Abstract
The transcription factor nuclear factor of activated T cell 5 (NFAT5) is activated by the stress of hypertonicity (e.g., high NaCl). Increased expression of NFAT5 target genes causes accumulation of protective organic osmolytes and heat shock proteins. Under normotonic conditions (∼300 mosmol/kgH2O), NFAT5 is distributed between the nucleus and cytoplasm, hypertonicity causes it to translocate into the nucleus, and hypotonicity causes it to translocate into the cytoplasm. The mechanism of translocation is complex and not completely understood. NFAT5-T298 is a known contact site of NFAT5 with its specific DNA element [osmotic response element (ORE)]. In the present study, we find that mutation of NFAT5-T298 to alanine or aspartic acid not only reduces binding of NFAT5 to OREs (EMSA) but also proportionately reduces high NaCl-induced nuclear translocation of NFAT5. Combined mutation of other NFAT5 DNA contact sites (R293A/E299A/R302A) also greatly reduces both specific DNA binding and nuclear localization of NFAT5. NFAT5-T298 is a potential phosphorylation site, but, using protein mass spectrometry, we do not find phosphorylation at NFAT5-T298. Further, decreased high NaCl-induced nuclear localization of NFAT5 mutated at T298 does not involve previously known regulatory mechanisms, including hypotonicity-induced export of NFAT5, regulated by phosphorylation of NFAT5-S155, XPO1 (CRM1/exportin1)-mediated export of NFAT5 from the nucleus, or hypertonicity-induced elevation of NUP88, which enhances nuclear localization of NFAT5. We conclude that specific DNA binding of NFAT5 contributes to its nuclear localization, by mechanisms, as yet undetermined, but independent of ones previously described to regulate NFAT5 distribution.
Keywords: high NaCl, NUP88, leptomycin B, XPO1
the transcription factor nuclear factor of activated T-cells 5 [NFAT5, also called TonEBP (24) or OREBP (17)] is activated by the stress of high NaCl and other sources of hypertonicity. Increased expression of its target genes results in accumulation of protective organic osmolytes and heat shock proteins (2). Under normotonic conditions (∼300 mosmol/kgH2O), NFAT5 is distributed between the nucleus and cytoplasm, hypertonicity causes it to translocate into the nucleus (17, 24), and hypotonicity causes it to translocate into the cytoplasm (32). The mechanisms remain uncertain, however, since studies by different investigators of the tonicity-mediated nuclear localization of NFAT5 have led to markedly different conclusions. Thus the Ko laboratory (30, 34) identified several different sites in NFAT5, one of which is necessary for the hypertonicity-induced translocation of NFAT5 into the nucleus, and other sites necessary for export of NFAT5 from the nucleus. In contrast, the Kwon laboratory (19) concluded that a single site mediates both the hypertonicity-induced translocation of NFAT5 into the nucleus and the hypotonicity-induced export of NFAT5 from the nucleus.
The Ko laboratory, using HeLa cells, reported (30) a consensus bipartite nuclear localization signal (NLS, in amino acids 199–216 of NFAT5) where two clusters of basic amino acids, residues 202–204 (RKR) and residues 213–215 (KRR), are aligned in tandem. Mutation at the first basic cluster completely abolished hypertonicity-induced nuclear localization of NFAT5, but mutation of the second basic cluster had no effect on nuclear accumulation. They concluded that only the first portion of the bipartite NLS is necessary for hypertonicity-induced nuclear translocation of NFAT5. As we will see, this conclusion about the NLS is not controversial. However, there is disagreement about whether additional sites are involved in export of NFAT5 from the nucleus. Specifically, the Ko laboratory identified regions of NFAT5 that are necessary for its export from the nucleus. They found that leptomycin B (LMB), a potent inhibitor of the nuclear exportin XPO1 (CRM1), causes nuclear retention of NFAT5 under normotonic conditions but fails to prevent hypotonicity-induced nuclear export of NFAT5. LMB-induced nuclear retention of NFAT5 under normotonic conditions also occurs in mIMCD3 cells (1). The Ko laboratory identified two separate regions of NFAT5, one involved in its LMB-dependent nuclear export and the other involved in its LMB-independent nuclear export. For their nuclear export experiments, they used mutants of NFAT5-1-547-FLAG, which is appropriate since nuclear localization of NFAT5-1-547 responds to hypertonicity and hypotonicity in the same way as does full-length (1,531 amino acids) native NFAT5 (35). They concluded that under normotonic conditions nuclear export of NFAT5 is mediated by an XPO1-dependent, leucine-rich canonical nuclear export sequence (NES) located between amino acids 8 and 15. Disruption of the NES by site-directed mutagenesis yielded a mutant NFAT5 protein that accumulated in the nucleus under normotonic conditions but remained a target for hypotonicity-induced nuclear export. Further, they identified an auxiliary export domain (AED) located between amino acids 132 and 156 that is critical for the hypotonicity-induced nuclear export of NFAT5. Later (34), they identified the mechanism that regulates AED-mediated nuclear export of NFAT5, the evidence being that Ser-155 and Ser-158 of NFAT5 are both phosphorylated under hypotonic conditions, and phosphorylation of Ser-155 primes the phosphorylation of Ser-158; pharmacological inhibition of casein kinase 1 (CK1) by CKI-7 abolishes the phosphorylation of Ser-158 and impedes NFAT5 nuclear export; recombinant CK1 phosphorylates Ser-158; knockdown of CK1α1L, a novel isoform of CK1, inhibits hypotonicity-induced NFAT5 nuclear export; and alanine substitution at Ser-155 prevents hypotonicity-dependent nuclear export of NFAT5. They speculated that the multisite phosphorylation induces conformational changes that mask the NLS or expose the NES, thus favoring cytoplasmic localization.
In contrast, the Kwon laboratory, using COS cells, reported (19) that the NLS (amino acids 199–215) suffices for both tonicity-dependent import and export of NFAT5 with little or no role for the putative NES and AED. When short peptides containing the NLS were fused to constitutively cytoplasmic proteins, the fusion proteins displayed bidirectional tonicity-dependent nucleocytoplasmic trafficking like native NFAT5. In agreement with the Ko laboratory, the Kwon laboratory found that the NLS is indeed critical for hypertonicity-dependent nuclear translocation of NFAT5. Constructs lacking those amino acids were constitutively cytoplasmic even in hypertonic conditions. However, in contradiction to the Ko laboratory, and others (1), the Kwon laboratory found no evidence for an XPO1-dependent NES. In COS cells, the nuclear export of endogenous NFAT5 was not affected by LMB. Full-length GFP-NFAT5 fusion constructs where the putative NES was either deleted or mutated in the critical leucine residues did not display increased nuclear localization under normotonic conditions. Further, deletion or mutation of the AED caused only modest nuclear localization under hypotonic and normotonic conditions, which was interpreted as indicating that the AED provides only a modest nuclear export signal. Finally, although the XPO1-interacting NES from human immunodeficiency virus Rev protein displayed a clear nuclear export activity, an NFAT5 fragment containing the AED did not display significant nuclear export activity. The Kwon laboratory attributed the difference in their results from the Ko laboratory to the fact that the Kwon laboratory generally used full-length constructs of NFAT5, whereas the Ko laboratory had used constructs limited to NFAT5-1-581.
Previous investigations also revealed several pathways by which hypertonicity signals nuclear translocation of NFAT5 and, in some cases, implicated the particular amino acids in NFAT5 that are affected: 1) ataxia telangiectasia mutated (ATM) contributes to nuclear localization of native NFAT5 and of recombinant NFAT5-1-547 (35). High NaCl-induced nuclear translocation of NFAT5 is reduced in AT cells, which lack functional ATM, and is restored when the cells are reconstituted with ATM. 2) Inhibition of proteosomes contributes to nuclear localization of native NFAT5 in Madin-Darby canine kidney (MDCK) and Hep G2 cells (20, 33, 35). The proteosome inhibitor MG-132 reduces the hypertonicity-induced increase of NFAT5 in the nucleus. However, in contrast to the result in MDCK and Hep G2 cells, MG-132 did not inhibit high NaCl-induced nuclear translocation of NFAT5 in COS7 cells (35). 3) Activation of CDK5 by high NaCl increases phosphorylation of NFAT5 at threonine 135, which contributes to its rapid nuclear localization in HEK293 cells (9). The high NaCl-induced nuclear localization of NFAT5 is reduced if threonine 135 is mutated to alanine or if CDK5 is inhibited. Further, hyperosmolality increases phosphorylation of NFAT5-T135 in the rat renal inner medulla in vivo. 4) High NaCl-induced phosphorylation of tyrosine 143 in NFAT5 contributes to nuclear localization of NFAT5 by providing a binding site for phospholipase C-γ1 (PLC-γ1; Ref. 13). High NaCl increases phosphorylation of NFAT5 at Y143, and PLC-γ1 associates with wild-type (WT) NFAT5 but not NFAT5-Y143A. High NaCl-induced nuclear localization of NFAT5 is reduced if cells lack PLC-γ1, if dominant negative PLC-γ1 (mutated in its SH2C domain) is overexpressed, or if tyrosine 143 in NFAT5 is mutated to alanine. Expression of recombinant PLC-γ1 restores high NaCl-induced nuclear localization of WT NFAT5 in PLC-γ1 null cells but not of NFAT5-Y143A. High NaCl-induced phosphorylation of NFAT5-Y143 is catalyzed by c-Abl kinase, which is activated by high NaCl (10), and NFAT5-Y143 is dephosphorylated by SHP-1 phosphatase, which is inhibited by high NaCl (36). 5) High NaCl increases expression of Nucleoporin 88 (Nup88, a component of a nuclear pore complex) in mIMCD3 cells (1), and silencing of Nup88 prevents hypertonicity-induced nuclear translocation of NFAT5.
In addition, exclusion of NFAT5 from mitotic chromatin resets its nucleo-cytoplasmic distribution in interphase (4). This mechanism, together with export signals acting in interphase, relocalizes NFAT5 to the cytoplasm after mitosis in each cell cycle, preventing nuclear accumulation and association with DNA of NFAT5 in the absence of hypertonic stress.
We initiated the present study with a Scansite (26) search of NFAT5 since this program had previously identified NFAT5 T135 and Y143 as binding and/or phosphorylation sites important in regulating its cellular distribution (9, 10, 13). A Scansite search for predicted binding sites in NFAT5 revealed that phosphorylated NFAT5-T298 is a consensus binding site for 14-3-3 proteins. The 14-3-3 proteins can contribute to regulation of transcription factors by several mechanisms, including control of nuclear localization (31). We performed mass spectrometry to determine phosphorylation at NFAT5-T298 but, regardless of tonicity, were able only to identify the nonphosphorylated peptide containing T298. Since 14-3-3 binding requires residue phosphorylation, we did not pursue 14-3-3 further. However, when we mutated NFAT5-T298 to test whether threonine 298 is important for the function of NFAT5, we observed a remarkable decrease in high NaCl-induced nuclear translocation. The remainder of the present study was aimed at discovering the basis for this effect.
We used full-length recombinant NFAT5 for our analysis of the effect of mutations of NFAT5-T298 on its subcellular localization and DNA binding. We could have used a truncated version of NFAT5, containing the known DNA binding sites NLS and NESs. However, the use of full-length NFAT5 obviated any question of possible interactions with other parts of the protein. To provide an objective and quantitative measurement of subcellular localization, we used Western analysis of nuclear and cytoplasmic proteins (6).
MATERIALS AND METHODS
Cell culture.
WT and DNA binding domain eliminated NFAT5 (Mut) mouse embryonic fibroblast (MEF) cell lines (11) were provided by Dr. W.Go. MEF cells were grown in DMEM with 10% FBS. Human embryonic kidney 293 (HEK293) cells were grown in EMEM with 10% FBS and 2 mM l-glutamine.
Western analysis.
Whole cell proteins were extracted using PhosphoSafe Extraction Reagent (EMD Chemicals, Gibbstown NJ). Nuclear and cytoplasmic proteins were separately extracted using the NE-PER nuclear and cytoplasmic extraction reagent kit (Thermo Scientific, Rockford IL), according to the manufacturer's instructions. Concentration of extracted proteins was measured using BCA protein assay reagent (Thermo Scientific). Then, 10 to 30 μg of total protein were migrated on a NuPAGE Novex 3–8% tris-acetate gel (Invitrogen) and transferred to nitrocellulose membrane (Invitrogen) by electrophoresis. The membrane was incubated in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h and then incubated with primary antibody overnight. After the incubation with IR dye-labeled secondary antibody for 1 h, the membrane was scanned using the Odyssey imaging system (LI-COR BioSciences). The nuclear to cytoplasmic ratio (n:c) was calculated as previously described (6): 1) [lysate volume (μl)] * [protein concentration (μg/μl)] = total protein in a fraction (μg); 2) [protein loaded in gel (μg)]/[total protein in fraction (μg)] = percentage of protein loaded; 3) [densitometry of NFAT5 band]/[percentage of protein loaded] = NFAT5 in nuclear or cytoplasmic fraction; and 4) NFAT5 in nucleus/NFAT5 in cytoplasm = n:c ratio.
Transfection.
Transfection of plasmids used Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Cells were transfected 40 h before an experimental change of the medium.
Reagents, antibodies, and plasmids.
Rabbit polyclonal anti-NFAT5 antibody (sc-13035) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-V5 antibody (MCA-1360) was purchased from AbD Serotec (Kidlington, Oxford, UK). Mouse monoclonal anti-α tubulin antibody (MP Biomedicals, Solon, OH) was used as a cytoplasmic marker and rabbit monoclonal anti-CREB antibody (9197; Cell Signaling Technology, Boston, MA) as a nuclear marker. Mouse monoclonal anti-Nup88 antibody (sc-365868) was purchased from Santa Cruz Biotechnology. LMB, purchased from Sigma-Aldrich (St. Louis, MO), was used at 10 ng/ml, dissolved in 0.03% methanol; the methanol was also added to the controls. pCMV6-XL5 empty and pCMV6-XL5 Nup88 (NP_0025.2) constructs were purchased from Origene (Rockville, MD). Human NFAT5 cDNA clone KIAA0827 was a gift from Dr. Takahiro Nagase (Kazusa DNA Research Institute, Chiba, Japan). Sequence coding for amino acids 1–1531 of KIAA0827 was cloned into expression vector pcDNA6V5-His (Invitrogen) to generate 1–1531V5-His, as previously described (14).
EMSA.
The osmotic response element (ORE) DNA probe was previously described (15). It contains bp −1,238 to −1,104 of the human aldose reductase gene. It includes three ORE elements (18) and is labeled with IR 800. After transfection of WT, T298A, T298D, and/or S155A NFAT5-V5 plasmids, the concentration of NaCl was changed in the medium, and a whole cell extract was prepared using cell lysis buffer [50 mM Tris·HCl pH8.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1 mM DTT, 1% phosphatase inhibitor cocktail 1 and 2, respectively (Sigma), and 1 tablet/10 ml Complete protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis IN)]. Then, 10–20 μg of whole cell protein extract were mixed with 300 fmol ORE probe in 20-μl reaction buffer. After 20 min of incubation at room temperature, proteins were migrated in a 0.4% agarose gel at 80 V for 2.5 h. The gel was scanned directly with the Odyssey imaging system to visualize the IR 800 labeled probe. Equal expression of transfected NFAT5-V5 constructs was confirmed by Western analysis before the EMSAs were run.
FoldX analysis.
Theoretical prediction of the effect of mutating or phosphorylating NFAT5-T298 on the stability of binding of NFAT5 to OREs utilized FoldX (http://foldx.crg.es/; Ref. 28). The DNA sequence that was used contains the 280 DNA residues surrounded by NFAT5 in the crystal structure of NFAT5 bound to DNA (29). In the simulation, threonine 298 is replaced by 20 other native amino acids or is phosphorylated, and stability of the NFAT5/DNA complex is calculated relative to threonine 298.
Targeted ion selection mass spectrometry to test for phosphorylation of NFAT5-T298.
HEK293 cells were transfected with NFAT5-1-547-V5 at 300 mosmol/kgH2O and then medium was changed to the same osmolality, lowered to 200 mosmol/kg, or raised to 500 mosmol/kgH2O for 1 h (NaCl varied). The experiment was performed (n = 3) at 300, at 500, and at 200 mosmol/kgH2O. NFAT5-V5 was immunoprecipitated with anti-V5 agarose beads and eluted by 2% SDS with constant vortexing at room temperature. The eluted NFAT5-V5 was separated on a Bis-Tris 4–12% gel (Invitrogen). The portion of the lane containing NFAT5-1-547-V5 was sliced out, and the proteins in it were digested by trypsin (Promega). Phosphopeptides were enriched via iron IMAC (Pierce), both the retained phosphopeptides and the flow through from the column were analyzed via targeted ion selection on an Eksigent nanoLC Ultra coupled with a Thermo LTQ-Orbitrap Velos mass spectrometer. Calculated m/z values of T298-containing peptides (peptides: [294–302], [296–302], [296–306], [294–306]) with up to 2 miss cleavages and 0–3 phosphorylation sites were targeted in the targeted ion selection (TIS).
RESULTS
Role of threonine 298 of NFAT5 in determining nuclear localization of NFAT5.
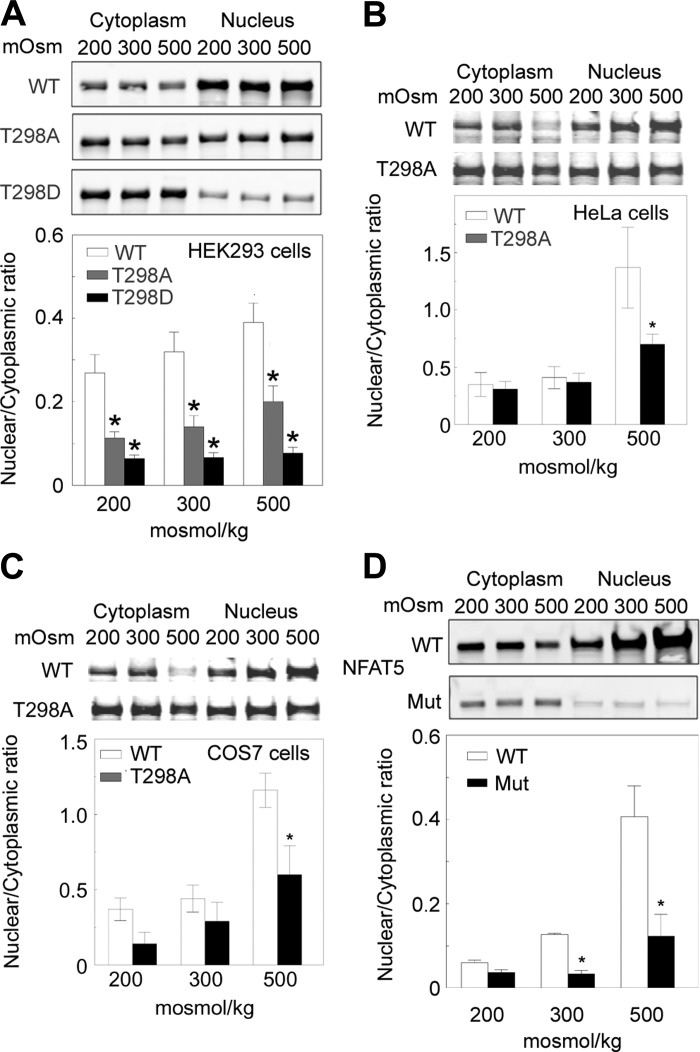
Threonine 298 in NFAT5 is a possible site of phosphorylation. To determine whether phosphorylation of NFAT5-T298 might affect nuclear localization of NFAT5, we mutated NFAT5-T298 to alanine, which cannot be phosphorylated, or to aspartate, which, being negatively charged, mimics the negative charge of phosphorylation. We transfected HEK293 cells with WT or mutant (T298A or T298D) NFAT5-V5 at 300 mosmol/kgH2O and then exchanged the medium for one in which osmolality was kept at 300 mosmol/kgH2O, decreased to 200 mosmol/kgH2O, or increased to 500 mosmol/kgH2O for 2 h by varying NaCl. We measured NFAT5-V5 separately in nuclear and cytoplasmic extracts and calculated the nuclear to cytoplasmic ratio (n:c ratio) of NFAT5. The n:c ratio of NFAT5-WT increases with concentration of NaCl (Fig. 1A). Mutating NFAT5-T298 to alanine decreases the NFAT5 n:c ratio, and mutating T298 to aspartic acid decreases the n:c ratio even further (Fig. 1A). To determine whether this effect is peculiar to HEK293 cells, we also tested the effect of mutating threonine 298 to alanine on the n:c ratio of NFAT5-V5 in HeLa and COS7 cells. In HeLa (Fig. 1B) and COS7 (Fig. 1C) cells, the n:c ratio of NFAT5-V5-T298A is less than that of WT NFAT5 at 500 mosmol/kgH2O. We conclude that threonine 298 of NFAT5 is important for high NaCl-induced nuclear localization of NFAT5. We next attempted to see whether NFAT5-T298 is phosphorylated.
Fig. 1.
Mutation of nuclear factor of activated T cell 5 NFAT5 threonine 298 to alanine or aspartic acid reduces its nuclear to cytoplasmic ratio (n:c). A: HEK293 cells transfected with NFAT5-V5, NFAT5-T298A-V5, or NFAT5-T298D-V5 at 300 mosmol/kgH2O were exposed to 200, 300, or 500 mosmol/kgH2O (NaCl varied) for 2 h and then V5 proteins were measured by quantitative Western analysis in cytoplasmic and nuclear extracts. Top: representative Western blots. Bottom: combined results. B: as in A, except with HeLa cells. C: as in A, except with COS7 cells. D: elimination of the DNA binding domain in NFAT5 greatly reduces its nuclear localization. Wild type (WT) mouse embryonic fibroblasts (MEFs) and MEFs in which NFAT5 is mutated to eliminate its DNA binding domain (Mut) were grown at 300 mosmol/kgH2O and then the medium was changed for 2 h to 200, 300, or 500 mosmol/kgH2O by varying NaCl. NFAT5 was measured by quantitative Western analysis in separate cytoplasmic and nuclear extracts and the NFAT5 n:c was calculated. Top: representative Western blots. Bottom: combined results. (means ± SE; n = 3; *P < 0.05 vs. WT).
Search for phosphorylation at NFAT5-T298.
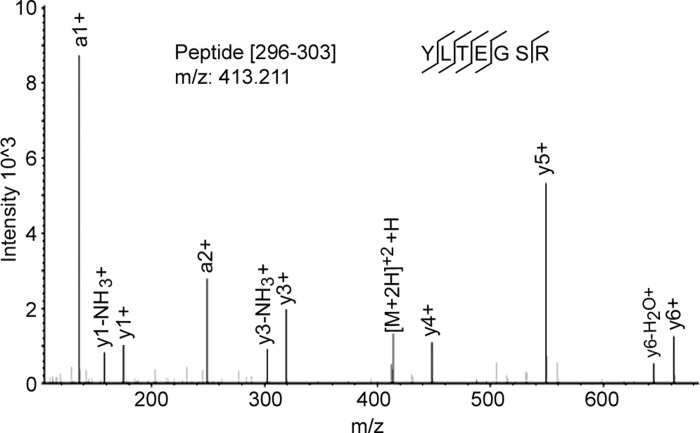
The antibody that we produced against phospho-NFAT5-T298 was not specific for phosphorylation at that site, so we used TIS mass spectrometry to search for phosphorylation. We transfected HEK293 cells with NFAT5-1-547-V5 at 300 mosmol/kgH2O and then changed the medium for 1 h to the same osmolality or lowered osmolality to 200 mosmol/kgH2O by reducing NaCl or raised osmolality to 500 by adding NaCl, followed by immunoprecipitation and in gel trypsin digestion. We performed the experiment three times at 200, at 300, and at 500 mosmol/kgH2O. We found the tryptic peptide containing unphosphorylated NFAT5-T298 (Fig. 2) in all samples but never found the peptide containing phosphorylated NFAT5-T298. Being unable to establish that there is phosphorylation of NFAT5-T298, which would provide a binding site for 14-3-3 proteins, we attempted to discover another basis for the role of NFAT5-T298 in nuclear localization of NFAT5.
Fig. 2.
Spectrum of nonphosphorylated peptide NFAT5–296-302. No phosphorylated peptides were detected.
DNA binding domain of NFAT5 is necessary for NFAT5 nuclear localization.
NFAT5-T298 is one of the contact sites between the DNA binding domain of NFAT5 and the DNA element to which it binds specifically (29). To determine whether specific DNA binding might be a mechanism promoting NaCl-induced nuclear localization of NFAT5, we measured the n:c ratio of NFAT5 in WT MEFs and in MEFs in which the DNA binding domain of NFAT5 is eliminated (11). Two hours after osmolality is changed by varying NaCl, the NFAT5 n:c ratio increases directly with NaCl concentration in WT MEFs. However, high NaCl does not elevate the n:c ratio of NFAT5 lacking its DNA binding domain in the mutant MEFs (Fig. 1D). This result raised the possibility that NFAT5-T298 might affect nuclear localization of NFAT5 by supporting specific DNA binding of NFAT5.
Theoretical prediction of the effect on its specific DNA binding of mutating or phosphorylating NFAT5-T298.
NFAT5 binds to specific DNA elements (OREs) in its target genes (7). To predict effects on the stability of the NFAT5/ORE complex of mutations and of phosphorylation, we conducted a theoretical analysis by Foldx (28) of the effect of mutating threonine 298 to alanine or aspartate or of phosphorylating threonine 298 (Table 1). Foldx is a program that predicts protein-DNA stability based on the change in unfolding free energy (ΔG) of an amino acid substitution on the protein structure. ΔΔG = ΔGwt − ΔGmut where ΔGwt and ΔGmut are the free energy of unfolding for WT and mutant proteins, respectively. Negative and positive ΔΔG (kcal/mol) values correspond to stabilizing and destabilizing effects of mutations, respectively (28). Mutating T298 to alanine decreases the calculated stability of NFAT5/ORE complex (0.3 vs. 0, Table 1), and mutating to aspartic acid decreases the stability even more (1.49 vs. 0, Table 1), presumably because of the negative charge of aspartic acid. Phosphorylating NFAT5-T298 also greatly lowers the stability of the NFAT5/ORE complex.
Table 1.
Analysis by Foldx of the stability of the NFAT5/ORE complex with other amino acids substituted for T298
| Amino Acid Replacing T298 | Decrease of Binding Stability, kcal/mol |
|---|---|
| Phospho-T298 | 4.29 |
| Aspartic acid (D) | 1.49 |
| Tryptophan (W) | 1.42 |
| Glutamic acid (E) | 0.96 |
| Lysine (K) | 0.88 |
| Histidine (H) | 0.71 |
| Tyrosine (Y) | 0.64 |
| Arginine (R) | 0.49 |
| Glutamine (Q) | 0.46 |
| Glycine (G) | 0.4 |
| Proline (P) | 0.32 |
| Alanine (A) | 0.3 |
| Methionine (M) | 0.17 |
| Phenylalanine (F) | 0.02 |
| Threonine (T) | 0 |
| Valine (V) | −0.1 |
| Leucine (L) | −0.18 |
| Serine (S) | −0.26 |
| Asparagine (N) | −0.31 |
| Cysteine (C) | −0.61 |
| Isoleucine (I) | −1.53 |
Stability decreases with increase in kcal/mol.
Effect of mutation of NFAT5-T298 on specific DNA binding of NFAT5.
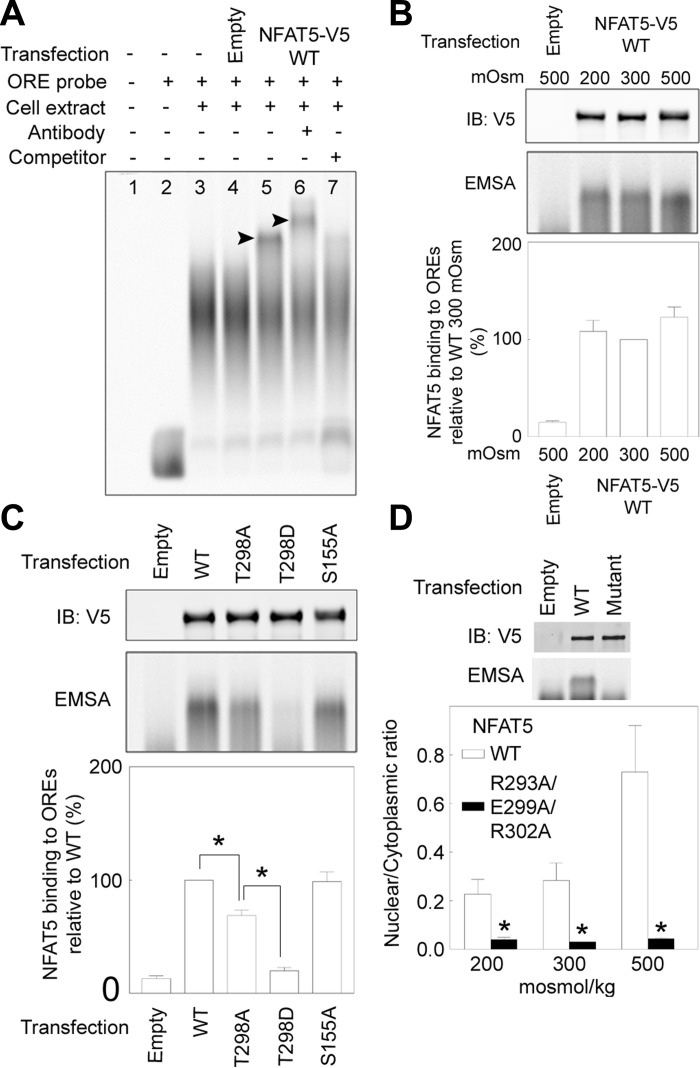
We used EMSA with an IR-800 labeled ORE DNA probe to test experimentally for the effect of mutations of NFAT5-T298 on its specific binding to ORE elements (Fig. 3). Whole cell extracts were prepared from HEK293 cells that had been transfected with various NFAT5-V5 constructs or with empty vector-V5. Specificity of the EMSA is demonstrated by finding that the mobility shift (Fig. 3A, lane 5) does not occur in cells transfected with empty vector-V5 (Fig. 3A, lane 4), is eliminated by 10× unlabeled probe (Fig. 3A, lane 7), and is increased (“supershift”) by anti-NFAT5 antibody (Fig. 3A, lane 6). Interestingly, the mobility shift is independent of the osmolality to which the cells are exposed (Fig. 3B), suggesting that tonicity does not affect specific DNA binding of NFAT5. Effects on EMSA of mutating NFAT5-T298-V5 are shown in Fig. 3C. Protein extracts were prepared from HEK293 cells expressing equal amounts of either WT or mutant NFAT-V5. The amount of NFAT5-WT-V5 bound to the shifted probe is reduced by the T298A mutation and is further reduced by the T298D mutation (Fig. 3C). Thus mutations that reduce nuclear localization of NFAT5 also proportionately reduce its specific DNA binding, a correlation that supported the possibility that specific DNA binding of NFAT5 contributes to its high NaCl-induced nuclear localization. The following experiment was aimed at testing that possibility further by determining whether mutation of other amino acids in NFAT5 that are specific DNA contact sites also affect both the specific DNA binding and nuclear localization of NFAT5.
Fig. 3.
A: EMSA demonstrating specificity of NFAT5-V5 binding to the IR 800 labeled osmotic response element (ORE) probe. Proteins extracted from HEK293 cells transfected with NFAT5-WT-V5 produce a shifted band (lane 5) but not extracts from cells transfected with empty vector (lane 4). Addition of anti-NFAT5 antibody supershifts the band (lane 6). Competitor DNA (non-IR 800-labeled ORE probe) reduces the intensity of the shifted band (lane 7). B: lack of effect of the NaCl concentration to which HEK293 cells are exposed on specific DNA binding of NFAT5. HEK293 cells were transfected with NFAT5-WT-V5 and then incubated at 200, 300, or 500 mosmol/kgH2O (NaCl varied) for 2 h. Equivalent expression of NFAT5-WT-V5 is confirmed by Western blot (top). Bottom: means ± SE; n = 3. C: effect of mutating NFAT5-T298-V5 on its specific binding to OREs. HEK293 cells were transfected with NFAT5-WT-V5, NFAT5-T298A-V5, NFAT5-T298D-V5, or NFAT5-S155A-V5 at 300 mosmol/kgH2O and then incubated at 500 mosmol/kgH2O (NaCl added) for 2 h. Equivalent expression of NFAT5-V5 is confirmed by Western blot (top). Bottom: intensity of the specifically shifted band (means ± SE; n = 3; *P < 0.05). D: effect of simultaneous mutation to alanine of three amino acids in NFAT5 that are contact sites with OREs. HEK293 cells were transfected with NFAT5-WT-V5 or NFAT5-R293A/E299A/R302A-V5, then incubated at 200, 300, or 500 mosmol/kgH2O (NaCl varied) for 2 h. Top: immunoblot (IB) of whole cell extracts from cells at 300 mosmol/kgH2O demonstrates equal expression of NFAT5-V5. EMSA demonstrates that the triple mutant does not bind to the ORE DNA probe. Bottom: n:c ratio of the triple mutant is greatly reduced at all NaCl concentrations (means ± SE; n = 3; *P < 0.05).
Effect on specific DNA binding and nuclear localization of NFAT5 of mutating specific DNA contact sites other than T298.
Arginine 293, glutamic acid 299, and arginine 302 are specific DNA contact sites in NFAT5 (29). We mutated all of them to alanine in combination and tested the effect of the triple mutation on specific DNA binding and the n:c ratio of NFAT5. The specific binding of NFAT5-R293A/E299A/R302A-V5 to OREs is much less than that of WT NFAT5-V5, as illustrated by the representative EMSA in Fig. 3D, top. Binding of the mutant NFAT5 was only 4 and 7% of that of WT NFAT in two independent experiments. Further, nuclear localization of the triple mutant is also greatly inhibited at all NaCl concentrations (Fig. 3D, bottom). Note, that unlike NFAT5-T298 none of the other mutated amino acids can be phosphorylated, which makes it unlikely that altered phosphorylation contributes to the effects of the mutations. We conclude that reduced specific binding to ORE DNA elements correlates with the reduced nuclear localization of NFAT5-T298A, -T298D, and -R293A/E299A/R302A.
The following experiments were aimed at testing the possibility that a mechanism already reported to regulate nuclear localization of NFAT5 is involved in the reduced nuclear localization of NFAT5 that is mutated at threonine 298. The mechanisms are hypotonicity-induced export of NFAT5, regulated by phosphorylation of NFAT5-S155 (34); XPO1 (CRM1/exportin1)-mediated export of NFAT5 from the nucleus (30); and hypertonicity-induced increase of NUP88, which supports high NaCl-induced increase of NFAT5 in the nucleus of mIMCD3 cells (1).
Tests of whether enhanced export of NFAT5, mediated by phosphorylation of NFAT5-S155, is involved in the effect of NFAT5-T298 on nuclear localization of NFAT5.
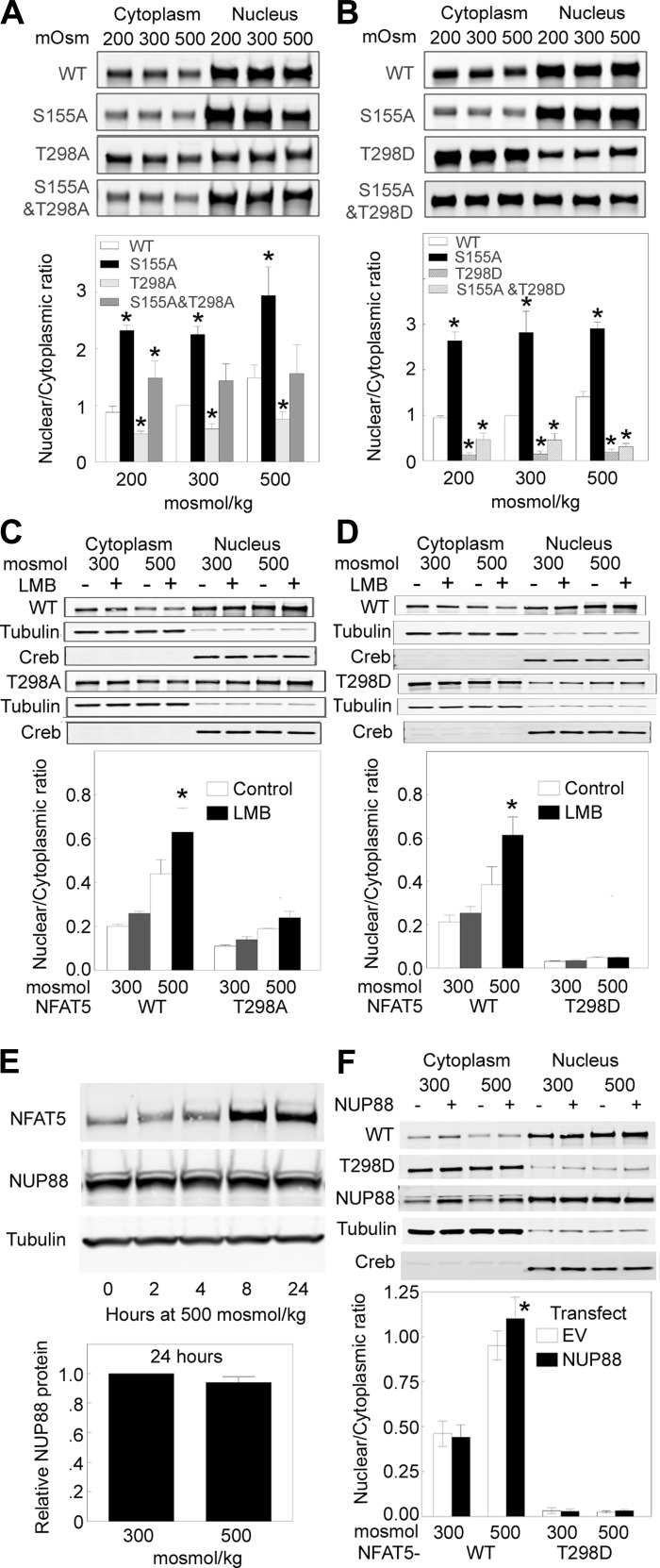
When osmolality bathing HeLa cells is reduced to 260 mosmol/kgH2O by lowering NaCl, NFAT5-1-581 becomes phosphorylated at S155 and S158, which reduces its nuclear localization (34). Mutating NFAT5-1-581-S155 to alanine, which cannot be phosphorylated, prevents low NaCl-induced reduction of NFAT5-1-581-FLAG in the nucleus of HeLa cells (34). In agreement, we find that low NaCl (200 mosmol/kgH2O) reduces the n:c ratio of WT NFAT5-1-1531-V5 and that mutating NFAT5 serine 155 to alanine increases the n:c ratio of NFAT51–1531-S155A-V5 at 200 mosmol/kgH2O in HEK293 cells (Fig. 4, A and B). In addition, we find that the n:c ratio of NFAT5-1-1531-S155A is also elevated in HEK 293 cells at 300 and at 500 mosmol/kgH2O (Fig. 4, A and B). Thus NFAT5-S155 is an important regulator of NFAT5 nuclear localization, regardless of the level of NaCl.
Fig. 4.
Effect of mutations of NFAT5-T298 and -S155, of leptomycin B (LMB), and of overexpression of NUP88 on n:c ratio of NFAT5 (means ± SE; n = 3; *P < 0.05). A and B: NFAT5-S155 and -T298. HEK293 cells transfected with NFAT5-V5-WT or mutants at 300 mosmol/kgH2O were exposed to 200, 300, or 500 mosmol/kgH2O (NaCl varied) for 2 h and then V5 proteins were measured by quantitative Western analysis of cytoplasmic and nuclear extracts. A: n:c ratio of NFAT5-S155A-V5 is greater than WT, NFAT5-T298A-V5 is less than WT, and NFAT5-S155A/T298A is intermediate. B: n:c ratio of NFAT5-S155A-V5 is greater than WT, NFAT5-T298D-V5 is close to zero, and NFAT5-S155A/T298D is only slightly more than NFAT5-T298D-V5. Conclusion: reduction of n:c ratio by mutation of NFAT5-T298 is not overcome by the mutation of NFAT5-S155 that elevates n:c ratio of NFAT5. C and D: effect of the XPO1 inhibitor LMB; as in A and B except that cells were incubated at 300 or 500 mosmol/kgH2O with LMB or its vehicle for 5 h before measurement of n:c ratio of NFAT5-V5. C: LMB increases n:c ratio of NFAT5-WT-V5 at 500 mosmol/kgH2O but has only small and statistically insignificant effects on n:c ratio of NFAT5-T298A-V5. D: LMB increases n:c ratio of NFAT5-WT-V5 at 500 mosmol/kgH2O but has no effect on n:c ratio NFAT5-T298D-V5. Conclusion: Reduction of n:c ratio of NFAT5 by mutation of NFAT5-T298 does not involve increase of XPO1- mediated export of NFAT5 from the nucleus. E and F: NUP88. E: effect of high NaCl on expression of NUP88 in HEK293 cells. High NaCl does not affect the abundance of NUP88 protein. F: effect of overexpression of NUP88. HEK293 cells were transfected with NUP88 or empty vector plus NFAT5-WT-V5 or NFAT5-T298D-V5 and then were incubated at 300 or 500 mosmol/kgH2O for 2 h before measuring n:c ratio of NFAT5. Overexpression of NUP88 increases n:c ratio of NFAT5-WT-V5 at 500 mosmol/kgH2O but has no effect on the low n:c ratio of NFAT5-T298D-V5.
Since specific binding to OREs, mediated by NFAT5-T298, might be involved in nuclear localization of NFAT5, we performed EMSA to test whether mutation of NFAT5 serine 155 to alanine also affects specific binding to OREs. NFAT5-1-1531-S155A binds to OREs as efficiently as does WT NFAT5-1-1531 (Fig. 3C). Thus the effect of NFAT5-S155 on nuclear localization of NFAT5 does not involve specific binding to OREs.
We next tested the n:c of NFAT5-T298A/S155A-V5 and NFAT5-T298D/S155A-V5 in HEK293 cells to test whether reduced n:c of NFAT5-T298A and NFAT5-T298D depends on the mechanism regulated by phosphorylation of NFAT5-S155. The NFAT5-T298A/S155A n:c ratio is intermediate between the high levels of NFAT5-S155A and the low levels of NFAT5-T298A and is not affected by the concentration of NaCl (Fig. 4A). The NFAT5-S155A/T298D n:c ratio is very low, and remains low at all NaCl concentrations (Fig. 4A). Evidently, the absence of phosphorylation of NFAT5-S155 (NFAT5-S155A/T298A and NFAT5-S155A/T298D) does not negate the effect of the NFAT5-T298 mutations, indicating that the reduced the n:c of the T298 mutants does not depend to any important degree on whether NFAT5-S155 can be phosphorylated.
Lack of effect of inhibition of XPO1-mediated nuclear export of NFAT5 on nuclear localization of NFAT5-T298A and NFAT5-T298D.
Under normotonic conditions (300 mosmol/kgH2O), NFAT5 apparently shuttles actively between the nucleus and cytoplasm (30). LMB, a specific inhibitor of the nuclear export receptor XPO1 (8, 27), causes nuclear retention of NFAT5 at 300 mosmol/kgH2O in HeLa (30) and mIMCD3 (1) cells. We used LMB to test whether enhanced XPO1-mediated nuclear export might be involved in the reduced nuclear localization of NFAT5-T298A and NFAT5-T298D. In contrast to the result in HeLa and mIMCD3 cells, LMB does not significantly raise the n:c ratio of WT NFAT5 at 300 mosmol/kgH2O in HEK293 cells (Fig. 4, C and D). However, LMB does increase the WT NFAT5 n:c ratio at 500 mosmol/kgH2O in HEK293 cells (Fig. 4, C and D), which was not tested in the previous studies of HeLa and mIMCD3 cells. In contrast to the result with WT NFAT5, LMB does not significantly affect the n:c ratio of NFAT5-T298A (Fig. 4C) or NFAT5-T298D (Fig. 4D). Thus we do not find evidence that acceleration of XPO1-mediated export of either NFAT5-T298A or NFAT5-T298D from the nucleus contributes to its low nuclear localization.
Lack of effect of overexpression of NUP88 on the reduced nuclear localization of NFAT5-T298D.
Nup88 protein increases in mIMCD3 cells within 4 h after NaCl is elevated to 550 mosmol/kgH2O, and it remains elevated for at least 48 h (1). In mIMCD3 cells, silencing of Nup88 prevents high NaCl-induced nuclear localization of NFAT5-1-267-GFP (1). In contrast, we do not find any effect of high NaCl on protein abundance of NUP88 in HEK293 cells (Fig. 4E). The n:c ratio of NFAT5-T298D in HEK293 cells is already close enough to zero (Fig. 4, B, D, and F) to make it unlikely that any further reduction by knock down of NUP88 could be seen. Therefore, we tested for the possible opposite effect, that overexpression of NUP88 might increase the n:c ratio of NFAT5-T298D. Overexpression of NUP88 increases the n:c ratio of WT NFAT5 at 500 mosmol/kgH2O but has no effect on the n:c ratio of NFAT5-T298D (Fig. 4E). Thus we do not find that the low nuclear localization of NFAT5-T298D involves NUP88.
DISCUSSION
NFAT5 isoforms a (NFAT5a) and c (NFAT5c).
Alternative splicing of NFAT5 results in isoforms NFAT5a and NFAT5c. The isoforms are identical except that NFAT5a lacks the first 76 amino acids of NFAT5c, resulting in different numbering of the amino acids in their identical portion. All of the experiments referred to up to this point used NFAT5c. NFAT5a is myristoylated at glycine 2 and palmitoylated at cysteine 5 (3). The lipid anchors result in binding to the plasma membrane. In contrast, NFAT5c is neither myristoylated nor palmitoylated and is diffusely spread within the cytoplasm. Hypertonicity elicits translocation of NFAT5a from the plasma membrane into the nucleus despite the lipid anchoring. We will refer to isoform a again later, and when we do we will specify not only the actual numbering of its amino acids but also of the comparable amino acids in NFAT5c to facilitate comparison.
Relation between specific DNA binding and nuclear localization.
We find that in HEK293 cells mutations in NFAT5 that reduce its specific binding to OREs also reduce its nuclear localization. NFAT5-T298A mutation reduces both EMSA (Fig. 3C) and the n:c ratio (Fig. 4A) by approximately one-half, whereas NFAT5-T298D mutation virtually completely inhibits both EMSA (Fig. 3C) and the n:c ratio (Fig. 4B). NFAT5-R293A/E299A/R302A mutation also virtually completely inhibits both EMSA and the n:c ratio (Fig. 3D). In HeLa (Fig. 1B) and COS7 (Fig. 1C) cells, NFAT5-T298A also causes comparable reductions in EMSA and high NaCl-induced n:c. Surprisingly, previous investigations did not find that mutations that prevent specific DNA binding of NFAT5 also reduce its nuclear localization. Thus the Kwon laboratory reported (19) virtually no specific DNA binding of NFAT5-T298A/E299A/R302A-FLAG from COS7 cells, but the mutated form displayed normal tonicity-dependent nuclear localization. Similarly, NFAT5a-T222A/E223A/R226A (equivalent to NFATc-T298A/E299A/R302A) mutants that did not bind specifically to DNA in HeLa cells, nevertheless translocated to the nucleus following hypertonicity (4). Those results evidently differ from ours, but the reason remains speculative.
Previously, mutations in STAT transcription factors were also observed to reduce both their specific DNA binding and their stimulus-induced nuclear translocation. For example, a functional DNA binding domain is required for growth hormone (GH)-induced nuclear accumulation of Stat5B (12). When coexpressed with GH receptor in COS7 cells, GFP-Stat5B is tyrosyl-phosphorylated, forms dimers, and binds DNA in response to GH. GFP-Stat5B accumulates in the nucleus upon GH stimulation. A mutation (466VVVI469 replaced by alanines) that prevented binding to DNA also abrogated GH-stimulated nuclear localization. Another example involves a nuclear export signal located within the DNA-binding domain of the STAT1transcription factor (22). Nuclear accumulation of STAT1 requires specific tyrosine phosphorylation. The presence of STATs in the nucleus is transient, however. Within hours the STATs reappear in the cytoplasm. STAT1 can be dephosphorylated in the nucleus and actively exported by the XPO1 export receptor. XPO1 recognizes a specific amino acid sequence located within the DNA-binding domain of STAT1. This region shares sequence and functional properties of characterized nuclear export signals. The location of this sequence within STAT1 suggests that it is not accessible to XPO1/CRM1 when STAT1 is bound to DNA. The authors proposed that STAT1 is tyrosine dephosphorylated in the nucleus and dissociates from DNA, allowing recognition by XPO1 and nuclear export. A third example is a nonclassical arginine/lysine-rich NLS within the DNA-binding domain of STAT1 and STAT2 (23). Mutations R378A/K379A, K410A/K413A, or K413A/R418A in STAT1 and R409A/K415A in STAT2 reduced INF-induced nuclear accumulation and specific DNA binding. STAT1-K410 and STAT1-K413 are important for nuclear localization of STAT1/STAT2 dimers because these sites directly interact with importin alpha 5 (NPI-1; Ref. 5). None of the studies of the relation between DNA binding of STATs and their nuclear localization found evidence that DNA binding per se retains the STATs in the nucleus.
Some of the possibilities proposed for the relation between DNA binding and nuclear localization of STAT transcription factors could also apply to NFAT5. Thus the mutations might disrupt the structure of the protein at a more distant location, preventing the interaction with proteins necessary for nuclear import (12). Alternatively, specific binding to DNA might conceal a site otherwise accessible to XPO1 (e.g., an NES in NFAT5), resulting in reduced export from the nucleus (22). The latter mechanism does not appear likely since, at least in HEK293 cells, inhibition of XPOI that acts via the NFAT5 NES at amino acids 8–15 (29) has only modest effect on WT NFAT5 (Fig. 4, C and D). Also to be considered is the possibility of another, as yet unidentified, NES in NFAT5. There are several hydrophobic residues (I284, V285, V286, V305, F313, and V316) in the vicinity of the DNA contact sites mutated in the present study (R293, T298, E299, and R302). If there is another NES, it might interact with an exportin other than XPOI. NFAT5 has an NLS at amino acids 199–216 (29). There are also basic residues, characteristic of nuclear localization sequences, near the DNA contact sites mutated in the present study. They are K280, L282, K283, K306, and R308. Finally, modulation of nucleocytoplasmic transport of transcription factors by serine/threonine phosphorylation is well established (16). Since several of the sites whose mutation reduces both specific DNA binding of NFAT5 and its nuclear localization cannot be directly phosphorylated, any effect involving phosphorylation would have to be indirect or involve other NFAT5 amino acids.
At this point, the conclusion that specific DNA binding contributes directly to nuclear localization of NFAT5 is based on the correlation that we found between the effect of particular mutations of NFAT5 on its specific DNA binding and on its nuclear localization. Although we consider it likely that specific DNA binding directly enhances nuclear localization of NFAT5, we recognize that more evidence, particularly as to mechanism, is necessary to convincingly support the conclusion.
Methodological considerations.
The subcellular location of NFAT5 differs between individual cells. Under normotonic conditions, NFAT5 is predominantly located in the cytoplasm of some cells and in the nucleus of others in the same dish. Hypertonicity increases the fraction of cells in which NFAT5 is predominantly in the nucleus and hypotonicity decreases the fraction of cells in which it is predominantly nuclear. Pictures of cells containing colorfully labeled NFAT5 (anti-NFAT5 immunocytochemistry or NFAT5-GFP) distinguish NFAT5 in the nucleus from that in the cytoplasm in a direct and convincing manner. However, although color-labeled NFAT5 can be separately quantified in the nucleus and cytoplasm by computer-based image analysis, such objective image analysis has not generally been employed with NFAT5. Instead, investigators have counted cells in which NFAT5 appears to be nuclear, in which it appears to be cytoplasmic, and in which it appears to be in both nucleus and cytoplasm. This method introduces a subjective element. Supportive illustration showing cells does not avoid the problem since subjectivity is inherent in selecting the cells to be displayed. Our own preference is the Western analysis of nuclear and cytoplasmic proteins that we employed here, since this method provides objective, quantitative results (6).
The Kwon laboratory (19) emphasized the importance of using full-length NFAT5 when investigating the effect of mutations, rather than using constructs containing only part of NFAT5. We are not convinced that this is critical, but nevertheless note that only full-length NFAT5 constructs were used in the present studies.
Physiological significance.
High NaCl stresses cells and, when excessive, can be lethal. Interstitial NaCl normally is very high in kidney medullas and can vary considerably (2). Interstitial NaCl also is normally high in some other organs, and hypernatremia occurs in a number of pathophysiological states (25). Adaptation to high NaCl depends critically on NFAT5 (21). High NaCl-induced nuclear translocation of NFAT5 plays an important role in activation of NFAT5. The mechanisms regulating high NaCl-induced nuclear translocation of NFAT5 are complex and not fully understood. The present study calls attention to the possibility that specific DNA binding of NFAT5 contributes to it nuclear translocation. This insight is important not only for understanding the physiology of renal medullary cells but also for understanding the physiology of cells in other tissues in which NaCl is high, as well as for better understanding of the cellular responses to pathological hypernatremia.
GRANTS
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.I., M.B.B., and J.D.F. conception and design of research; Y.I., J.L., and C.V. performed experiments; Y.I., J.L., C.V., K.H., M.B.B., and J.D.F. analyzed data; Y.I., J.L., C.V., K.H., M.B.B., and J.D.F. interpreted results of experiments; Y.I., J.L., M.B.B., and J.D.F. prepared figures; Y.I., M.B.B., and J.D.F. drafted manuscript; Y.I., J.L., M.B.B., and J.D.F. edited and revised manuscript; Y.I., J.L., C.V., K.H., M.B.B., and J.D.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the staff of the Proteomics Core of Intramural Research Program of the National Heart, Lung, and Blood Institute for helpful advice and assistance.
REFERENCES
- 1.Andres-Hernando A, Lanaspa MA, Rivard CJ, Berl T. Nucleoporin 88 (Nup88) is regulated by hypertonic stress in kidney cells to retain the transcription factor tonicity enhancer-binding protein (TonEBP) in the nucleus. J Biol Chem 283: 25082–25090, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Eisenhaber B, Sammer M, Lua WH, Benetka W, Liew LL, Yu W, Lee HK, Koranda M, Eisenhaber F, Adhikari S. Nuclear import of a lipid-modified transcription factor: mobilization of NFAT5 isoform a by osmotic stress. Cell Cycle 10: 3897–3911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estrada-Gelonch A, Aramburu J, Lopez-Rodriguez C. Exclusion of NFAT5 from mitotic chromatin resets its nucleo-cytoplasmic distribution in interphase. PLos One 4: e7036, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagerlund R, Kinnunen L, Kohler M, Julkunen I, Melen K. NF-κB is transported into the nucleus by importin α3 and importin α4. J Biol Chem 280: 15942–15951, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Ferraris JD, Burg MB. Tonicity-regulated gene expression. Methods Enzymol 428: 279–296, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ferraris JD, Williams CK, Jung KY, Bedford JJ, Burg MB, Garcia-Perez A. ORE, a eukaryotic minimal essential osmotic response element. The aldose reductase gene in hyperosmotic stress. J Biol Chem 271: 18318–18321, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90: 1051–1060, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Gallazzini M, Heussler GE, Kunin M, Izumi Y, Burg MB, Ferraris JD. High NaCl-induced activation of CDK5 increases phosphorylation of the osmoprotective transcription factor TonEBP/OREBP at threonine 135, which contributes to its rapid nuclear localization. Mol Biol Cell 22: 703–714, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallazzini M, Yu MJ, Gunaratne R, Burg MB, Ferraris JD. c-Abl mediates high NaCl-induced phosphorylation and activation of the transcription factor TonEBP/OREBP. FASEB J 24: 4325–4335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA 101: 10673–10678, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrington J, Rui L, Luo G, Yu-Lee LY, Carter-Su C. A functional DNA binding domain is required for growth hormone-induced nuclear accumulation of Stat5B. J Biol Chem 274: 5138–5145, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Irarrazabal CE, Gallazzini M, Schnetz MP, Kunin M, Simons BL, Williams CK, Burg MB, Ferraris JD. Phospholipase C-g1 is involved in signaling the activation by high NaCl of the osmoprotective transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 107: 906–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irarrazabal CE, Liu JC, Burg MB, Ferraris JD. ATM, a DNA damage-inducible kinase, contributes to activation by high NaCl of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 101: 8809–8814, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irarrazabal CE, Williams CK, Ely MA, Birrer MJ, Garcia-Perez A, Burg MB, Ferraris JD. Activator protein-1 contributes to high NaCl-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J Biol Chem 283: 2554–2563, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Jans DA, Hubner S. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol Rev 76: 651–685, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Ko BC, Turck CW, Lee KW, Yang Y, Chung SS. Purification, identification, and characterization of an osmotic response element binding protein. Biochem Biophys Res Commun 270: 52–61, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Ko BCB, Ruepp B, Bohren KM, Gabbay KH, Chung SS. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J Biol Chem 272: 16431–16437, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kwon MS, Lee SD, Kim JA, Colla E, Choi YJ, Suh PG, Kwon HM. Novel nuclear localization signal regulated by ambient tonicity in vertebrates. J Biol Chem 283: 22400–22409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammers PE, Beck JA, Chu S, Kempson SA. Hypertonic upregulation of betaine transport in renal cells is blocked by a proteasome inhibitor. Cell Biochem Funct 23: 315–324, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Rodriguez C, Antos CL, Shelton JM, Richardson JA, Lin F, Novobrantseva TI, Bronson RT, Igarashi P, Rao A, Olson EN. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci USA 101: 2392–2397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBride KM, McDonald C, Reich NC. Nuclear export signal located within theDNA-binding domain of the STAT1transcription factor. EMBO J 19: 6196–6206, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melen K, Kinnunen L, Julkunen I. Arginine/lysine-rich structural element is involved in interferon-induced nuclear import of STATs. J Biol Chem 276: 16447–16455, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA 96: 2538–2542, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhofer W. Role of NFAT5 in inflammatory disorders associated with osmotic stress. Curr Genomics 11: 584–590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31: 3635–3641, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science 278: 141–144, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Schymkowitz J, Borg J, Stricher F, Nys R, Rousseau F, Serrano L. The FoldX web server: an online force field. Nucleic Acids Res 33: W382–W388, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroud JC, Lopez-Rodriguez C, Rao A, Chen L. Structure of a TonEBP-DNA complex reveals DNA encircled by a transcription factor. Nat Struct Biol 9: 90–94, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Tong EH, Guo JJ, Huang AL, Liu H, Hu CD, Chung SS, Ko BC. Regulation of nucleocytoplasmic trafficking of transcription factor OREBP/TonEBP/NFAT5. J Biol Chem 281: 23870–23879, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta 1813: 1938–1945, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Woo SK, Dahl SC, Handler JS, Kwon HM. Bidirectional regulation of tonicity-responsive enhancer binding protein in response to changes in tonicity. Am J Physiol Renal Physiol 278: F1006–F1012, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Woo SK, Maouyo D, Handler JS, Kwon HM. Nuclear redistribution of tonicity-responsive enhancer binding protein requires proteasome activity. Am J Physiol Cell Physiol 278: C323–C330, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Xu SX, Wong CC, Tong EH, Chung SS, Yates JR, III, Yin YB, Ko BC. Phosphorylation by casein kinase 1 regulates tonicity-induced OREBP/TonEBP nucleocytoplasmic trafficking. J Biol Chem 283: 17624–17634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Z, Ferraris J, Irarrazabal CE, Dmitireva NI, Park JH, Burg MB. Ataxia-telangiectasia mutated (ATM), a DNA damage-inducible kinase, contributes to high NaCl-induced nuclear localization of the transcription factor TonEBP/OREBP. Am J Physiol Renal Physiol 289: F506–F511, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Gallazzini M, Burg MB, Ferraris JD. Contribution of SHP-1 protein tyrosine phosphatase to osmotic regulation of the transcription factor TonEBP/OREBP. Proc Natl Acad Sci USA 107: 7072–7077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]