 84: 1229–1238, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Chaplen FW, Fahl WE, Cameron DC. Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci USA 95: 5533–5538, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest 110: 203–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook DL, Satin LS, Ashford ML, Hales CN. ATP-sensitive K+ channels in pancreatic beta-cells. Spare-channel hypothesis. Diabetes 37: 495–498, 1988 [DOI] [PubMed] [Google Scholar]
- 13. Croker B, Crozat K, Berger M, Xia Y, Sovath S, Schaffer L, Eleftherianos I, Imler JL, Beutler B. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nat Genet 39: 1453–1460, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Department of Health and Human Services National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States 2011. Atlanta, GA: Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 15. Desai KM, Chang T, Wang H, Banigesh A, Dhar A, Liu J, Untereiner A, Wu L. Oxidative stress and aging: is methylglyoxal the hidden enemy? Can J Physiol Pharmacol 88: 273–284, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Desai KM, Wu L. Free radical generation by methylglyoxal in tissues. Drug Metabol Drug Interact 23: 151–173, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Ellis EM. Reactive carbonyls and oxidative stress: potential for therapeutic intervention. Pharmacol Ther 115: 13–24, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han Y, Randell E, Vasdev S, Gill V, Gadag V, Newhook LA, Grant M, Hagerty D. Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes. Mol Cell Biochem 305: 123–131, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–22, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kane GC, Lam CF, O'Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knockout of the KCNJ8-encoded Kir6.1 K(ATP) channel imparts fatal susceptibility to endotoxemia. FASEB J 20: 2271–2280, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res 44: 65–81, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Krymkiewicz N. Reactions of methylglyoxal with nucleic acids. FEBS Lett 29: 51–54, 1973 [DOI] [PubMed] [Google Scholar]
- 24. Lapolla A, Flamini R, Dalla Vedova A, Senesi A, Reitano R, Fedele D, Basso E, Seraglia R, Traldi P. Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med 41: 1166–1173, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol 38: 43–49, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol 29: 305–311, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med 8: 466–472, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 92: 151–158, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Miyoshi H, Nakaya Y. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Res Cardiol 90: 332–336, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Mukohda M, Morita T, Okada M, Hara Y, Yamawaki H. Long-term methylglyoxal treatment impairs smooth muscle contractility in organ-cultured rat mesenteric artery. Pharmacol Res 65: 91–99, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Mukohda M, Yamawaki H, Okada M, Hara Y. Methylglyoxal enhances sodium nitroprusside-induced relaxation in rat aorta. J Pharm Sci 112: 176–183, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Ogawa S, Nakayama K, Nakayama M, Mori T, Matsushima M, Okamura M, Senda M, Nako K, Miyata T, Ito S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 56: 471–476, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Papoulis A, al-Abed Y, Bucala R. Identification of N2-(1-carboxyethyl)guanine (CEG) as a guanine advanced glycosylation end product. Biochemistry 34: 648–655, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 77: 1165–1232, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Ross J. mRNA stability in mammalian cells. Microbiol Rev 59: 423–450, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schneider M, Quistad GB, Casida JE. N2,7-bis(1-hydroxy-2-oxopropyl)-2'-deoxyguanosine: identical noncyclic adducts with 1,3-dichloropropene epoxides and methylglyoxal. Chem Res Toxicol 11: 1536–1542, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol 293: R191–R199, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Shi W, Cui N, Wu Z, Yang Y, Zhang S, Gai H, Zhu D, Jiang C. Lipopolysaccharides up-regulate Kir6.1/SUR2B channel expression and enhance vascular KATP channel activity via NF-kappaB-dependent signaling. J Biol Chem 285: 3021–3029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shi Y, Chen X, Wu Z, Shi W, Yang Y, Cui N, Jiang C, Harrison RW. cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J Biol Chem 283: 7523–7530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shi Y, Cui N, Shi W, Jiang C. A short motif in Kir6.1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J Biol Chem 283: 2488–2494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Rojas A, Ha BT, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by β-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol 293: R1205–R1214, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun X, Cao K, Yang G, Huang Y, Hanna ST, Wang R. Selective expression of Kir6.1 protein in different vascular and non-vascular tissues. Biochem Pharmacol 67: 147–156, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Thornalley PJ. Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans 31: 1343–1348, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Thornalley PJ. Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem J 254: 751–755, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344: 109–116, 1999 [PMC free article] [PubMed] [Google Scholar]
- 48. Vaca CE, Fang JL, Conradi M, Hou SM. Development of a 32P-postlabelling method for the analysis of 2′-deoxyguanosine-3′-monophosphate and DNA adducts of methylglyoxal. Carcinogenesis 15: 1887–1894, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Wang H, Liu J, Wu L. Methylglyoxal-induced mitochondrial dysfunction in vascular smooth muscle cells. Biochem Pharmacol 77: 1709–1716, 2009 [DOI] [PubMed] [Google Scholar]
- 50. Wang X, Desai K, Chang T, Wu L. Vascular methylglyoxal metabolism and the development of hypertension. J Hypertens 23: 1565–1573, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Wang X, Desai K, Clausen JT, Wu L. Increased methylglyoxal and advanced glycation end products in kidney from spontaneously hypertensive rats. Kidney Int 66: 2315–2321, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium 38: 397–407, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Wu L, Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension 39: 809–814, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Yamawaki H, Saito K, Okada M, Hara Y. Methylglyoxal mediates vascular inflammation via JNK and p38 in human endothelial cells. Am J Physiol Cell Physiol 295: C1510–C1517, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, Trower TC, Zhang S, Jiang C. Molecular basis and structural insight of vascular K(ATP) channel gating by S-glutathionylation. J Biol Chem 286: 9298–9307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem 285: 38641–38648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, Zhu D, Jiang C. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta 1778: 88–96, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem 282: 31038–31045, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol Cell Physiol 274: C25–C37, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Yu L, Jin X, Yang Y, Cui N, Jiang C. Rosiglitazone inhibits vascular K(ATP) channels and coronary vasodilation produced by isoproterenol. Br J Pharmacol, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang F, Jin S, Yi F, Xia M, Dewey WL, Li PL. Local production of O2- by NAD(P)H oxidase in the sarcoplasmic reticulum of coronary arterial myocytes: cADPR-mediated Ca2+ regulation. Cell Signal 20: 637–644, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu G, Zhang Y, Xu H, Jiang C. Identification of endogenous outward currents in the human embryonic kidney (HEK 293) cell line. J Neurosci Methods 81: 73–83, 1998 [DOI] [PubMed] [Google Scholar]