Abstract
A pH-responsive, luminescent, dimetallic Eu(III)-containing complex has been synthesized and exhibits a unique mechanism of response. The luminescence-decay rate of the complex is slow, due to a lack of water molecules coordinated to the Eu(III) ions. However, the luminescence-decay rate decreases with increasing pH over a biologically relevant range of 4 to 8. Physical characterization and computational analysis suggest that the pH response is due to protonation of a bridging alkoxide at lower pH values. Modulation of the luminescence-decay rate is independent from the concentration of Eu(III), which we expect to be useful in the non-invasive imaging of in vivo pH.
Molecular imaging with fluorescence microscopy is a powerful tool in biochemical and biomedical research. Organic fluorescent probes that respond to the presence of specific molecular analytes are limited by photobleaching, broad emission bands (~100 nm), small Stokes shifts (<20 nm), and short-lived emissions (<100 ns). Lanthanide-based probes overcome many of the limitations associated with organic fluorophores because they offer atomic-based emissions that do not photobleach, emit line-like bands (<20 nm bandwidth), have large Stokes shifts, and exhibit long luminescence lifetimes (ms). Due to these advantages, many examples of lanthanide-based probes have been reported for the detection of biologically important cations,1 anions,2 neutral species,3 proteins and peptides,4 and DNA.5 Many of these probes rely on the ratio of two emission peaks to determine analyte concentration, but the luminescence-decay rate of Eu(III)-containing complexes should enable the determination of analyte concentration without knowledge of probe concentration or extinction coefficient. Reports describing time-resolved microscopy,6 luminescence-lifetime imaging,7 and phosphorescence-quenching microscopy8 demonstrate that luminescence-decay rates can be determined from microscopy and are, therefore, a useful parameter for sensing applications. Lanthanide-based probes often exhibit low sensitivity that can be addressed by the use of antennae or through he use of multimetallic complexes that increase sensitivity additively.9 In targeting the multimetallic strategy, we synthesized a new dimetallic Eu(III)-containing complex, 1, that responds to pH over a physiologically relevant range of 4 to 8 by a new mechanism, based on luminescence-decay rates, that is independent of the probe concentration and selective for only proton concentration. To our knowledge, no system of this type has been reported to respond to pH by luminescence-decay rate.
We hypothesized that bridging two Eu(III) ions with an alcohol would decrease electron density on the bridging oxygen and increase the acidity of the alcohol compared to an uncoordinated alcohol. We anticipated that the acid-base equilibrium of the bridging oxygen would result in a pH-dependent change in the luminescence-decay rate because the luminescence-decay rate of Eu(III) increases with the number of hydroxyl oscillators coordinated to the metal ion.10 We expected this change to be independent of the concentration of 1 because luminescence-decay rate is not dependent on metal concentration. Further, we expected that this mechanism would be selective for protons because hydroxyl oscillators have the correct vibrational energy levels to efficiently quench Eu(III) luminescence. Here, we present the synthesis, characterization, and spectroscopic and computational analyses of dimetallic Eu(III)-containing complex 1. Additionally, we propose a mechanism for the pH-dependent luminescence-decay rate supported by our experimental and computational data.
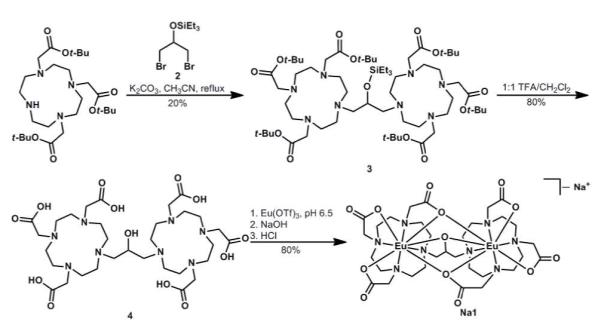
Our ligand design incorporates two Eu(III)-containing 2,2′,2″-(1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetate complexes bridged by an iso-propanol linker that is positioned to form two ortho-fused five-membered rings containing the two metal ions. The synthesis of 1 was completed in four steps (Scheme 1). Briefly, 1,3-dibromo-2-propanol was converted to triethylsilyl ether 2, which was used to alkylate two equivalents of 1,4,7,10-tetraazacyclododecane-1,4,7-tris(t-butyl acetate) yielding the protected ligand 3. We found it imperative that the alcohol be protected for the success of this alkylation. Ligand 4 was obtained by global deprotection of 3 with trifluoroacetic acid (TFA), and Eu(III) complexation was achieved by addition of an aqueous solution of Eu(III) trifluoromethanesulfonate (OTf) to ligand 4 at pH 6.5. Dialysis yielded dimetallic Eu(III)-containing complex 1. A xylenol orange test indicated that there was no unchelated Eu(III) ions present at pH 5.8 even after storing in solution at ambient temperature for 24 h.11
Scheme 1. Synthetic route to 1.
To assess the coordination environment of the Eu(III) ions, the number of water molecules coordinated to the Eu(III) ion, q, was determined using the method developed by Horrocks and coworkers.10 Complex 1 was found to have a q value of 0 in the absence of buffer (pH 5.4), suggesting that no water was bound to the Eu(III) ions. When we considered possible structures to satisfy this data, we were unable to construct a structure lacking a bridge between Eu(III) ions that contained no water molecules bound to the Eu(III) ions. This q data indicated that the Eu(III) ions are bridged by an alcohol donor from the ligand. Because coordinated OH oscillators influence the luminescence-decay rate of Eu(III) ions, we hypothesized that there would be a decrease in the luminescence-decay rate of 1 with increasing pH above 5.4 due to deprotonation of the bridging alcohol.
To test this hypothesis, we prepared 0.5 mM solutions of 1 in a series of buffers from pH 4.0 to 8.0 in increments of 0.2 pH units. The luminescence-decay rate of each solution was determined and plotted against pH values (Figure 1). We confirmed that the pH response is independent of the concentration of 1 by repeating the luminescence-decay rate measurements with 1 mM solutions of 1. These experiments resulted in identical pH-dependent changes in luminescence-decay rates. The resulting plot supports our hypothesis that the luminescence-decay rate depends on pH and indicates that the pH response extends over a biologically relevant range of 4 to 8. The second derivative of this plot was used to estimate the pKa of the protic source at 5.8, placing the maximum sensitivity of the probe very close to the pH of diseased tissue.12
Figure 1.
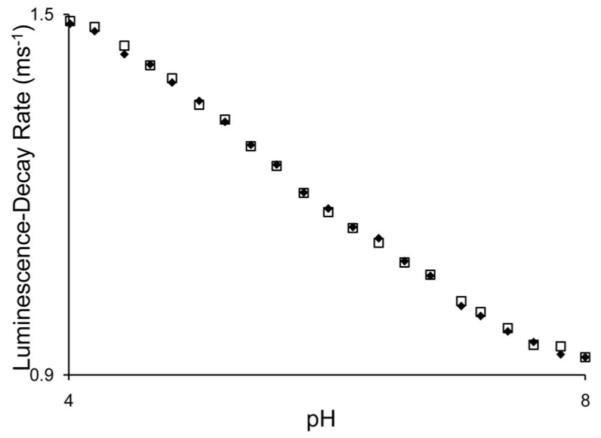
Luminescence-decay rate of 0.5 (♦) and 1.0 mM (□) 1 as a function of pH (50 mM citrate, 50 mM phosphate, λex = 395 nm, λem = 595 nm).
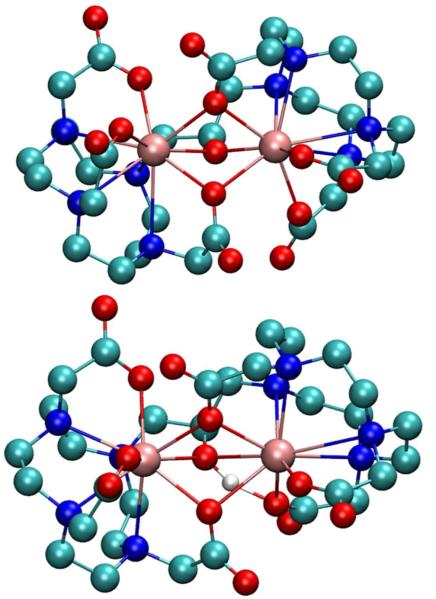
Structures of H1 and 1− were optimized with Gaussian 09 at the PBE0/SDDall level (small core for all atoms) using the default convergence criteria.13 The optimized structures suggest that the alcohol bridges the Eu(III) ions and that two carboxylate oxygens are also bridging (Figure 2). This tri-bridged structure is consistent with a 9-coordinate Eu(III) environment that is saturated by the ligand and corroborates the luminescence-decay data that indicate the absence of water molecules bound to Eu(III). Interestingly, to obtain a suitable initial guess for the optimization, it was necessary to include the two bridging carboxylates. To achieve this, the orientation of the carboxylate arms on the two chelates had to be in opposite conformations from each other (Δ vs Λ) to minimize the bridged structure. Further, the steric constraints of the ligand prevent a bridge as small as alkoxide without two bridging carboxylates. These structures corroborate both the exceptionally long luminescence lifetime at higher pH values and the luminescence quenching that results from lowering pH.
Figure 2.
Optimized structures of 1− (top) and H1 (bottom): Eu = pink, C = cyan, N = blue, O = red, and H = white. Non-exchangeable hydrogen atoms are omitted for clarity.
To further elucidate the mechanism of the pH response, we compared the emission spectra of 1 at different pH values (Figure 3 and Figure S3). At pH 4.4, we observed that the multiplicity of the ΔJ = 1 manifold (centered at 592 nm) is at the maximum of three peaks. This high multiplicity indicates that the symmetry around the Eu(III) ions is low. However, the spectrum would indicate that the symmetry of the left and right chelates is similar because we do not observe greater than 2J+1 peaks for any mani-fold in the spectrum. Surprisingly, the spectrum of 1 at pH 7.4 contains four peaks in the ΔJ = 1 manifold and two major peaks in the ΔJ = 2 manifold. The source of these additional peaks at high pH is not immediately obvious. One explanation that is consistent with all other data is that the loss of the intramolecular hydrogen bond at high pH values increases the flexibility of the ligand, resulting in a change in conformation about one or both of the Eu(III) ions. Previous studies of similar monometallic Eu(III) complexes by Horrocks and coworkers demonstrated that inter-converting isomers can be observed by luminescence spectroscopy.14 NMR analyses of interconverting lanthanide macrocyclic complexes is also well established and these methods are currently under investigation.
Figure 3.
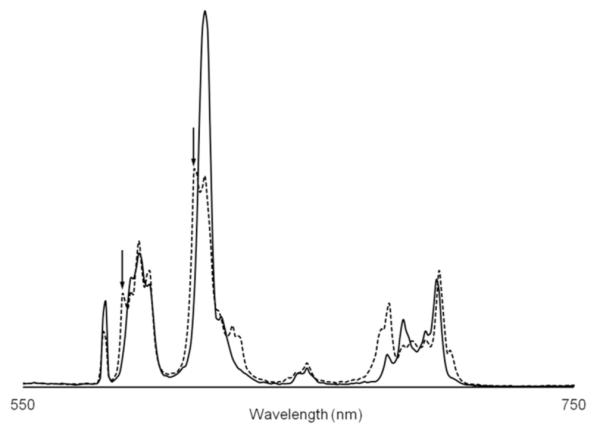
Comparison of emission spectra of 1 at pH 4.4 (solid line) and pH 7.4 (dashed line) (0.5 mM 1, 50 mM citrate, 50 mM phosphate, λex = 395 nm). Arrows point to peaks that may result from an second Eu(III) environment.
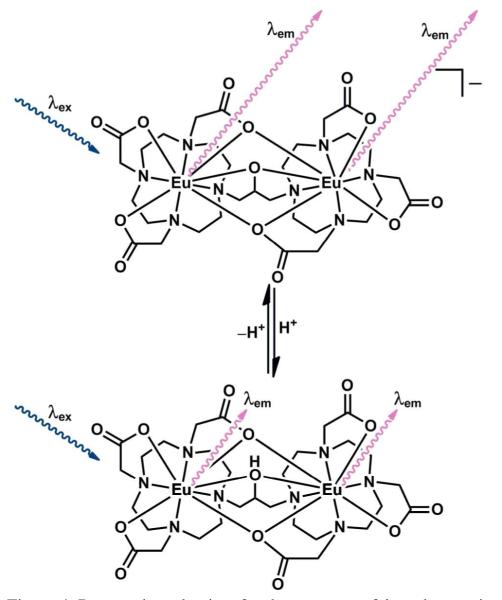
The spectroscopic and computational data suggest a novel mechanism of pH response wherein protonation of the bridging alkoxide under acidic conditions introduces a hydroxyl oscillator in the Eu(III) coordination sphere (Figure 4). The inner-sphere hydroxyl oscillator quenches the Eu(III) excited state resulting in a faster luminescence-decay rate. Further, because coordination of other cations to the alkoxide will not produce oscillators with the proper energy to quench the Eu(III) emission, we expect that this system will specifically respond to proton concentration. Moreover, this process is reversible such that continuous monitoring of pH is possible. To our knowledge, no other system of this kind has been reported to respond to pH by luminescence-decay rate.
Figure 4.
Proposed mechanism for the response of 1 to changes in pH. At high pH (top), the lack of OH oscillators leads to long-lived luminescence (long wavy arrows, λem). At low pH (bottom), the bridging OH oscillator quenches the Eu(III) excited state leading to rapid luminescence decay (short wavy arrows, λem).
We have demonstrated a concentration-independent luminescence response to pH achieved through the protic equilibrium of an alkoxide in 1. Our characterization indicates that the Eu(III) ions have no coordinated water molecules resulting in relatively slow luminescence-decay rates, especially at pH values above 7. The absence of coordinated water molecules makes this system less sensitive to changes in the composition of the surrounding media relative to systems that contain coordinated water molecules. Spectral changes with pH are indicative of a change in the coordination sphere in agreement with our proposed mechanism. Computationally optimized structures also are consistent with our experimental observations. Complex 1 represents a unique system using a new mechanism by which pH can be detected and offers a useful method for concentration-independent, non-invasive, continuous pH imaging. We expect that this research is a step toward powerful tools for biomedical and biochemical research because pH is often an indicator of disease.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by Wayne State University (GAC and MJA) and the Elsa U. Pardee Foundation (MJA). JDM was supported by a Summer Dissertation Fellowship from Wayne State University. GAC and RLL thank Wayne State University Computing & Information Technology for computing support. Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number 1R13EB015773-01.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Synthetic details, characterization, table with optimized distances, and the complete Gaussian citation. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) Parker D, Senanayake K, Williams JAG. Chem. Commun. 1997:1777–1778. [Google Scholar]; (b) Aime S, Barge A, Botta M, Howard JAK, Kataky R, Lowe MP, Moloney JM, Parker D, de Sousa AV. Chem. Commun. 1999:1047–1048. [Google Scholar]; (c) Parker D. Coord. Chem. Rev. 2000;205:109–130. [Google Scholar]; (d) Lowe MP, Parker D, Reany O, Aime S, Botta M, Castellano G, Gianolio E, Pagliarin R. J. Am. Chem. Soc. 2001;123:7601–7609. doi: 10.1021/ja0103647. [DOI] [PubMed] [Google Scholar]; (e) Woods M, Sherry AD. Inorg. Chem. 2003;42:4401–4408. doi: 10.1021/ic0300823. [DOI] [PubMed] [Google Scholar]; (f) Pal R, Parker D. Chem. Commun. 2007:474–476. doi: 10.1039/b616665b. [DOI] [PubMed] [Google Scholar]; g) Hanaoka K, Kikuchi K, Kobayashi S, Nagano T. J. Am. Chem. Soc. 2007;129:13502–13509. doi: 10.1021/ja073392j. [DOI] [PubMed] [Google Scholar]; (h) Pal R, Parker D. Org. Biomol. Chem. 2008;6:1020–1033. doi: 10.1039/b718993a. [DOI] [PubMed] [Google Scholar]; (i) Thibon A, Pierre VC. J. Am. Chem. Soc. 2009;131:434–435. doi: 10.1021/ja8077889. [DOI] [PubMed] [Google Scholar]; (j) Andrews M, Amoroso AJ, Harding LP, Pope SJA. Dalton Trans. 2010;39:3407–3411. doi: 10.1039/b923988j. [DOI] [PubMed] [Google Scholar]; (k) Weitz EA, Pierre VC. Chem. Commun. 2011;47:541–543. doi: 10.1039/c0cc02637a. [DOI] [PubMed] [Google Scholar]
- (2).(a) Dickins RS, Gunnlaugsson T, Parker D, Peacock RD. Chem. Commun. 1998:1643–1644. [Google Scholar]; (b) Dickins RS, Aime S, Batsanov AS, Beeby A, Botta M, Bruce JI, Howard JAK, Love CS, Parker D, Peacock RD, Puschmann H. J. Am. Chem. Soc. 2002;124:12697–12705. doi: 10.1021/ja020836x. [DOI] [PubMed] [Google Scholar]; (c) Atkinson P, Bretonniere Y, Parker D. Chem. Commun. 2004:438–439. doi: 10.1039/b313496m. [DOI] [PubMed] [Google Scholar]; (d) Bretonniere Y, Cann MJ, Parker D, Slater R. Org. Biomol. Chem. 2004;2:1624–1632. doi: 10.1039/b400734b. [DOI] [PubMed] [Google Scholar]; (e) Parker D, Yu J. Chem. Commun. 2005:3141–3143. doi: 10.1039/b502553b. [DOI] [PubMed] [Google Scholar]; (f) Poole RA, Bobba G, Cann MJ, Frias J-C, Parker D, Peacock RD. Org. Biomol. Chem. 2005;3:1013–1024. doi: 10.1039/b418964g. [DOI] [PubMed] [Google Scholar]; (g) Harte AJ, Jensen P, Plush SE, Kruger PE, Gunnlaugsson T. Inorg. Chem. 2006;45:9465–9474. doi: 10.1021/ic0614418. [DOI] [PubMed] [Google Scholar]; (h) Poole RA, Kielar F, Richardson SL, Stenson PA, Parker D. Chem. Commun. 2006:4084–4086. doi: 10.1039/b611259e. [DOI] [PubMed] [Google Scholar]; (i) Poole RA, Montgomery CP, New EJ, Congreve A, Parker D, Botta M. Org. Biomol. Chem. 2007;5:2055–2062. doi: 10.1039/b705943d. [DOI] [PubMed] [Google Scholar]; (j) Pålsson L-O, Pal R, Murray BS, Parker D, Beeby A. Dalton Trans. 2007:5726–5734. doi: 10.1039/b710717j. [DOI] [PubMed] [Google Scholar]; (k) Plush SE, Gunnlaugsson T. Org. Lett. 2007;9:1919–1922. doi: 10.1021/ol070339r. [DOI] [PubMed] [Google Scholar]; (l) Kielar F, Montgomery CP, New EJ, Parker D, Poole RA, Richardson SL, Stenson PA. Org. Biomol. Chem. 2007;5:2975–2982. doi: 10.1039/b709062e. [DOI] [PubMed] [Google Scholar]; (m) Murray BS, New EJ, Pal R, Parker D. Org. Biomol. Chem. 2008;6:2085–2094. doi: 10.1039/b803895c. [DOI] [PubMed] [Google Scholar]; (n) Andolina CM, Morrow JR. Eur. J. Inorg. Chem. 2011:154–164. [Google Scholar]
- (3).(a) Song B, Wang G, Tan M, Yuan J. J. Am. Chem. Soc. 2006;128:13442–13450. doi: 10.1021/ja062990f. [DOI] [PubMed] [Google Scholar]; (b) Page SE, Wilke KT, Pierre VC. Chem. Commun. 2010;46:2423–2425. doi: 10.1039/b923912j. [DOI] [PubMed] [Google Scholar]; (c) Pershagen E, Nordholm J, Borbas KE. J. Am. Chem. Soc. 2012;134:9832–9835. doi: 10.1021/ja3004045. [DOI] [PubMed] [Google Scholar]
- (4).(a) Yu J, Parker D, Pal R, Poole RA, Cann MJ. J. Am. Chem. Soc. 2006;128:2294–2299. doi: 10.1021/ja056303g. [DOI] [PubMed] [Google Scholar]; (b) Halim M, Tremblay MS, Jockusch S, Turro NJ, Sames D. J. Am. Chem. Soc. 2007;129:7704–7705. doi: 10.1021/ja071311d. [DOI] [PubMed] [Google Scholar]; (c) Kielar F, Congreve A, Law G.-l., New EJ, Parker D, Wong K-L, Castreňo P, de Mendoza J. Chem. Commun. 2008:2435–2437. doi: 10.1039/b803864c. [DOI] [PubMed] [Google Scholar]
- (5).Bobba G, Frias JC, Parker D. Chem. Commun. 2002:890–891. doi: 10.1039/b201451n. [DOI] [PubMed] [Google Scholar]
- (6).Jin D, Piper JA. Anal. Chem. 2011;83:2294–2300. doi: 10.1021/ac103207r. [DOI] [PubMed] [Google Scholar]
- (7).(a) Beeby A, Botchway SW, Clarkson IM, Faulkner S, Parker AW, Parker D, Williams JAG. J. Photochem. Photobiol., B. 2000;57:83–89. doi: 10.1016/s1011-1344(00)00070-1. [DOI] [PubMed] [Google Scholar]; (b) You Y, Han Y, Lee Y-M, Park SY, Nam W, Lippard SJ. J. Am. Chem. Soc. 2011;133:11488–11491. doi: 10.1021/ja204997c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) You Y, Lee S, Kim T, Ohkubo K, Chae W-S, Fukuzumi S, Jhon G-J, Nam W, Lippard SJ. J. Am. Chem. Soc. 2011;133:18328–18342. doi: 10.1021/ja207163r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Holst G, Kohls O, Klimant I, König B, Kühl M, Richter T. Sens. Actuators, B. 1998;51:163–170. [Google Scholar]
- (8).Helmlinger G, Yuan F, Dellian M, Jain RK. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- (9).Moore JD, Allen MJ. Recent Patents on Nanomedicine. 2011;1:88–100. doi: 10.2174/1877912311101020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Supkowski RM, Horrocks WD., Jr Inorg. Chim. Acta. 2002;340:44–48. [Google Scholar]; (b) Horrocks WD, Jr., Sudnick DR. J. Am. Chem. Soc. 1979;101:334–340. [Google Scholar]
- (11).Barge A, Cravotto G, Gianolio E, Fedeli F. Contrast Med. Mol. Imaging. 2006;1:184–188. doi: 10.1002/cmmi.110. [DOI] [PubMed] [Google Scholar]
- (12).a) Gibbs EL, Lennox WG, Nims LF, Gibbs FA. J. Biol. Chem. 1942;144:325–332. [Google Scholar]; (b) Wike-Hooley JL, van den Berg AP, van der Zee J, Reinhold HS. Eur. J. Cancer Clin. Oncol. 1985;21:785–791. doi: 10.1016/0277-5379(85)90216-0. [DOI] [PubMed] [Google Scholar]; (c) Gerweck LE, Seetharaman K. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- (13).(a) Frisch MJ, et al. Gaussian 09 Rev. A.1. Gaussian Inc.; Wallingford CT: 2009. [Google Scholar]; (b) Adamo C, Barone V. J. Chem. Phys. 1999;110:6158–6170. [Google Scholar]
- (14).Albin M, Horrocks WD, Jr., Liotta F. J. Chem. Phys. Lett. 1982;85:61–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.