Abstract
The large conductance voltage- and Ca2+-activated K+ (BK) channel is a major regulator of detrusor smooth muscle (DSM) excitability and contractility. Recently, we showed that nonselective phosphodiesterase (PDE) inhibition reduces guinea pig DSM excitability and contractility by increasing BK channel activity. Here, we investigated how DSM excitability and contractility changes upon selective inhibition of PDE type 1 (PDE1) and the underlying cellular mechanism involving ryanodine receptors (RyRs) and BK channels. PDE1 inhibition with 8-methoxymethyl-3-isobutyl-1-methylxanthine (8MM-IBMX; 10 μM) increased the cAMP levels in guinea pig DSM cells. Patch-clamp experiments on freshly isolated DSM cells showed that 8MM-IBMX increased transient BK currents and the spontaneous transient hyperpolarization (STH) frequency by ∼2.5- and ∼1.8-fold, respectively. 8MM-IBMX hyperpolarized guinea pig and human DSM cell membrane potential and significantly decreased the intracellular Ca2+ levels in guinea pig DSM cells. Blocking BK channels with 1 μM paxilline or inhibiting RyRs with 30 μM ryanodine abolished the STHs and the 8MM-IBMX inhibitory effects on the DSM cell membrane potential. Isometric DSM tension recordings showed that 8MM-IBMX significantly reduced the spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and tone of DSM isolated strips. The electrical field stimulation-induced DSM contraction amplitude, muscle force integral, and duration were also attenuated by 10 μM 8MM-IBMX. Blocking BK channels with paxilline abolished the 8MM-IBMX effects on DSM contractions. Our data provide evidence that PDE1 inhibition relaxes DSM by raising cellular cAMP levels and subsequently stimulates RyRs, which leads to BK channel activation, membrane potential hyperpolarization, and decrease in intracellular Ca2+ levels.
Keywords: contractility, patch-clamp, 8MM-IBMX, paxilline, ryanodine
detrusor smooth muscle (DSM) myogenic activity is characterized by spontaneous phasic contractions that are dependent on Ca2+ entry through L-type voltage-gated Ca2+ (CaV) channels (6, 19, 39, 41). DSM contractility is also regulated by autonomic neuronal pathways (3). Alterations in myogenic or neurogenic DSM mechanisms may lead to involuntary spontaneous contractions during the bladder filling phase of urodynamic testing. This is termed detrusor overactivity (DO) and is often clinically associated with urinary frequency, urgency, incontinence, and the clincal syndrome of overactive bladder (OAB; Refs. 1, 3, 46).
The large conductance voltage- and Ca2+-activated K+ (BK) channel is a key regulator of DSM cell membrane potential and thereby the Ca2+ entry through CaV channels, thus controlling DSM excitability and contractility (7, 9, 27, 39, 41, 56). BK channel pharmacological inhibition increases DSM myogenic phasic contractions (7, 11, 26–28, 36, 47, 56). Impaired BK channel activity contributes to DO in patients with benign prostatic hyperplasia and patients with some types of neurogenic bladder dysfunction (8, 25, 36). Direct or indirect pharmacological activation of BK channels reduces DSM excitability and contractility (8, 27, 28, 36, 41, 42, 47, 56). Thus pharmacological modulation of BK channel activity can be a novel approach to treat urinary bladder dysfunctions, such as DO or urinary retention (39).
In DSM, BK channels are transiently activated by localized Ca2+ releases from the ryanodine receptors (RyRs), known as “Ca2+ sparks,” which generate transient BK currents (TBKCs; Refs. 23, 27, 41). A macromolecular complex, consisting of RyRs and protein kinase-A (PKA), regulates Ca2+ sparks (4, 10). The activation of the cAMP/PKA pathway stimulates RyRs to release Ca2+ from the sarcoplasmic reticulum resulting in an increase in BK channel activity and hyperpolarization of the cell membrane potential (7, 27, 41, 56). DSM cells from mice with knockout type 2 RyR have reduced Ca2+ sparks and TBKC activity (24). Inhibition of RyRs abolished DSM cell membrane potential hyperpolarization induced by stimulation of the β3-adrenergic receptor–cAMP/PKA signaling pathway (7, 27).
cAMP is hydrolyzed by phosphodiesterases (PDEs), a group of key enzymes controlling intracellular cAMP levels. Pharmacological inhibition of PDEs results in a substantial increase in the cAMP levels and activation of PKA (5). Our recent study (56) demonstrated that the nonselective PDE inhibition by IBMX leads to guinea pig DSM relaxation via BK channel activation and cell membrane potential hyperpolarization. This suggests that PDE inhibition remains a novel pharmacotherapy for OAB associated with DO. Indeed, several PDE5 inhibitors have already been utilized to treat lower urinary tract symptoms (15, 54). However, information on the regulation of BK channels with respect to specific PDE isoenzyme inhibition is lacking. The next critical step is to identify the DSM-specific PDE isoenzymes involved in BK channel regulation.
In the human bladder, the level of PDE1 mRNA is the second highest among all PDEs following PDE5 (31). PDE1 is the only isoenzyme of all 11 PDE superfamily members that is activated by Ca2+/calmodulin and all PDE1 isoforms are predominately located in the cell cytosol (5). Previous studies (43, 52, 53) demonstrated that PDE1 inhibition with vinpocetine relaxes carbachol precontracted rabbit, porcine, and human DSM isolated strips. The relaxation effect of vinpocetine is mediated mainly by the cAMP pathway even though PDE1 hydrolyzes both cAMP and cGMP (43, 52, 53). Clinical studies (50, 51) provided data suggesting that PDE1 could be a promising therapeutic target for OAB treatment. However, the mechanism underlying the regulation of DSM excitability and contractility by PDE1 inhibition is unknown. A clear understanding of the cellular mechanisms is essential for developing strategies for targeting PDE1 to treat OAB/DO.
In the current study, we used the highly selective PDE1 inhibitor 8-methoxymethyl-3-isobutyl-1-methylxanthine (8MM-IBMX; 10 μM; Ref. 16). The IC50 values of 8MM-IBMX for PDE1 isoforms are lower than 12 μM, whereas the IC50 values for other major human cAMP-PDEs are >130 μM (45). Here, for the first time, we investigated the functional role of selective PDE1 inhibition in the regulation of guinea pig and human DSM excitability and guinea pig DSM contractility, and we further explored the signaling pathways involving BK channel activation. We employed a multidisciplinary approach, including a cAMP enzyme immunoassay, perforated whole cell patch-clamp, and live-cell Ca2+-imaging techniques on freshly isolated DSM cells, as well as isometric DSM tension recordings on isolated DSM strips.
MATERIALS AND METHODS
DSM tissue collection.
A total of 41 male Hartley Albino guinea pigs (Charles River Laboratory, Raleigh, NC) with an average weight of 439.6 ± 13.1 g were used in this study. Guinea pigs were euthanized with CO2 inhalation followed by thoracotomy according to the animal use protocol number 1747 reviewed and approved by the Institutional Animal Care and Use Committee of the University of South Carolina. Human DSM tissue specimens were obtained from routine open bladder surgeries for bladder cancer according to protocol HR 16918, which was reviewed and approved by the Medical University of South Carolina (MUSC) Institutional Review Board. All specimens were obtained from patients without a preoperative history of OAB. We used four male and one female patients, (4 Caucasian and 1 African-American), 46 to 78 yr of age (mean age: 62.4 ± 5.2 yr). DSM strips (∼2- to 4-mm wide and ∼5- to 8-mm long) were prepared by removing the mucosa and were used for single DSM cell isolation, isometric DSM tension recordings, and cAMP enzyme immunoassay.
DSM single cell isolation.
DSM single cells were freshly isolated as previously described (2, 26, 28, 38, 56). Briefly, one to two DSM strips were incubated at 37°C for 12–18 min in 2 ml dissection solution (DS) supplemented with 1 mg/ml BSA, 1 mg/ml papain, and 1 mg/ml dl-dithiothreitol. The DSM tissues were then transferred to 2 ml DS supplemented with 1 mg/ml BSA, 0.5 mg/ml type II collagenase, 0.5 mg/ml trypsin inhibitor, and 100 μM CaCl2 and incubated at 37°C for 12–15 min for guinea pig DSM or 20–25 min for human DSM, followed by three washouts with DS supplemented with 1 mg/ml BSA. The DSM tissues were then gently triturated with a fire-blunted Pasteur pipette to disperse single DSM cells, which were used for patch-clamp and Ca2+ imaging experiments.
cAMP measurement in DSM isolated strips.
Total cAMP levels in DSM strips were measured using cAMP enzyme immunoassay (cAMP enzyme immunoassay kit, Cayman Chemical) following the manufacturer's instructions. Briefly, the experiments were conducted on DSM isolated strips frozen immediately in liquid nitrogen after isometric DSM tension recordings. The frozen strips were ground to a fine powder in liquid nitrogen and homogenized in cold 5% trichloroacetic-acid in a glass-Teflon tissue grinder (10 ml/mg-tissue) and centrifuged at ≥600 g for 10 min. The supernatants were extracted with 3 vol of water-saturated ether and dried. The reconstituted samples were run directly in the assay. The nontreated DSM strips were used as negative controls and the strips treated with 10 μM IBMX as positive controls. The total cAMP levels were expressed as picomoles per milligrams of DSM tissue. The ELX808 Ultra Microplate Reader (BioTek, Winooski, VT) was used to read the plates.
Electrophysiological recordings.
The amphotericin-B perforated whole cell patch-clamp technique was used for electrophysiological recordings from freshly isolated DSM single cells as previously described (2, 7, 26–28, 38, 56). Briefly, a few drops of the DSM cell suspension were placed into a recording chamber and the cells were allowed to adhere to the glass bottom for ∼20 min. Patch-clamp recordings were conducted using an Axopatch 200B amplifier controlled by pCLAMP 10.2 software and Digidata 1440A (all from Molecular Devices, Union City, CA). The currents were filtered using an eight-pole Bessel filter model 900CT/9L8L (Frequency Devices, Ottawa, IL). The patch-clamp pipettes were made from borosilicate glass (Sutter Instruments, Novato, CA) and pulled using a Narishige PP-830 vertical puller (Narishige Group, Tokyo, Japan). The pipettes were polished with a Micro Forge MF-830 fire polisher (Narishige Group). Pipette resistance was 4 to 6 MΩ. DSM cell resting membrane potential was recorded using the current-clamp mode of the patch-clamp technique (Ih = 0). The cell membrane potential was measured as the average of the last 5-min recording under each experimental condition. All patch-clamp experiments were conducted at room temperature (22–23 °C).
Ca2+ imaging in freshly isolated DSM cells.
The intracellular Ca2+ levels were monitored using a ratiometric fluorescent calcium probe fura-2 AM as previously described (28). Briefly, a suspension of freshly isolated DSM cells was added into a 35-mm glass bottom dish coated with poly-l-lysine and incubated for 30 min at room temperature to allow cells to adhere to the coverslip and then the supernatant was removed. The extracellular solution (250 μl; see Solutions and drugs) containing 2 μM fura 2-AM was added into the dish and stored in the dark for 30 min. The fura 2-AM solution was then removed and cells were washed three times with extracellular solution. DSM cells were imaged with an OLYMPUS IX81 motorized inverted research microscope equipped with a ×40 oil objective and MetaFluor 7.7.2.0 software (Molecular Devices). The fura-2 fluorescence intensity ratio was determined with 200-ms exposure at 340 and 380 nm, and the ratio of the emission intensities was calculated at 510 nm every 3 s. All Ca2+-imaging experiments were carried out at room temperature (22–23°C).
Isometric DSM tension recordings.
Isometric contractions of DSM isolated strips were measured as previously described (2, 38, 56). In brief, DSM strips were secured to isometric force-displacement transducers and were suspended in temperature-controlled (37°C) water-jacketed tissue baths containing 10 ml physiological saline solution (PSS) aerated with 95% O2-5% CO2 at pH 7.4. The DSM strips were initially stretched to 1 g of tension and washed with fresh PSS every 15 min during an equilibration period of 45–60 min. To minimize the potential effects of neurotransmitters released from neurons in the DSM tissue, the spontaneous phasic contractions were recorded in the presence of 1 μM tetrodotoxin, a selective blocker of the neuronal Na+ channels.
Nerve-evoked contractions were induced by electrical field stimulation (EFS) using a pair of platinum electrodes mounted in the tissue baths parallel to the DSM strips. The EFS pulses were generated using a PHM-152I stimulator (MED Associates, St. Albans, VT). The EFS pulse parameters were as follows: 0.75-ms pulse width, 20-V pulse amplitude, and 3-s stimulus duration. Polarity was reversed for alternating pulses. After the equilibration period, the DSM strips were subjected to continuous repetitive EFS with a frequency of 20 Hz at 1-min intervals. The DSM contractions were recorded using a Myomed myograph system (MED Associates).
Data analysis and statistics.
The TBKC and STH frequency and amplitude were analyzed using MiniAnalysis (Synaptosoft, Decatur, GA). The data were further analyzed with GraphPad Prism 4.03 software (GraphPad Software, La Jolla, CA). The cell membrane potential was analyzed using Clampfit 10.2 (Molecular Devices, Union City, CA). The parameters of the DSM phasic contractions (phasic contraction amplitude, muscle force integral, contraction duration, contraction frequency, and muscle tone) were also analyzed using MiniAnalysis. The phasic contraction parameters for spontaneous and 20-Hz EFS-induced contractions were normalized to the control (taken to be 100%) and expressed as percentages. Net muscle force (muscle force integral) was determined by integrating the area under the phasic contraction force-time baseline curve. The relative change of the muscle tone was determined by measuring changes of the phasic contraction baseline curve. Data were expressed as means ± SE; n = the number of cells or strips, and N = the number of guinea pigs or patients, respectively. Statistical significance was performed using two-way ANOVA followed by Bonferroni's posttest or paired Student's t-test, and P < 0.05 was considered significant.
Solutions and drugs.
The nominally Ca2+-free DS contained the following (in mM): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2, pH 7.3, adjusted with NaOH. The extracellular solution for whole cell patch-clamp and Ca2+-imaging experiments contained the following (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH adjusted to 7.4 with NaOH. The patch pipette solution contained the following (in mM): 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 10 HEPES, and 0.05 EGTA, pH adjusted to 7.2 with NaOH and supplemented with freshly dissolved (every 1–2 h) 200 μg/ml amphotericin-B. The Ca2+-containing PSS was prepared daily and contained the following (in mM): 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose, and was aerated with 95% O2-5% CO2 to obtain pH 7.4. Trypsin inhibitor, BSA, and amphotericin-B were obtained from Thermo Fisher Scientific (Fair Lawn, NJ). Papain was purchased from Worthington Biochemical (Lakewood, NJ). Trichloroacetic-acid was purchased from RICCA Chemical (Arlington, TX). Paxilline, fura 2-AM, collagenase (type II), and tetrodotoxin in citrate buffer were purchased from Sigma-Aldrich (St. Louis, MO). 8MM-IBMX and ryanodine (9,21-dehydro-ryanodine) were purchased from Enzo Life Sciences (Farmingdale, NY). Amphotericin-B, paxilline, fura 2-AM, 8MM-IBMX, and ryanodine were dissolved in DMSO. All other chemicals were dissolved in double distilled water or as indicated. cAMP enzyme immunoassay kit was purchased from Cayman Chemical.
RESULTS
Selective PDE1 inhibition with 8MM-IBMX increased the total intracellular cAMP levels in intact guinea pig DSM tissue.
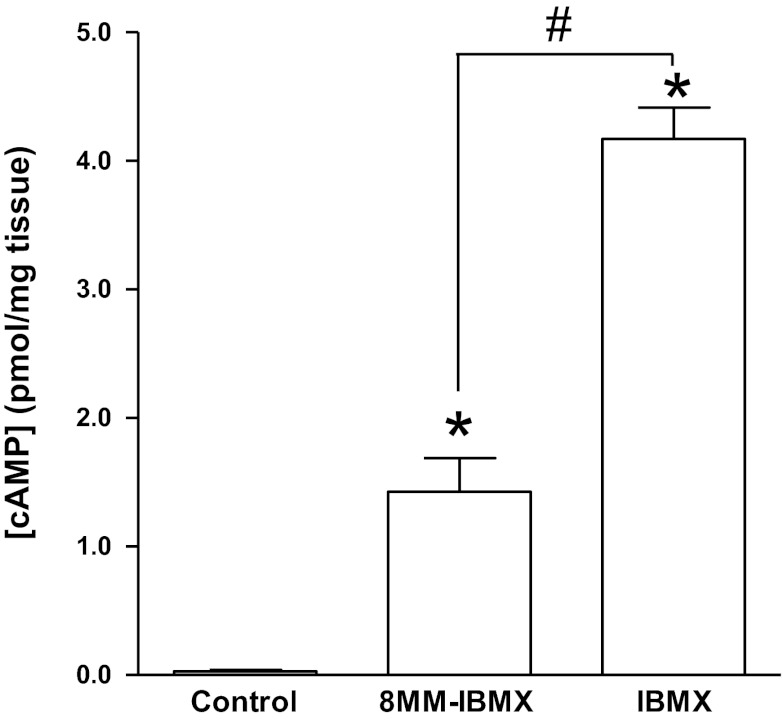
It has been reported that the effect of PDE1 inhibition on the contractility of carbachol-pretreated DSM strips was mainly mediated by the cAMP pathway (43, 53). Therefore, we measured the total cAMP levels in DSM isolated strips using an enzyme immunoassay. cAMP levels were very low with an average of 0.03 ± 0.01 pmol/mg tissue in control strips. In one of the four strips, the cAMP content was undetectable. The cAMP levels increased to 1.4 ± 0.3 pmol/mg tissue in strips treated with 8MM-IBMX (10 μM) (n = 4, N = 4; P < 0.05 vs. control) and increased to 4.2 ± 0.2 pmol/mg-tissue in strips treated with the nonselective PDE inhibitor IBMX (10 μM; n = 4, N = 4; P < 0.05 vs. 8MM-IBMX; P < 0.05 vs. control; Fig. 1). These data indicate that selective PDE1 inhibition with 8MM-IBMX increases the cAMP levels in DSM cells.
Fig. 1.
Phosphodiesterase 1 (PDE1) inhibition with 8-methoxymethyl-3-isobutyl-1-methylxanthine (8MM-IBMX) increases the cAMP levels in guinea pig detrusor smooth muscle (DSM). Summary data showing that 8MM-IBMX (10 μM) increased the total cAMP levels and nonselective PDEs inhibition with IBMX (10 μM) increased cAMP to a higher level in guinea pig DSM. cAMP was measured using enzyme immunoassay. Data were obtained from 4 strips each from a different guinea pig and all measurements were in duplicates. (n = 4, N = 4; *P < 0.05 vs. control; #P < 0.05 vs. 8MM-IBMX).
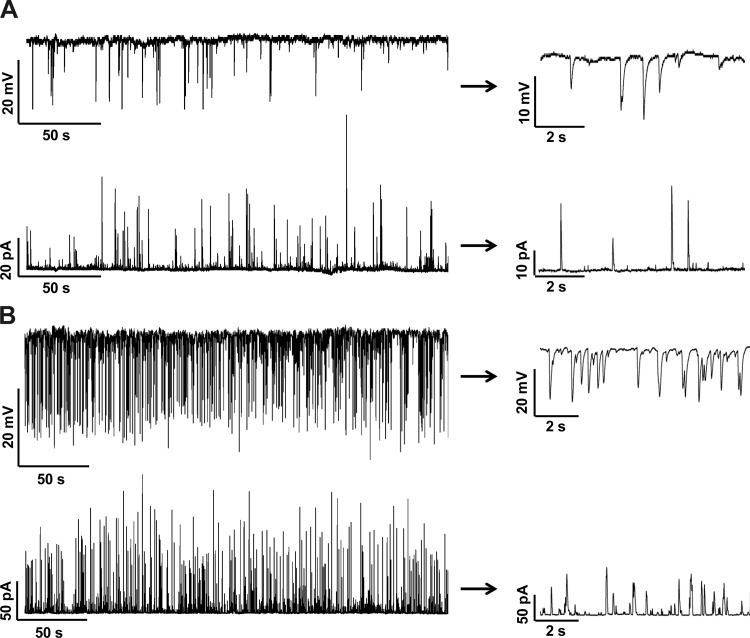
TBKC frequency determines STH frequency in guinea pig DSM cells.
In DSM cells, localized Ca2+ sparks from RyRs activate the closely located BK channels and generate TBKCs (23, 39, 41). The TBKCs were recorded with the perforated patch-clamp technique in voltage-clamp mode in freshly isolated DSM cells at a holding potential of −20 mV. The STHs were then recorded using the current-clamp mode (Ih = 0) on the same cells (Fig. 2). The low-frequency STHs corresponded to low-frequency TBKCs in the same cell. In cells exhibiting high frequency TBKCs, the STH frequency was also proportionally high (Fig. 2). All tested cells showed a clear correlation between the frequencies of TBKCs and STHs (n = 5, N = 4). Next, we investigated the effect of PDE1 inhibition on the TBKCs and STHs.
Fig. 2.
Transient BK currents (TBKCs) determine the spontaneous transient hyperpolarization (STHs). A: original recordings obtained from the same guinea pig DSM cell illustrate that low frequency TBKCs generate low frequency STHs. B: original recordings obtained from the same DSM cell illustrate that high frequency TBKCs generate high frequency STHs. Portions of the recordings are shown on an expended time scale at right. Top traces: current-clamp recordings. Bottom traces: voltage-clamp recordings. TBKCs were recorded at a holding potential of −20 mV.
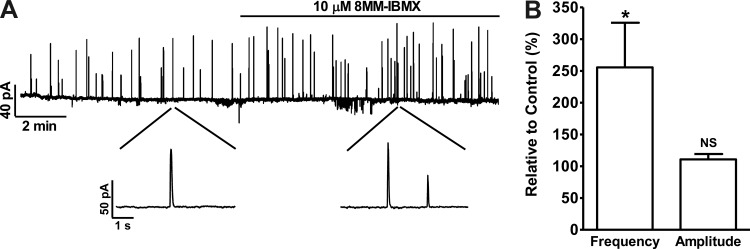
Selective PDE1 inhibition with 8MM-IBMX increased the TBKC frequency.
The TBKCs were recorded in isolated DSM cells at a holding potential of −40 mV, a potential close to the physiological resting membrane potential of intact guinea pig DSM preparations (40). PDE1 inhibition increased the TBKC frequency to 255.6 ± 70.2% (P < 0.05) of the control activity without a significant effect on the average TBKC amplitude (n = 7, N = 6; P > 0.05; Fig. 3). The activation of TBKCs hyperpolarizes the cell membrane potential in the form of STHs (Fig. 2). Therefore, we next evaluated the 8MM-IBMX effect on DSM cell resting membrane potential.
Fig. 3.
PDE1 inhibition with 8MM-IBMX increases the TBKC frequency in guinea pig DSM isolated cells. A: original voltage-clamp recording illustrating that 8MM-IBMX (10 μM) increased the frequency of TBKCs in a single DSM cell recorded with the perforated patch-clamp technique at a holding potential of −40 mV. A portion of the recording is shown on an expanded time scale before and after 8MM-IBMX application. B: summary data showing that 8MM-IBMX (10 μM) significantly increased the TBKC frequency without a significant change in the average TBKC amplitude (n = 7, N = 6; *P < 0.05; NS, nonsignificant). TBKC frequency and amplitude under control conditions were taken to be 100%, respectively.
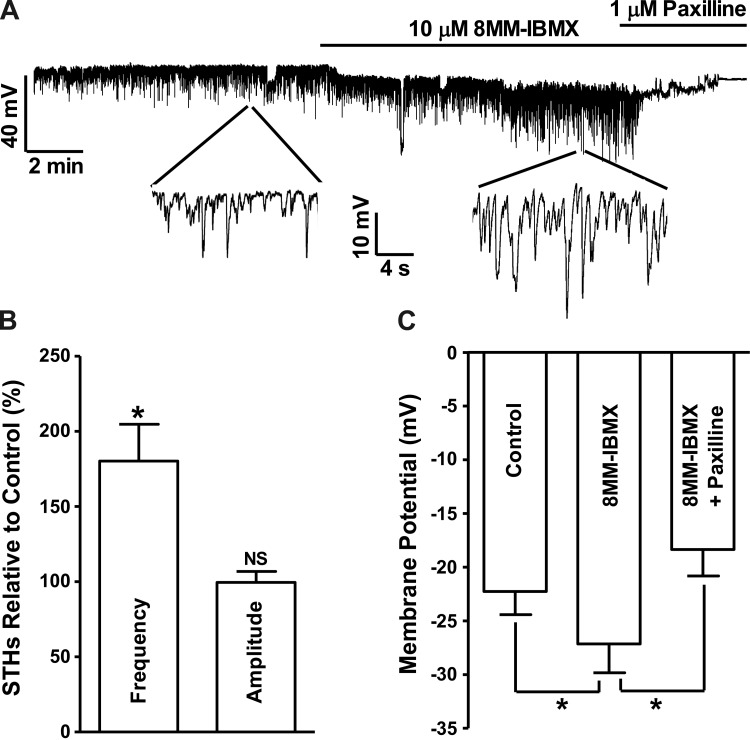
Selective PDE1 inhibition with 8MM-IBMX increased STH frequency and hyperpolarized the resting membrane potential of freshly isolated guinea pig DSM cells.
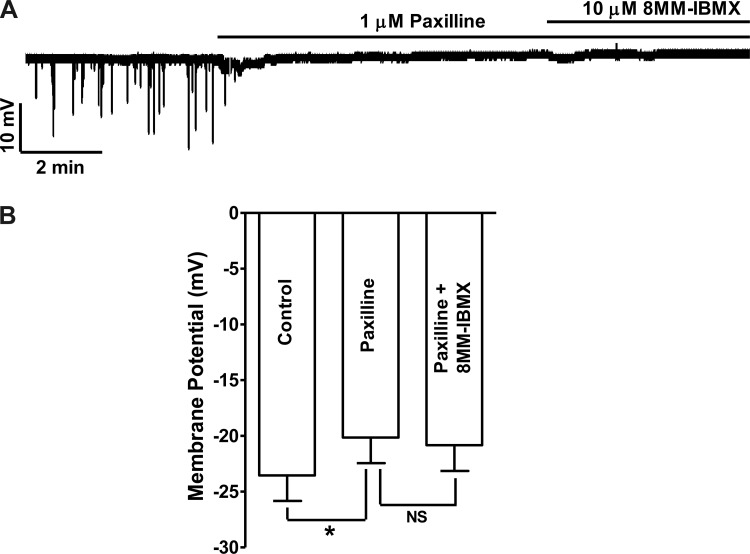
In cells exhibiting STHs, 8MM-IBMX (10 μM) increased STH frequency to 180.3 ± 24.5% of the control values without significant effect on the average STH amplitude (n = 5, N = 5; P < 0.05; Fig. 4). 8MM-IBMX hyperpolarized the guinea pig DSM cell membrane potential from −22.3 ± 2.2 to −27.2 ± 2.7 mV (n = 8, N = 8; P < 0.05; Fig. 4). Blocking BK channels with paxilline (1 μM) following 8MM-IBMX application abolished STHs and depolarized the cell resting membrane potential to −18.4 ± 2.5 mV (n = 8, N = 8; P < 0.05 vs. 8MM-IBMX; Fig. 4). The role of BK channels in DSM cell membrane potential hyperpolarization induced by PDE1 inhibition was further confirmed in separate experiments by applying paxilline before the addition of 8MM-IBMX. Paxilline (1 μM ) pretreatment abolished the STHs and depolarized DSM cell membrane potential from −23.5 ± 2.3 to −20.1 ± 2.3 mV (n = 7, N = 7; P < 0.05; Fig. 5). In the presence of 1 μM paxilline, the subsequent addition of 8MM-IBMX did not have any significant effect on the membrane potential (n = 7, N = 7; P > 0.05 vs. paxilline; Fig. 5).
Fig. 4.
Selective PDE1 inhibition with 8MM-IBMX increases the STH frequency and hyperpolarizes guinea pig DSM cell membrane potential. A: original current-clamp recording illustrating that 8MM-IBMX (10 μM) increased the STH frequency, and hyperpolarized the DSM cell membrane potential. Subsequent addition of paxilline (1 μM) abolished the STHs and depolarized the membrane potential. A portion of the recording is shown on an expanded time scale. B: summary data showing that 8MM-IBMX (10 μM) significantly increased STH frequency and had no effect on the STH amplitude (n = 5, N = 5; *P < 0.05; NS, nonsignificant). C: summary data showing that 8MM-IBMX (10 μM) significantly hyperpolarized the cell membrane potential and that subsequent inhibition of BK channels with paxilline (1 μM) abolished the hyperpolarizing effect of PDE1 inhibition and depolarized the membrane potential (n = 8, N = 8; *P < 0.05).
Fig. 5.
Inhibition of BK channels with paxilline abolishes the hyperpolarizing effect of 8MM-IBMX in guinea pig DSM isolated cells. A: original current-clamp recording illustrating that 1 μM paxilline abolished STHs and depolarized the membrane potential and that the subsequent addition of 8MM-IBMX (10 μM) did not have any additional effect on the membrane potential. B: summary data showing that paxilline (1 μM) depolarized the membrane potential (n = 7, N = 7; *P < 0.05) and the subsequent addition of 10 μM 8MM-IBMX did not cause any significant change in the membrane potential (n = 7, N = 7; NS, nonsignificant vs. paxilline).
Selective PDE1 inhibition with 8MM-IBMX hyperpolarized the resting membrane potential of freshly isolated human DSM cells.
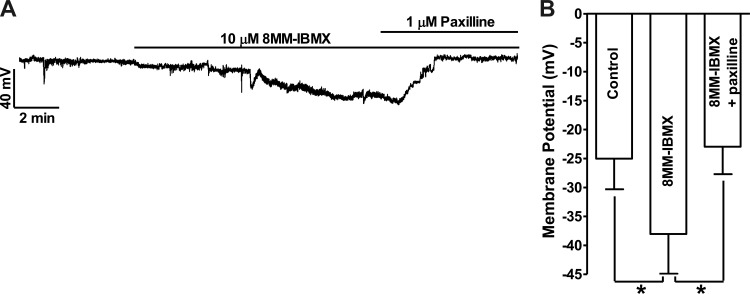
8MM-IBMX (10 μM) hyperpolarized human DSM cell membrane potential from −25.0 ± 5.3 mV under control conditions to −38.0 ± 6.9 mV (n = 5, N = 5; P < 0.05 vs. control), and the subsequent addition of 1 μM paxilline reversed the membrane potential to −23.0 ± 4.7 mV (n = 5, N = 5; P < 0.05 vs. 8MM-IBMX; Fig. 6). These results indicate that the hyperpolarizing effect of 8MM-IBMX is mediated by the BK channels, which are activated by Ca2+ released from RyRs in both guinea pig and human DSM cells. We next investigated whether RyRs are affected following PDE1 inhibition.
Fig. 6.
Selective PDE1 inhibition with 8MM-IBMX hyperpolarizes human DSM cell membrane potential. A: an original current-clamp recording illustrating that 8MM-IBMX (10 μM) hyperpolarized human DSM cell membrane potential. Subsequent inhibition of the BK channels with paxilline (1 μM) reversed the hyperpolarization effect of 8MM-IBMX on human DSM cell membrane potential. B: summary data showing that 8MM-IBMX (10 μM) significantly hyperpolarized the cell membrane potential and that subsequent addition of paxilline (1 μM) reversed the hyperpolarizing effect of PDE1 inhibition (n = 5, N = 5; *P < 0.05).
RyR inhibition abolished STHs and the membrane potential hyperpolarization induced by selective PDE1 inhibition with 8MM-IBMX in guinea pig DSM cells.
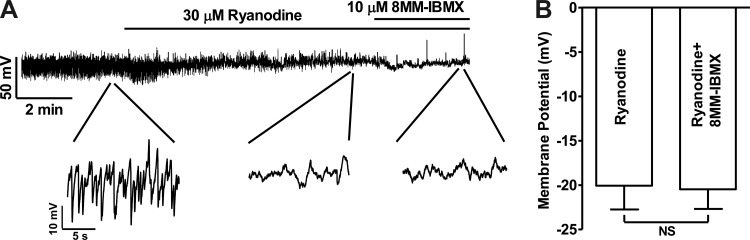
RyR inhibition by ryanodine (30 μM) eliminated the STHs but did not significantly change the membrane potential, and the subsequent addition of 10 μM 8MM-IBMX did not induce any STHs nor membrane hyperpolarization (n = 9, N = 4; Fig. 7A). The values of the membrane potential were −18.3 ± 2.8, −19.3 ± 2.5, and −20.7 ± 2.0 mV under control conditions, after 30 μM ryanodine treatment, and after 10 μM 8MM-IBMX in the presence of 30 μM ryanodine, respectively (n = 9, N = 4; P > 0.05; Fig. 7B). These data suggest that RyR activation is a critical step in DSM cell membrane potential hyperpolarization induced by PDE1 inhibition. Since the changes of cell membrane potential regulate the Ca2+ entry through CaV channels, we next determined the effect of selective PDE1 inhibition on intracellular Ca2+ levels.
Fig. 7.
Inhibition of ryanodine receptors (RyRs) eliminates the STHs and the 8MM-IBMX hyperpolarizing effect in guinea pig DSM isolated cells. A: original current-clamp recording illustrating that RyRs inhibition with ryanodine (30 μM) abolished STHs, and the subsequent addition of PDE1 inhibitor 8MM-IBMX did not have further effects on the membrane potential. A portion of the recording is shown on an expanded time scale. B: summary data indicating that ryanodine and 8MM-IBMX did not significantly change the membrane potential (n = 9, N = 4; NS, nonsignificant).
Selective PDE1 inhibition with 8MM-IBMX decreased the intracellular Ca2+ levels in guinea pig DSM cells.
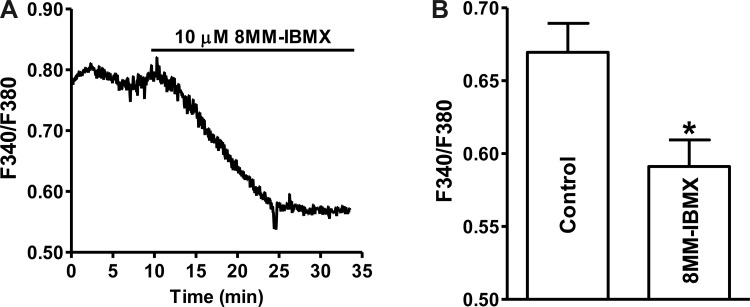
PDE1 inhibition with 10 μM 8MM-IBMX significantly decreased the intracellular Ca2+ levels (F340/F380 nm) from 0.67 ± 0.01 under control condition to 0.59 ± 0.02 in the presence of 8MM-IBMX (n = 36, N = 5; P < 0.05; Fig. 8). Next, we investigated whether 8MM-IBMX-induced reduction in intracellular Ca2+ levels leads to relaxation of DSM intact preparations.
Fig. 8.
Selective PDE1 inhibition with 8MM-IBMX reduces the intracellular Ca2+ levels in freshly isolated guinea pig DSM cells. A: an original recording illustrating that 8MM-IBMX (10 μM) decreased the intracellular Ca2+ level in a single DSM cell shown as a ratio of fura-2 fluorescence emission intensities at 510 nm with excitation at 340 and 380 nm. B: summary data indicating that 8MM-IBMX (10 μM) significantly decreased the intracellular Ca2+ levels (n = 36, N = 5; *P < 0.05).
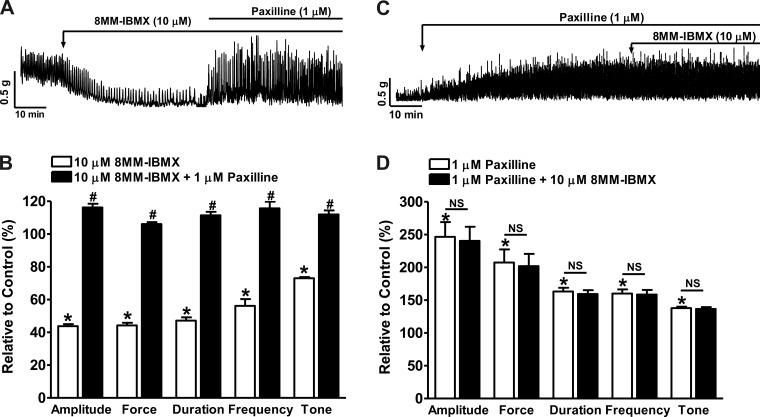
Selective PDE1 inhibition with 8MM-IBMX reduced spontaneous phasic (myogenic) contractions of guinea pig DSM isolated strips.
In DSM isolated strips, 8MM-IBMX (10 μM) reduced the spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and tone down to 44.7 ± 1.4, 43.9 ± 1.3, 46.4 ± 1.7, 54.9 ± 3.4, and 73.6 ± 0.8% of the control activity, respectively (n = 5, N = 5; P < 0.05 vs. control; Fig. 9, A and B). Subsequent inhibition of the BK channels with paxilline (1 μM) reversed the relaxation effect of 8MM-IBMX on DSM spontaneous contractility and recovered the phasic contraction amplitude, muscle force integral, duration, frequency, and tone to 117.0 ± 1.9, 106.6 ± 1.0, 111.7 ± 1.6, 120.0 ± 5.4, and 112.4 ± 1.9% of the control values, respectively (n = 5, N = 5; P < 0.05 vs. 8MM-IBMX; Fig. 9, A and B).
Fig. 9.
Selective PDE1 inhibition with 8MM-IBMX significantly reduces spontaneous phasic contractions of guinea pig DSM isolated strips. A: representative recording of a DSM strip exhibiting spontaneous phasic contractions and illustrating that 8MM-IBMX (10 μM) inhibited the contractions. Blocking the BK channels with paxilline (1 μM) reversed the inhibitory effects of 8MM-IBMX on the spontaneous phasic contractions. B: summary data showing that 8MM-IBMX reduced the spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and tone; and that blocking the BK channels with paxilline (1 μM) abolished the 8MM-IBMX effect and further increased the contraction amplitude, muscle force integral, duration, frequency, and tone (n = 5, N = 5; *P < 0.05 vs. control; #P < 0.05 vs. 8MM-IBMX). C: representative recording of a DSM isolated strip exhibiting spontaneous phasic contractions showing that the BK channel inhibitor paxilline enhanced the contractions and that the subsequent addition of 8MM-IBMX did not have any further effect on the contractions. D: summary data showing that paxilline increased the spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and muscle tone and that the subsequent addition of 8MM-IBMX did not have any further effect on the contraction parameters (n = 5, N = 3; *P < 0.05 vs. control; NS, nonsignificant vs. paxilline).
Blocking BK channels with paxilline eliminated the 8MM-IBMX inhibitory effect on guinea pig DSM spontaneous phasic contractions.
We further explored if the BK channels play a major role in the reduction of DSM spontaneous phasic contractions induced by selective PDE1 inhibition with 8MM-IBMX (10 μM). Blocking BK channels by pretreating the DSM strips with 1 μM paxilline increased the spontaneous phasic contraction amplitude, muscle force integral, duration, frequency, and tone to 246.6 ± 22.4, 207.4 ± 20.0, 163.2 ± 5.7, 160.0 ± 6.3%, and 137.8 ± 2.2% of the control values, respectively (n = 5, N = 3; P < 0.05 vs. control; Fig. 9, C and D), and the addition of 8MM-IBMX did not cause any further change to the phasic contraction amplitude, muscle force integral, duration, frequency, and tone measured as 240.4 ± 21.5, 201.8 ± 18.7, 159.5 ± 5.8, 158.5 ± 7.1, and 136.6 ± 2.7% of the control values (n = 5, N = 3; P > 0.05 vs. paxilline; Fig. 9, C and D). These data further demonstrate that BK channels are key elements in the reduction of DSM myogenic contraction upon selective PDE1 inhibition. Next, we investigated the 8MM-IBMX effects on the nerve-evoked DSM contractions.
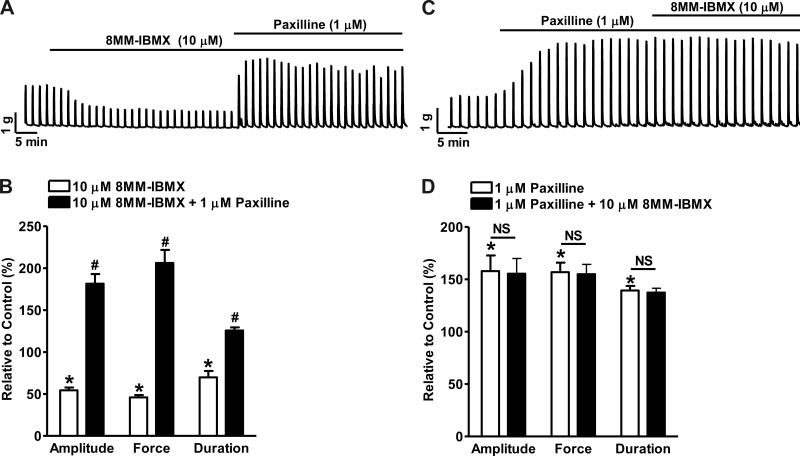
Selective PDE1 inhibition with 8MM-IBMX reduced the EFS-induced guinea pig DSM contractions.
Nerve-evoked DSM contractions are critical for the periodic voiding of urine (3, 12). EFS causes release of excitatory neurotransmitters and subsequently induces high-amplitude DSM contractions. PDE1 inhibition with 10 μM 8MM-IBMX attenuated the 20-Hz EFS-induced contraction amplitude, muscle force integral, and duration to 54.4 ± 3.3, 45.9 ± 2.8, and 69.8 ± 7.6% of the control values, respectively (n = 5, N = 3; P < 0.05 vs. control; Fig. 10, A and B). Subsequently blocking the BK channels with paxilline (1 μM) reversed the relaxation effect of PDE1 inhibition and further increased the contraction amplitude, muscle force integral, and duration to 181.4 ± 11.5, 206.2 ± 15.5, and 125.6 ± 3.7% of the control values (n = 5, N = 3; P < 0.05 vs. 8MM-IBMX; Fig. 10, A and B). Paxilline (1 μM) pretreatment of DSM strips increased the EFS-induced phasic contraction amplitude, muscle force integral, and duration to 157.8 ± 15.1, 156.9 ± 9.0, and 139.4 ± 4.3% of the control values, respectively (n = 5, N = 5; P < 0.05 vs. control; Fig. 10, C and D). The subsequent addition of 8MM-IBMX did not further change the contraction parameters measured as 155.3 ± 14.6, 154.9 ± 9.3, and 137.4 ± 4.1% of the control values, respectively (n = 5, N = 5; P > 0.05 vs. paxilline; Fig. 10, C and D). These results indicate that selective PDE1 inhibition suppresses the neurogenic DSM contractions via BK channel activation.
Fig. 10.
Selective PDE1 inhibition with 8MM-IBMX significantly reduces 20-Hz EFS-induced contractions of guinea pig DSM isolated strips. A: representative recording illustrating that 8MM-IBMX (10 μM) inhibited the 20-Hz EFS-induced contractions. Blocking the BK channels with paxilline (1 μM) reversed the inhibitory effects of 8MM-IBMX on DSM contractions. B: summary data showing that 8MM-IBMX reduced the 20-Hz EFS-induced contraction amplitude, muscle force integral, and duration; and that blocking BK channels with paxilline abolished the 8MM-IBMX inhibitory effect and further increased the contraction parameters (n = 5, N = 3; *P < 0.05 vs. control; #P < 0.05 vs. 8MM-IBMX). C: representative recording showing that paxilline (1 μM) increased the 20-Hz EFS-induced phasic contractions; and that the subsequent addition of 8MM-IBMX did not have any further effects on DSM contractions. D: summary data showing that paxilline increased the 20-Hz EFS-induced contraction amplitude, muscle force integral, and duration and that the subsequent addition of 8MM-IBMX did not have any further effects on the contraction parameters (n = 5, N = 5; *P < 0.05 vs. control; NS, nonsignificant vs. paxilline).
DISCUSSION
The data presented here reveal for the first time that selective PDE1 inhibition with 8MM-IBMX significantly: 1) increases the intracellular cAMP levels in guinea pig DSM, 2) increases TBKC frequency in guinea pig DSM cells, 3) increases the STH frequency in guinea pig DSM cells and hyperpolarized guinea pig and human DSM cell membrane potential, 4) decreases the intracellular Ca2+ levels of guinea pig DSM isolated cells, and 5) attenuates guinea pig DSM spontaneous phasic and nerve-evoked contractions. The results provide the first evidence that selective PDE1 inhibition suppresses DSM excitability and contractility via the activation of RyRs, thus leading to an increase in TBKC frequency.
cAMP, but not cGMP, has been demonstrated to play a major role in DSM relaxation (3, 35). Our recent study (56) demonstrates that nonselective PDE inhibition with IBMX relaxes guinea pig DSM via TBKC activation and subsequent DSM cell membrane hyperpolarization. PDEs are key enzymes controlling cellular cAMP levels (14). PDE isoenzymes are differentially located in subcellular compartments, and the localization of specific PDE isoenzymes ensures the cAMP signaling specificity for regulating distinct cellular functions (14, 32, 49, 57). PDE1 proteins are expressed in animal and human bladders and are predominately located in the cytosol (5, 43, 44, 52). In DSM, PDE1 inhibition had a more significant relaxation effect than other PDEs (43, 44, 53). However, the underlying cellular mechanisms have never been explored. Here, for the first time, we investigate the signaling pathway by which the selective PDE1 inhibition causes DSM relaxation.
Previous studies (43, 53) demonstrated that the relaxation effect of PDE1 inhibition on DSM contractility is mainly mediated by the cAMP pathway. Consistently, our data show that 8MM-IBMX significantly increased the total cAMP levels in guinea pig DSM (Fig. 1) The nonselective PDE inhibitor IBMX increased cAMP to a much higher level than the PDE1 selective inhibitor, suggesting that in DSM cells cAMP levels are controlled by multiple PDEs in addition to PDE1 (Fig. 1). The specific PDE isoenzymes are key factors determining the spatial distribution of cellular cAMP, and the spatially localized cAMP is critical to regulation of particular cellular processes (48, 58). Manipulation of particular PDE isoform expression and/or activity can be an efficient way to regulate cAMP levels in the distinct subcellular compartments, thereby regulating cellular functions with high specificity (57). The selective inhibition of specific PDEs as a therapeutic treatment could limit collateral unwanted side effects (13). Even though the measured increase of total cAMP by PDE1 inhibition is lower than that mediated by the nonselective PDEs inhibition, the potentially compartmentalized cAMP pool is crucial to activate the downstream signaling cascades including RyRs and BK channels (29, 32, 48).
The Ca2+ sparks released from RyRs transiently activate BK channels and generate TBKCs and STHs, and their frequencies are tightly correlated to each other (Fig. 2) (23, 41). In agreement with our recently published study (56), PDE inhibition increased BK channel activity (Fig. 3). The increase of TBKC frequency induced by PDE1 inhibition is comparable to that of nonselective PDE inhibition, even though the total cAMP increase by PDE1 inhibition is much smaller (56). This suggests that the likely localized cAMP increase by PDE1 inhibition, not the global cAMP, is critical to stimulate the BK channel activity and thus to increase the TBKC frequency. The PDE1 inhibition increased STH frequency and hyperpolarized the cell membrane potential via BK channel activation, which is evidenced by the fact that the BK channel selective inhibitor paxilline abolished STHs and the hyperpolarization effect of 8MM-IBMX (Figs. 4–6).
Our recently published data (56) showed that PDEs inhibition increased the TBKCs but did not have any effect on the steady-state BK currents when all of the Ca2+ sources for BK channel activation were blocked. The TBKC activity is selectively stimulated by Ca2+ sparks via sarcoplasmic reticulum RyRs (27, 41). Therefore, the pharmacological manipulation of RyRs can be an efficient way to regulate BK channel activity and thereby the cell membrane potential and DSM contractility (27). It has been observed that PDE inhibition increases RyR activity and Ca2+ sparks via highly compartmentalized cAMP/PKA pathways in various cell types (4, 30, 32). The selective inhibition of PDE1 hyperpolarized guinea pig and human DSM cell membrane potential (Figs. 4 and 6). In agreement with the previous findings (26, 27), inhibition of RyRs blocked STHs and attenuated the hyperpolarization effect of PDE1 inhibition (Fig. 7). This suggests that RyRs are the key mediators of TBKC activation and the reduction in DSM excitability induced by PDE1 inhibition.
The hyperpolarization of the membrane potential reduces Ca2+ entry through CaV channels and consequently inhibits the DSM excitability and contractility (18, 39, 40). Indeed, the data presented here demonstrate that PDE1 inhibition reduced the intracellular Ca2+ levels (Fig. 8). Intracellular Ca2+ directly regulates DSM contractility. The myogenic DSM spontaneous phasic contractions originate from a transient elevation of Ca2+ concentration due to Ca2+ influx through CaV channels (17, 39). Ca2+ flows into the cell through CaV channels coincident with membrane potential depolarization during an action potential (6, 19, 21). In guinea pig DSM, BK channel activation hyperpolarizes the cell membrane potential, prevents Ca2+ entry, and thus can reduce action potential firing, thereby attenuating the DSM spontaneous phasic contractions (20, 22, 39). The data presented here demonstrate that PDE1 inhibition reduced DSM cell excitability via BK channel activation and thus reduced the myogenic DSM contractility (Fig. 9). The role of BK channels was confirmed by using paxilline, which blocked the relaxation effect of PDE1 inhibition (Fig. 9). This suggests that PDE1 could be a target for the treatment of OAB resulting from a myogenic origin.
Autonomic neuronal pathways regulate DSM contractility by releasing excitatory neurotransmitters, such as ATP and acetylcholine. The abnormal neuronal activity could excessively increase DSM contractility causing DO (3). Our data showed that PDE1 inhibition attenuated the nerve-evoked DSM contractions. Pre- and posttreatment with a BK channel blocker abolished the DSM relaxation effect of PDE1 inhibition (Fig. 10), suggesting a BK channel-dependent mechanism of DSM relaxation induced by PDE1 inhibition. These results demonstrate that PDE1 inhibition can also regulate neurogenic DSM contractility through a mechanism of BK channel activation and cellular Ca2+ reduction.
It is well known that BK channels can be directly activated by channel-selective pharmacological openers (8, 11, 28, 36, 42, 47). The data presented here demonstrate that PDE1 inhibition relaxes DSM by stimulating BK channel activity indirectly via cAMP/PKA pathways. Individual PDE isoenzymes, such as PDE1, have distinct tissue-expression patterns, subcellular localization, and regulation mechanisms (48, 58). Each individual PDE isoenzyme contributes to the formation of localized cyclic nucleotides microdomains, which regulate specific cellular signaling pathways (34, 37). In human bladder, PDE1 mRNA expression level is the highest compared with all peripheral tissues, except the heart (31). PDE1 is predominately located in the cytosol (5). Therefore, specific and highly selective PDE1 inhibitors can activate localized cAMP/PKA pathways, and this ability makes PDE1 inhibitors the plausible tools to solve the specificity issue of a pharmacological intervention (5). In contrast, the BK channel is ubiquitously expressed in excitable cells and regulates a variety of physiological functions (33, 55). Unfortunately, the currently available BK channel openers lack tissue specificity. Therefore, pharmacological treatment of OAB with selective PDE1 inhibitors could be a better option with fewer possible adverse effects compared with direct pharmacological activation of the BK channels.
In summary, for the first time, our study revealed the cellular mechanism by which PDE1 inhibition increases cellular cAMP levels, stimulates RyR activity, activates BK channels, hyperpolarizes cell membrane potential, reduces intracellular Ca2+ levels, and consequently suppresses DSM contractility. Selective PDE1 inhibitors could offer novel treatment options for OAB with myogenic or neurogenic origin.
GRANTS
This study was supported National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-084284 (to G. V. Petkov).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.X., R.P.S., Q.C., and G.V.P. performed experiments; W.X., R.P.S., Q.C., and G.V.P. analyzed data; W.X., R.P.S., Q.C., E.S.R., and G.V.P. interpreted results of experiments; W.X., R.P.S., Q.C., and G.V.P. prepared figures; W.X., R.P.S., Q.C., and G.V.P. drafted manuscript; W.X., R.P.S., Q.C., E.S.R., and G.V.P. edited and revised manuscript; W.X., R.P.S., Q.C., E.S.R., and G.V.P. approved final version of manuscript; G.V.P. conception and design of research.
ACKNOWLEDGMENTS
We thank MUSC Urology staff surgeons: Drs. Thomas Keane, Harry Clarke, Stephen Savage, Ross Rames, Jonathan Picard, and Ahmed M. El-Zawahry; as well as the MUSC Urology Residents: Matthew McIntyre, Jonathan N. Hamilton, Robin Bhavsar, Timothy R. Yoost, Vinh Q. Trang, Lydia Labocetta, Elizabeth Peacock, Matthew Young, Erin Burns, Vaughan Taylor, and Samuel Walker Nickles for help with human tissue collection; and Drs. John Malysz, Shankar Parajuli, Kiril Hristov, Ms. Amy Smith, and Mr. Serge Afeli for the critical evaluation of the manuscript.
REFERENCES
- 1.Abrams P. Describing bladder storage function: overactive bladder syndrome and detrusor overactivity. Urology 62: 28–37; discussion 40–22, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Afeli SAY, Hristov KL, Petkov GV. Do β3-adrenergic receptors play a role in guinea pig detrusor smooth muscle excitability and contractility? Am J Physiol Renal Physiol 302: F251–F263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Pharmacol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bellinger AM, Reiken S, Dura M, Murphy PW, Deng SX, Landry DW, Nieman D, Lehnart SE, Samaru M, LaCampagne A, Marks AR. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci USA 105: 2198–2202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. Beta-adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol 295: F1149–F1157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang S, Gomes CM, Hypolite JA, Marx J, Alanzi J, Zderic SA, Malkowicz B, Wein AJ, Chacko S. Detrusor overactivity is associated with downregulation of large-conductance calcium- and voltage-activated potassium channel protein. Am J Physiol Renal Physiol 298: F1416–F1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M, Petkov GV. Identification of large conductance calcium activated potassium channel accessory beta4 subunit in rat and mouse bladder smooth muscle. J Urol 182: 374–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Pharmacol Rev 89: 411–452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darblade B, Behr-Roussel D, Oger S, Hieble JP, Lebret T, Gorny D, Benoit G, Alexandre L, Giuliano F. Effects of potassium channel modulators on human detrusor smooth muscle myogenic phasic contractile activity: potential therapeutic targets for overactive bladder. Urology 68: 442–448, 2006 [DOI] [PubMed] [Google Scholar]
- 12.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br J Pharmacol 147, Suppl 2: S25–S40, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Visser YP, Walther FJ, Laghmani el H, Steendijk P, Middeldorp M, van der Laarse A, Wagenaar GT. Phosphodiesterase 4 inhibition attenuates persistent heart and lung injury by neonatal hyperoxia in rats. Am J Physiol Lung Cell Mol Physiol 302: L56–L67, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Pharmacol Rev 91: 651–690, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Giuliano F. Phosphodiesterase type 5 inhibitors improve male lower urinary tract symptoms. Eur Urol 53: 1121–1123; discussion 1123–1124, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Han P, Werber J, Surana M, Fleischer N, Michaeli T. The calcium/calmodulin-dependent phosphodiesterase PDE1C down-regulates glucose-induced insulin secretion. J Biol Chem 274: 22337–22344, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Heppner TJ, Werner ME, Nausch B, Vial C, Evans RJ, Nelson MT. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol 587: 5275–5288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hotta S, Morimura K, Ohya S, Muraki K, Takeshima H, Imaizumi Y. Ryanodine receptor type 2 deficiency changes excitation-contraction coupling and membrane potential in urinary bladder smooth muscle. J Physiol 582: 489–506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hristov K, Afeli S, Kellett W, Rovner E, Petkov G. Neurogenic detrusor overactivity is associated with decreased BK channel activity: electrophysiological and functional studies on human detrusor smooth muscle. J Urol 183: e73–e74, 2010 [Google Scholar]
- 26.Hristov KL, Chen M, Kellett WF, Rovner ES, Petkov GV. Large-conductance voltage- and Ca2+-activated K+ channels regulate human detrusor smooth muscle function. Am J Physiol Cell Physiol 301: C903–C912, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hristov KL, Cui X, Brown SM, Liu L, Kellett WF, Petkov GV. Stimulation of beta3-adrenoceptors relaxes rat urinary bladder smooth muscle via activation of the large-conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 295: C1344–C1353, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hristov KL, Parajuli SP, Soder RP, Cheng Q, Rovner ES, Petkov GV. Suppression of human detrusor smooth muscle excitability and contractility via pharmacological activation of large conductance Ca2+-activated K+ channels. Am J Physiol Cell Physiol 302: C1632–C1641, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jurevicius J, Fischmeister R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by beta-adrenergic agonists. Proc Natl Acad Sci USA 93: 295–299, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multicomponent signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci 114: 3167–3176, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology 59: 367–374, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Lehnart SE, Wehrens XHT, Reiken S, Warrier S, Belevych AE, Harvey RD, Richter W, Jin SLC, Conti M, Marks AR. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123: 25–35, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol 570: 65–72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mika D, Leroy J, Vandecasteele G, Fischmeister R. PDEs create local domains of cAMP signaling. J Mol Cell Cardiol 52: 323–329, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Morita T, Tsujii T, Dokita S. Regional difference in functional roles of cAMP and cGMP in lower urinary tract smooth muscle contractility. Urol Int 49: 191–195, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Oger S, Behr-Roussel D, Gorny D, Bernabe J, Comperat E, Chartier-Kastler E, Denys P, Giuliano F. Effects of potassium channel modulators on myogenic spontaneous phasic contractile activity in human detrusor from neurogenic patients. BJU Int 108: 604–611, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res 100: 309–327, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther 340: 114–123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petkov GV. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat Rev Urol 9: 30–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petkov GV, Heppner TJ, Bonev AD, Herrera GM, Nelson MT. Low levels of KATP channel activation decrease excitability and contractility of urinary bladder. Am J Physiol Regul Integr Comp Physiol 280: R1427–R1433, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol 288: C1255–C1263, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Ponte CG, McManus OB, Schmalhofer WA, Shen DM, Dai G, Stevenson A, Sur S, Shah T, Kiss L, Shu M, Doherty JB, Nargund R, Kaczorowski GJ, Suarez-Kurtz G, Garcia ML. Selective, direct activation of high-conductance, calcium-activated potassium channels causes smooth muscle relaxation. Mol Pharmacol 81: 567–577, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Qiu Y, Kraft P, Craig EC, Liu X, Haynes-Johnson D. Cyclic nucleotide phosphodiesterases in rabbit detrusor smooth muscle. Urology 59: 145–149, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Qiu Y, Kraft P, Craig EC, Liu X, Haynes-Johnson D. Identification and functional study of phosphodiesterases in rat urinary bladder. Urol Res 29: 388–392, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res 90: 151–157, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Semins MJ, Chancellor MB. Diagnosis and management of patients with overactive bladder syndrome and abnormal detrusor activity. Nat Clin Pract Urol 1: 78–84; quiz 109, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Soder RP, Petkov GV. Large conductance Ca2+ -activated K+ channel activation with NS1619 decreases myogenic and neurogenic contractions of rat detrusor smooth muscle. Eur J Pharmacol 670: 252–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stangherlin A, Zaccolo M. Phosphodiesterases and subcellular compartmentalized cAMP signaling in the cardiovascular system. Am J Physiol Heart Circ Physiol 302: H379–H390, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Terrenoire C, Houslay MD, Baillie GS, Kass RS. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem 284: 9140–9146, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Truss MC, Stief CG, Uckert S, Becker AJ, Schultheiss D, Machtens S, Jonas U. Initial clinical experience with the selective phosphodiesterase-I isoenzyme inhibitor vinpocetine in the treatment of urge incontinence and low compliance bladder. World J Urol 18: 439–443, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Truss MC, Stief CG, Uckert S, Becker AJ, Wefer J, Schultheiss D, Jonas U. Phosphodiesterase 1 inhibition in the treatment of lower urinary tract dysfunction: from bench to bedside. World J Urol 19: 344–350, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Truss MC, Uckert S, Stief CG, Kuczyk M, Jonas U. Cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human detrusor smooth muscle. I. Identification and characterization. Urol Res 24: 123–128, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Truss MC, Uckert S, Stief CG, Kuczyk M, Schulz-Knappe P, Forssmann WG, Jonas U. Effects of various phosphodiesterase-inhibitors, forskolin, and sodium nitroprusside on porcine detrusor smooth muscle tonic responses to muscarinergic stimulation and cyclic nucleotide levels in vitro. Neurourol Urodyn 15: 59–70, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Uckert S, Oelke M. Phosphodiesterase (PDE) inhibitors in the treatment of lower urinary tract dysfunction. Br J Clin Pharmacol 72: 197–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu RS, Marx SO. The BK potassium channel in the vascular smooth muscle and kidney: alpha- and beta-subunits. Kidney Int 78: 963–974, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Xin W, Cheng Q, Soder RP, Petkov GV. Inhibition of phosphodiesterases relaxes detrusor smooth muscle via activation of the large-conductance voltage- and Ca2+-activated K+ channel. Am J Physiol Cell Physiol 302: C1361–C1370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xin W, Tran TM, Richter W, Clark RB, Rich TC. Roles of GRK and PDE4 activities in the regulation of beta2 adrenergic signaling. J Gen Physiol 131: 349–364, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295: 1711–1715, 2002 [DOI] [PubMed] [Google Scholar]