Abstract
Excess amounts of abdominal subcutaneous (SAT) and visceral (VAT) adipose tissue (AT) are associated with insulin resistance, even in normal-weight subjects. In contrast, gluteal-femoral AT (GFAT) is hypothesized to offer protection against insulin resistance. Dynamic PET imaging studies were undertaken to examine the contributions of both metabolic activity and size (volume) of these depots in systemic glucose metabolism. Nonobese, healthy volunteers (n = 15) underwent dynamic PET imaging uptake of [18F]FDG at a steady-state (20 mU·m−2·min−1) insulin infusion. PET images of tissue [18F]FDG activity were coregistered with MRI to derive K values for insulin-stimulated rates of fractional glucose uptake within tissue. Adipose tissue volume was calculated from DEXA and MRI. VAT had significantly higher rates of fractional glucose uptake per volume than SAT (P < 0.05) or GFAT (P < 0.01). KGFAT correlated positively (r = 0.67, P < 0.01) with systemic insulin sensitivity [glucose disappearance rate (Rd)] and negatively with insulin-suppressed FFA (r = −0.71, P < 0.01). SAT (r = −0.70, P < 0.01) and VAT mass (r = −0.55, P < 0.05) correlated negatively with Rd, but GFAT mass did not. We conclude that rates of fractional glucose uptake within GFAT and VAT are significantly and positively associated with systemic insulin sensitivity in nonobese subjects. Furthermore, whereas SAT and VAT amounts are confirmed to relate to systemic insulin resistance, GFAT amount is not associated with insulin resistance. These dynamic PET imaging studies indicate that both quantity and quality of specific AT depots have distinct roles in systemic insulin resistance and may help explain the metabolically obese but normal-weight phenotype.
Keywords: insulin sensitivity, positron emission tomography imaging
more than 50 years ago, Vague (40) observed that upper body fat (the “apple” phenotype) confers greater cardiometabolic risk than lower body fat (the “pear” phenotype). Studies have since confirmed a distinct role for abdominal adipose tissue (AT) in insulin resistance (IR), type 2 diabetes, and metabolic syndrome (9). In the last 30 years, imaging studies have revealed that visceral AT is uniquely and strongly associated with IR (21). These associations are also evident in normal-weight subjects, advancing the “lean but metabolically obese” phenotype concept (35). In contrast, lower extremity subcutaneous AT has a weak connection with IR (11). Gluteal-femoral AT (GFAT) decreases cardiometabolic risk and mitigates IR risk, and loss of GFAT can increase IR risk (25, 36–38). Interestingly, women have a higher percentage of body fat vs. men, store more fat in the gluteal-femoral region, and have a lower risk of IR (5, 24).
Although useful, regional AT distribution and mass measurements may not fully explain AT's role in systemic IR. Studies into metabolic activity within specific fat depots may provide further insight into obesity and IR. Jensen (17) and Nielsen et al. (30) demonstrated a 40% increase in lipolysis in women compared with men with the same circulating free fatty acid (FFA) levels and that upper body subcutaneous fat has greater lipolytic activity and less insulin sensitivity (IS) compared with leg AT. However, studies by Karpe et al. (20) have advocated that increased AT mass does not necessarily lead to increased FFA. Virtanen and colleagues (41, 42) used positron emission tomography (PET) imaging to show IR in abdominal and GFAT in obese subjects but a greater insulin-mediated glucose uptake in visceral AT compared with the gluteal-femoral depot. Together, these studies demonstrate that both AT quantity and quality may affect IR in obesity. However, it remains unclear how AT quantity and quality account for the wide variability in systemic IS seen in normal-weight individuals. Because adipocytes are highly responsive to insulin, insight into the roles of specific regional AT depots and skeletal muscle in IS independent of obesity is invaluable. The current study used dynamic PET imaging and MRI of regional AT and skeletal muscle to assess IS within specific tissue depots and their contribution to systemic glucose metabolism in nonobese subjects. This approach significantly increases our understanding of the role of gluteal-femoral and abdominal AT with respect to systemic IS in nonobese individuals.
MATERIALS AND METHODS
Study subject characteristics.
Nonobese young adults (n = 15) were recruited from the general community through flyers. The study was described and discussed in detail with all participants, with all questions answered. Informed, written consent was obtained, followed by a medical examination and blood collection to assure good health. All participants met the following inclusion criteria: body mass index (BMI) 20–27 kg/m2, blood pressure <140 and <90 mmHg, glucose <100 mg/dl, Hb A1c <5.7%, triglycerides <150 mg/dl, total cholesterol 250 mg/dl, fasting insulin <12 μU/ml, no recent weight gain, no smoking, and no taking of medications known to affect adipose tissue metabolism. The protocol was approved by the University of Pittsburgh Institutional Review Board. Clinical characteristics are shown in Table 1.
Table 1.
Clinical characteristics
| All (n = 15) | Women (n = 9) | Men (n = 6) | |
|---|---|---|---|
| Age, yr | 31.5 ± 5.0 | 32.3 ± 6.1 (25/42) | 30.2 ± 2.9 (26/33) |
| Weight, kg | 73.7 ± 12.2 (53/98) | 66.7 ± 7.7** (53/80) | 84.1 ± 10.0 (72/98) |
| BMI | 25.1 ± 2.3 | 24.0 ± 2.0* (20.7/26.9) | 26.7 ± 1.8 (24.0/29.0) |
| %Body fat | 30.4 ± 8.4 | 35.2 ± 4.5** (28.3/43.3) | 23.3 ± 7.8 (12.6/34.3) |
| Ethnicity | 13 W/2 AA | 8 W/1 AA | 5 W/1 AA |
Values are means ± SD (minimum/maximum values in parentheses).
BMI, body mass index; W, white; AA, African-American.
P < 0.05;
P < 0.01, women vs. men.
Body composition tissue volume measurements.
Dual-energy X-ray absorptiometry (DEXA; Lunar Prodigy, Madison, WI) measured fat mass (FM) and fat-free mass (FFM). The DEXA images were analyzed (En Core for Windows version 9.30) to assess lower extremity FM and truncal FM. Abdominal FM was measured as described previously (18). The DEXA and MRI scans were combined to estimate abdominal AT quantity, and DEXA measurements were used to estimate GFAT quantity. Abdominal images obtained from MRI at the top of the diaphragm to the top of the femur were matched with DEXA images using the landmark of the greater edge of the superior trochanter that is easily identifiable on DEXA and has minimal adipose tissue. Lower and upper extremity lean body mass was used to estimate systemic skeletal muscle mass.
Metabolic studies.
Research volunteers were admitted the night prior to dynamic PET imaging to the University of Pittsburgh General Clinical Research Center and fasted overnight after dinner. The next morning, a catheter was placed in the antecubital vein for insulin infusion, dextrose infusion, and fluorodeoxyglucose ([18F]FDG) administration. A catheter was placed in the radial artery for blood sampling. All volunteers underwent an insulin infusion of (20 mU·m−2·min−1) for ∼4 h to suppress hepatic glucose output and achieve nearly full suppression of lipolysis (32, 39). Euglycemia was maintained with an adjustable 20% dextrose infusion using the glucose clamp method (7). A primed (200 mg) continuous (2 mg/min) infusion of [6,6-2H2]glucose was initiated 2 h prior and continuing through the insulin infusion to determine systemic glucose utilization rate [glucose disappearance rate (Rd)] and calculate endogenous glucose production (EGP). Arterial glucose samples were measured every 5 min using a YSI (Yellow Springs, OH) Glucose Analyzer, and plasma insulin levels were collected every 30 min and measured by ELISA (Linco Research). Plasma fatty acid samples were measured using the Agilent Gas Chromatograph (Agilent, Santa Clara, CA). Glucose enrichment of [6,6-2H2]glucose was measured using gas chromatography-mass spectrometry. Blood samples for measurement of adipokines [adiponectin, retinol-binding protein 4 (RBP4), leptin, IL-6, and TNFα] were obtained 3 h prior to the insulin infusion being started and measured with ELISA (ALPCO Diagnostics for adiponectin and RBP4, Linco Research for leptin, and R & D Systems for IL-6 and TNFα).
Dynamic PET imaging with [18F]FDG.
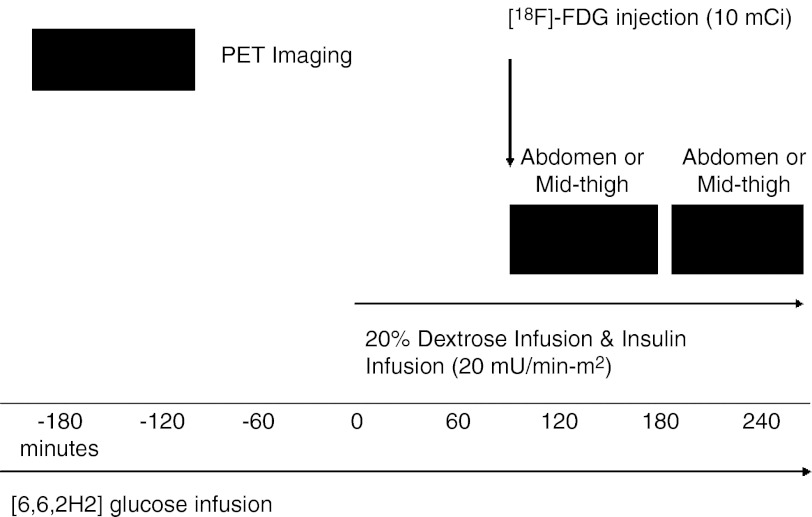
Dynamic PET imaging was obtained at the University of Pittsburgh PET Center using a Siemens/CTI ECAT HR+ PET scanner with a 3D imaging mode (63 parallel planes, axial field of view, 15.2 cm, slice width of 2.4 mm, image resolution 6 mm). The [18F]FDG was synthesized using a method modified by Hamacher et al. (14). Volunteers were positioned in a supine position on the PET scanner gantry so that the midthigh area or midabdominal area was in the center of the field of view. Subjects were positioned only after steady-state metabolic conditions were attained during the glucose clamp and continued during dynamic PET imaging. A transmission scan was performed to assess attenuation values (10–15 min). A 10-mCi dose of [18F]FDG was then injected at the beginning of 53 min of dynamic PET scanning with 27 total frames (8 frames for each 30-s duration, 9 frames for each 60-s duration, and 10 frames for each 4-min duration). Subsequently, the bed was repositioned to continue dynamic PET imaging of the other area (i.e., midthigh if abdominal area was imaged first and vice versa), and dynamic PET imaging continued for 40 min (10 frames for each 4-min duration), followed by a second transmission scan lasting 10–15 min. Arterial blood samples (0.5 ml) were hand-drawn to measure [18F]FDG arterial activity for a total of 95 min at approximately the following intervals: 10 samples in the 1st min, 8 samples from minutes 2 to 3, seven samples from minutes 3 to 10, and 20 samples from minutes 10 to 95. The blood was immediately centrifuged, and 100 μl of plasma was used for [18F] counting (>350 KeV). The study design is shown in Fig. 1.
Fig. 1.
Study design schematic. Shaded black boxes represent active dynamic positron emission tomography (PET) imaging after injection of [18F]FDG (fluorodeoxyglucose) radioactive tracer.
PET image tissue activity measurements.
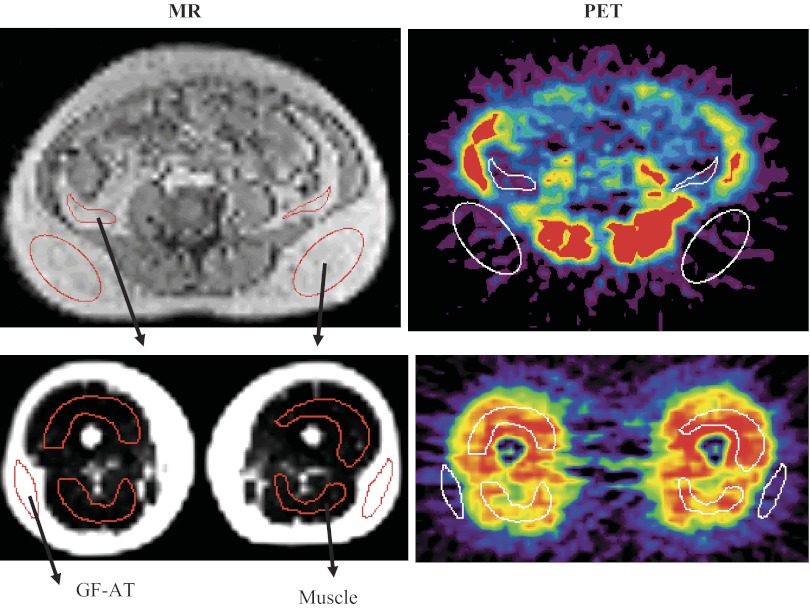
PET images were coregistered with their corresponding MRI images to allow a region of interest (ROI) transfer to the PET image using a previously described method (29, 45). A representative illustration of MR and PET images with ROIs is shown in Fig. 2. ROIs allow for highly specific anatomic localization of the specific AT depot on the MR image to eliminate or minimize spillover from other regions, organs, and vessels on PET imaging. The ROIs were placed on subcutaneous AT (GFAT; n = 15) and muscle in midthigh PET images and subcutaneous AT (SAT; n = 15) and visceral AT (VAT; n = 12) in abdominal PET images. It was technically not feasible to assess visceral adipose [18F]FDG uptake in three women because ROIs could not be applied consistently in sequential images due to lack of VAT depots from image to image. The ROIs were used to measure [18F]FDG activity within adipose or muscle. To minimize scatter effect, the middle 30 of 63 planes of each leg and abdomen were used. Tracer activity within the ROI was converted to a radioactivity concentration (μCi/ml) using an empiric phantom-based calibration factor (μCi·ml−1·PET counts per pixel−1).
Fig. 2.
Representative PET and MRI. Imaging with region of interest (ROI) placement. An example illustrating MRI and dynamic PET imaging after injection of [18F]FDG. Top pictures represent abdominal imaging, and bottom pictures represent thigh imaging, with MR images on the left and PET images on the right. After MR-PET coregistration, ROI (shown as red ovals) were generated on the MR image and applied to the corresponding coregistered PET image (shown as white ovals) to generate regional FDG time activity curves. GFAT, gluteal-femoral adipose tissue.
Patlak analysis and extrapolation to tissue mass.
Patlak graphical analysis modeled a macroscopic index of [18F]FDG uptake into AT and skeletal muscle, defined as a K (ml plasma·ml tissue−1·min−1) (26). Dynamic PET data acquired over 0–53 (1st imaging site) and 53–93 min (2nd imaging site) were averaged over 30 planes, and Patlak analysis was applied to the average for each table position, with a high degree of linearity shown in the Patlak plot data, with all regression values >0.9. Regional glucose uptake was calculated as the K product and arterial glucose concentration divided by the lumped constant, using 1.14 for adipose tissue and 1.2 for skeletal muscle (22, 42, 43).
Statistical analysis.
Statistical analysis was performed using Sigma Stat 3.0 (SAS institute). Data are presented as means ± SD unless otherwise indicated. To examine sex differences and differences between depots, analysis of variance was used. Pairwise comparisons were calculated with the Holm-Sidak multiple-comparison method procedure. Association between variables was examined with a Pearson or Spearman correlation. Spearman and Pearson correlations were used when appropriate to examine associations between variables. Stepwise regression analyses were used to adjust for other variables. A P value of <0.05 was considered significant.
RESULTS
Systemic and hepatic insulin sensitivity measurements.
Insulin infusion increased arterial insulin from 5.4 ± 2.0 μU/ml fasting to 23.8 ± 4.6 μU/ml with insulin (P < 0.01, n = 13). Free fatty acids were suppressed from 362 ± 120 μM fasting to 93 ± 16 μM with insulin (72 ± 11% suppression, P < 0.01). However, there was a broad range from 48 to 87%. Systemic glucose utilization rates during steady-state conditions doubled from 2.07 ± 0.34 (fasting) and 4.19 ± 1.45 mg·min−1·kg−1 (insulin stimulated). There was a broad, threefold range of insulin-stimulated systemic glucose utilization between 2.33 and 6.77 mg·min−1·kg−1. Greater FFA suppression was correlated with an increase in percentage stimulation of glucose Rd (r = −0.60, P < 0.05). EGP was suppressed to 91.4 ± 14.2%, whereas glucose Rd was stimulated to 98.4 ± 54.7% with insulin stimulation from baseline (n = 13). No sex differences were found with EGP suppression (P = 0.29) or glucose Rd (P = 0.65) with insulin stimulation.
Regional AT mass and IS.
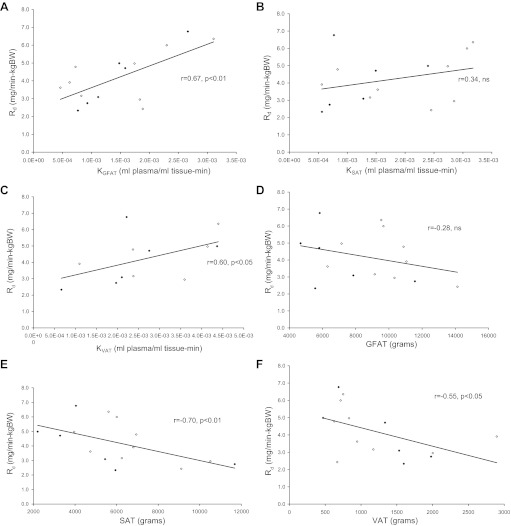
There was a negative association between total FM and glucose Rd (r = −0.51, P < 0.05). Truncal FM by DEXA was strongly and negatively associated with glucose Rd (r = −0.74, P < 0.01). When abdominal FM was subdivided using MRI, both SAT (r = −0.70, P < 0.01) and VAT (r = −0.55, P = 0.05) were associated with systemic glucose Rd (Fig. 4, E and F). However, absolute GFAT mass did not correlate with systemic glucose Rd [r = −0.28, not significant (NS)], although GFAT accounted for ∼40% of overall FM. A higher proportion of GFAT relative to total body fat tended to correlate positively with systemic glucose Rd (r = 0.45, P = 0.09). Interestingly, a higher proportion of GFAT was significantly correlated with systemic glucose Rd when measured as a proportion of combined regional AT: %GFAT/(GFAT + SAT) (r = 0.63, P = 0.01), %GFAT/(GFAT + SAT + VAT) (r = 0.64, P = 0.01).
Fig. 4.
Systemic insulin sensitivity correlations. ◆, Men; ◇, women. A: positive significance between the glucose rate of disappearance (Rd) and the overall activity (K) of GFAT. B: no significance between the overall activity of SAT and Rd. C: significance between the activity of VAT and Rd. D: no significance between the amount of GFAT and insulin sensitivity. E: negative correlation between SAT volume and insulin sensitivity. F: a negative correlation between VAT volume and insulin sensitivity.
AT PET imaging.
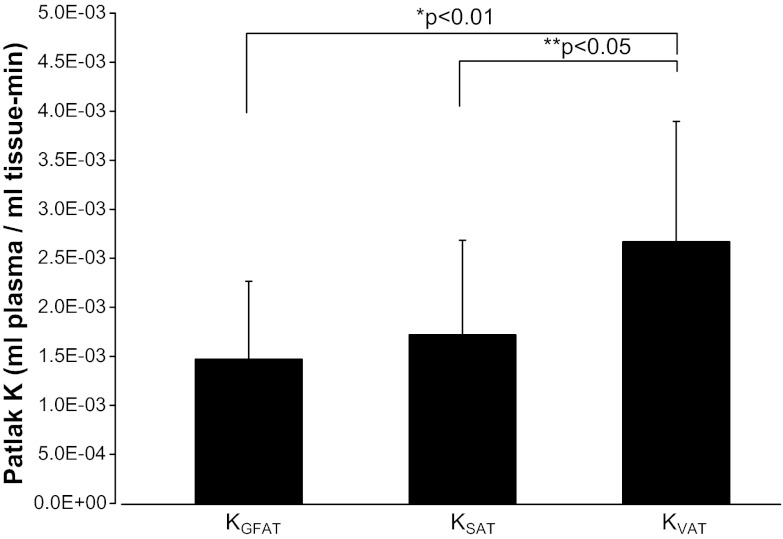
The [18F]FDG tissue activity within specific ROIs and arterial activity were used to calculate fractional FDG uptake expressed as K. The K value of [18F]FDG summarizes net effects of tracer delivery, transmembrane transport, and intracellular trapping of [18F]FDG 6-phosphate (2, 16). The mean values for K with insulin stimulation were similar and nonsignificant in GFAT (KGFAT) and SAT (KSAT) at 1.47 ± 0.79 and 1.72 ± 0.97 10−3 ml plasma·ml tissue−1·min−1, respectively, but VAT (KVAT) was significantly higher than both KGFAT (P < 0.01) and KSAT (P < 0.05) at 2.67 ± 1.23 (Fig. 3). Skeletal muscle (KMUS) was three to five times higher at 8.12 ± 3.19 10−3 ml plasma·ml tissue−1·min−1 (P < 0.001) than any AT depot (Table 2). KVAT could not be measured in three women with minimal VAT (<1 kg) due to the technical challenges of assigning proper ROIs within VAT. No sex differences in [18F]FDG uptake in any AT depot were observed in KVAT (P = 0.38) or KGFAT (P = 0.85). KSAT trended toward (P = 0.07) a higher uptake in women (2.07 ± 1.00 vs. 1.20 ± 0.69 10−3 ml plasma·ml tissue−1·min−1). No sex differences were observed in systemic glucose Rd [4.10 ± 1.69 (men) vs. 4.24 ± 1.37 mg·min−1·kg−1 (women), P = 0.87]. Each fat depot had a broad range in values, with a sixfold range in KGFAT and a sevenfold range in KSAT. KVAT also had a range, but this should be interpreted with caution due to the small cohort of nonobese, healthy, young adults (∼10–15% truncal adiposity).
Fig. 3.
Adipose tissue depot K values. FDG uptake in GFAT was similar to subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) differed significantly from both GFAT (*P < 0.01) and SAT (**P < 0.05), as shown above.
Table 2.
Adipose tissue mass and extrapolated tissue glucose uptake
| All (n = 15) | Women (n = 9) | Men (n = 6) | |
|---|---|---|---|
| Tissue mass, kg | |||
| GF-FM, kg | 8.6 ± 2.7 | 9.8 ± 2.3* | 6.9 ± 2.5 |
| ABD SAT, kg | 9.9 ± 3.3 | 10.1 ± 2.5 | 9.5 ± 4.4 |
| VAT, kg | 1.2 ± 0.7 | 1.2 ± 0.8 | 1.3 ±0.6 |
| Skeletal muscle, kg | 25.6 ± 7.9 | 20.3 ± 2.9** | 33.6 ± 5.9 |
| K values, 10−3 ml plasma·ml tissue−1·min−1 | |||
| KGFAT, min | 1.47 ± 0.79 | 1.50 ± 0.90 | 1.42 ± 0.68 |
| KSAT, min | 1.72 ± 0.97 | 2.07 ± 1.00† | 1.20 ± 0.69 |
| KVAT, min [n = 12 (6 men and 6 women)] | 2.67 ± 1.23 | 2.99 ± 1.27 | 2.34 ± 1.21 |
| KMUS, min | 8.12 ± 3.19 | 8.15 ± 3.12 | 8.07 ± 3.59 |
| Glucose uptake, mg/min | |||
| Rd, mg/min | 306 ± 116 | 278 ± 80 | 347 ± 155 |
| Rd, mg·min−1·kg body wt−1 | 4.19 ± 7.3 | 4.24 ± 1.37 | 4.10 ± 1.69 |
| GFAT, mg/min | 11.3 ± 7.3 | 13.4 ± 8.7 | 8.2 ± 3.1 |
| %Glucose uptake | 3.9 ± 2.7 | 4.8 ± 3.1‡ | 2.5 ± 0.8 |
| ABD SAT, mg/min | 15.0 ± 10.3 | 19.2 ± 11.6* | 8.7 ± 2.7 |
| %Glucose uptake | 5.7 ± 4.7 | 7.6 ± 5.3* | 3.0 ± 1.3 |
| VAT, mg/min (n = 12) | 2.7 ± 1.3 | 3.1 ± 1.6 | 2.3 ± 1.1 |
| %Glucose uptake | 1.0 ± 0.6 | 1.2 ± 0.8 | 0.8 ± 0.4 |
| Skeletal muscle, mg/min | 210 ± 115 | 163 ± 60 | 280 ± 147 |
| %Glucose uptake | 65.4 ± 20.2% | 57.4 ± 11.9% | 77.3 ± 25.1% |
Values are means ± SD.
GF-FM, gluteal-femoral fat mass; ABD, abdominal; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; GFAT, gluteal-femoral adipose tissue; KMUS, skeletal muscle; Rd, glucose disposal.
P < 0.05;
P < 0.01;
P = 0.07;
P = 0.06, women vs. men.
AT metabolic activity and IS measurements.
Values for KGFAT were positively correlated with systemic glucose Rd (Fig. 4A) and skeletal muscle (KMUS; r = 0.53, P < 0.05), indicating salutary physiological cross-talk between GFAT metabolism and systemic insulin sensitivity. Additionally, KGFAT values correlated negatively with insulin-suppressed FFA (r = −0.63, P < 0.05), but there was no association between KGFAT and GFAT mass (r = 0.02, NS). These observations further support the concept of dissociation between metabolic activity and quantity of GFAT and their role in systemic insulin sensitivity.
In contrast to KGFAT, KSAT did not correlate with systemic Rd (Fig. 4B). Interestingly, KVAT was positively correlated with glucose Rd. However, total estimated glucose uptake within VAT mass was much less than other fat depots. KMUS was strongly correlated with glucose Rd (r = 0.89, P < 0.01). Moreover, KMUS had a similar pattern of association with FM and the amounts of SAT, VAT, and GFAT, as described above with glucose Rd (data not shown). As a technical consideration, regression values (r2) as a fit of the Patlak K plot were determined for the following: KGFAT 0.97 ± 0.04, KSAT 0.96 ± 0.06, KVAT 0.93 ± 0.06, and KMUS 1.00 ± 0.00. As expected, the Patlak line fit was superior in muscle, owing to a much higher signal intensity and higher signal/noise ratio than in adipose tissue.
Stepwise regression analysis was performed to address more specifically how the amounts of specific tissue depots and specific tissue insulin sensitivities were associated with systemic IS. In GFAT, tissue IS (KGFAT) correlated with systemic glucose Rd and insulin-suppressed FFA even after adjustment for GFAT volume and age, sex, BMI, and ethnicity (P < 0.01 and P < 0.05, respectively). In contrast, SAT tissue volume negatively correlated with systemic Rd (P < 0.05) even after adjustment for SAT tissue IS.
Contributions of AT and skeletal muscle glucose uptake to overall systemic IS.
Estimated glucose uptake into each depot was performed by extrapolating [18F]FDG uptake to glucose using the lumped constant and the estimate of specific depot mass with values expressed in Table 2 (22, 43). Glucose uptake into GFAT was 4–6% of systemic glucose utilization, similar to SAT. Women tended to have a greater percentage of their systemic glucose uptake attributed to GFAT (4.7 ± 2.9 vs. 2.5 ± 0.8%, P = 0.06). Women had significantly higher SAT glucose uptake (19.2 ± 11.6 vs. 8.7 ± 2.7 mg/min, P < 0.05) and percentage of systemic glucose uptake into SAT (8.0 ± 6.1 vs. 3.0 ± 1.3%, P < 0.05) compared with men. VAT glucose uptake was significantly less than other adipose depots (P < 0.01). In total, these adipose depots represented ∼90% of FM, noting that upper extremity FM could not be estimated, and accounted for ∼13% of systemic glucose Rd in women and ∼6% of systemic glucose Rd in men (P < 0.05). The order in which fat depots were scanned with PET did not significantly affect glucose uptake (data not shown).
Estimation of skeletal muscle glucose uptake took into account appendicular skeletal muscle mass, representing ∼75% of total skeletal muscle mass in the body estimated from a whole body DEXA scan (31). Skeletal muscle accounted for ∼65% of systemic glucose utilization (Table 2). Skeletal muscle accounted for ∼57% of systemic Rd in women and ∼77% in men, but this difference was not statistically significant (P = 0.11). Considering that glucose uptake in the central nervous system is ∼1.0 mg·min−1·kg body wt−1 (100 g/day in a 70-kg person), the central nervous system accounts for the vast majority of the small remaining fraction of systemic glucose uptake (3). Of note, women had a higher percent body fat (41.5 ± 5.0 vs. 35.8 ± 5.1%, P = 0.05) and a greater amount of GFAT than men, but men had more skeletal muscle mass, with SAT and VAT being similar and nonsignificant (Table 2).
AT sensitivity and plasma adipokine levels.
KGFAT did not correlate with adipokines. KSAT correlated with RBP4 (r = −0.59, P < 0.05) and tended to correlate with TNFα (r = −0.42, P = 0.11). KMUS correlated positively with adiponectin (r = 0.53, P < 0.05) and negatively with IL-6 (r = −0.62, P < 0.05). The percent gluteal-femoral FM correlated positively with adiponectin (r = 0.78, P < 0.01) and negatively with RBP4 (r = −0.76, P < 0.01). Other body composition indices were not correlated with these adipokines. Adiponectin and RBP4 correlated with each other (r = −0.59, P < 0.05). Other significant correlations with body composition included leptin and total adiposity (r = 0.84, P < 0.01), IL-6 and trunk SAT (r = 0.55, P < 0.05), IL-6 and SAT (r = 0.53, P < 0.05), and TNFα and VAT (r = 0.57, P < 0.05). In regard to adipokine and systemic insulin sensitivity, adiponectin positively correlated with Rd (r = 0.58, P < 0.05). IL-6 negatively correlated with Rd (r = −0.52, P < 0.05), and adiponectin was negatively correlated with IL-6 (r = −0.52, P < 0.05).
DISCUSSION
Systemic IR is associated with generalized obesity but is also well known to manifest in normal-weight men and women. Body fat distribution in both normal-weight and obese people exhibits a stronger relationship with IR than total adiposity. Indeed, there is widespread acceptance in the literature that abdominal fat mass is associated with IR. In addition, studies in our laboratory have shown that subcutaneous thigh AT mass either did not associate with IR or is positively associated with higher systemic insulin sensitivity (1, 8, 11). Indeed, body fat distribution variations are often cited as key evidence for the “metabolically obese but normal-weight phenotype” (35). Our dynamic PET imaging studies revealed novel findings that intrinsic metabolic activity with GFAT and VAT can vary considerably among normal-weight subjects, and in combination with body fat distribution they may play key roles in determining systemic IR.
Our results indicated that higher metabolic activity (KGFAT) within GFAT is associated with better overall systemic glucose disposal (glucose Rd). A potential explanation for this observation is that subcutaneous adipose depots act as a protective “sink” by trapping excess fatty acids and preventing exposure to elevated lipid levels. In our nonobese individuals, greater KGFAT activity correlated with a lower absolute FFA value with insulin stimulation. Prior studies have shown that meal-derived fatty acids have greater uptake into abdominal adipose vs. lower body AT (23, 34). Our novel findings add to these studies by demonstrating that greater KGFAT activity was associated with lower systemic FFA values and improved IS. To the best of our knowledge, this has not been reported previously. In contrast, fractional glucose uptake within KSAT was not correlated with glucose Rd or insulin-stimulated decreased FFAs, indicating that the mass but not the intrinsic metabolic activity within this depot contributes to systemic IS, as reported previously using other methodologies (12).
Our novel data also indicate that higher VAT intrinsic metabolic activity (KVAT) is associated with systemic insulin sensitivity and more complete suppression of lipolysis, as reflected by the insulin-stimulated FFA levels. This was similar to GFAT and suggests that variation in VAT metabolic activity (KVAT) is related to systemic IS. To the best of our knowledge, this has not been reported previously. However, it appears that excess VAT mass may offset any potential positive influence that its intrinsic metabolic activity may have on systemic insulin sensitivity. Indeed, our study is in agreement with prior studies showing that VAT mass, but not GFAT mass, negatively correlated with systemic glucose Rd in nonobese individuals (35). Hence, our studies suggest opposing influences of metabolic activity and mass in VAT. However, we note that a limitation of this study is that we could not quantify different areas within VAT such as mesenteric, perirenal, and epididymal white AT depots. Moreover, additional studies are warranted to examine these associations among overweight and obese individuals.
Glucose uptake within AT accounted for ∼11% of total systemic glucose uptake, with GFAT and SAT contributing ∼4–6% each. This is similar to previous studies employing PET, which reported that total body AT accounted for 8% of systemic glucose uptake (42). This study, however, reported a slightly lower contribution of SAT (1.3%) to total systemic uptake (42). This previous study examined only men, and our higher contribution by AT was likely due to the women in our study who had more AT. Although this contribution by AT to total body glucose uptake might seem relatively minor, it could be important in the context of fat mass expansion, i.e., weight gain in which this contribution would increase. Further investigations are needed to examine how AT depot-specific metabolic activity may play a role in cardiometabolic risk according to sex difference. Prior isotope labeling studies in nonobese subjects showed meal fat uptake over 24 h did not predict regional fat body distribution over 8 wk (44). Because our study demonstrated differences only in regional glucose uptake and body fat distribution, other mechanisms need to be explored to further understand how regional AT distribution and “quality” of AT affect the variability in insulin sensitivity. For example, estrogen has been shown to promote insulin sensitivity in animal and human models through suppression of hepatic gluconeogenesis and enhancement of skeletal muscle glucose transport (27, 28, 33). We speculate that estrogen could also have direct effects on promoting glucose uptake directly into adipose tissue. Taken together, this would compliment prior studies that have observed sex-related differences in AT distribution and mass related to insulin resistance, type 2 diabetes, and cardiovascular disease risk.
Our adipokine data provide additional evidence that GFAT plays a positive role in systemic glucose metabolism. The percent GFAT mass positively correlated with adiponectin, a signaling protein secreted from AT known to affect insulin sensitivity and obesity by increasing glucose uptake, increasing IS, and promoting weight loss (6, 19). Percent GFAT mass negatively correlated with RBP4, a known protein secreted by AT that is elevated in IR and possibly contributes to IR (13, 46). Our data suggest that GFAT may have more than one mechanism influencing systemic insulin sensitivity, because both metabolic activity and secretion of specific adipokines affect systemic IS. Body composition indices of SAT did not show any significance, but KSAT correlated negatively with RBP4, indicating that SAT activity may play a role in altering systemic IS through adipokines. Increasing VAT mass correlated positively with TNFα as expected, indicating that increasing VAT mass may play a role in increasing TNFα, which is known to play a role in the pathogenesis of obesity (15).
There were several limitations to this study. Although our high-resolution MRIs allowed us to distinguish segment tissue activity specific to adipose tissue and muscle, it was technically challenging to assign proper ROIs within VAT for several of these nonobese subjects; thus we did not analyze [18F]FDG activity within VAT for all subjects. Future studies in obese subjects with greater VAT mass would allow for more precise discrimination of VAT activities. Prior studies have shown differences in SAT subdepot effects on systemic IS, but in our nonobese population it was technically difficult to properly coregister superficial and deep subcutaneous AT on MR and PET imaging, and additional studies with obese subjects could further investigate these subdepots (10). To address possible tissue activity contamination, we used the Bayesian approach that is also applied to account for tracer activity in blood. Another limitation was FDG signal decay depending upon the order of tissue imaging. Taking into account the long half-life of FDG (∼109 min), we applied a decay correction to the PET imaging studies, and our glucose uptake data revealed no significant differences depending on which depot was imaged first. We also recognize that ROIs are estimates of whole organ/tissue metabolism by means of extrapolation. However, the linear regression in the PATLAK modeling suggests this estimate is reasonable under the steady-state conditions provided by the euglycemic insulin clamp. We recognize that extrapolation of glucose uptake from metabolic activity relies on the lumped constant, and this has been verified independently for both muscle and AT in prior PET studies (22, 43). We recognize that a 20 mU·m−2·min−1 insulin infusion likely does not maximally stimulate peripheral glucose uptake, but our main emphasis was suppressing lipolysis and hepatic glucose metabolism to exert effects on AT metabolism. Further studies at higher insulin infusion rates may further delineate the specific roles of AT, muscle, and liver in regional glucose uptake. Additionally, we considered the possibility of the timing of the menstrual cycles affecting the female participants' insulin sensitivity, but evidence suggests that IS did not vary significantly with menstrual cycles (4).
In summary, these dynamic PET studies provide novel in vivo information regarding distinct roles of regional AT depot mass and metabolic activity affecting systemic glucose metabolism within nonobese subjects. This supports the hypothesis that AT “quality” is an important component in systemic IS in nonobese individuals, particularly thigh AT, as greater metabolic activity in thigh AT is associated with greater overall systemic glucose disposal, possibly through a greater suppression of FFAs with insulin stimulation. These findings potentially explain part of the interindividual variability in systemic IS among nonobese individuals of the same body phenotype (fat mass distribution). Future studies in lean and obese individuals may help further our understanding of the role of regional AT in systemic glucose metabolism through suppression of FFAs, release of adipokines, glucose uptake, or a combination of these mechanisms and may help explain the wide variation in cardiometabolic risk among nonobese men and women not fully explained by general adiposity or body fat distribution.
GRANTS
These studies were supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK-60555-02), the University of Pittsburgh General Clinical Research Center (5MO1RR00056), and the Obesity and Nutrition Research Center (NIDDK; P30-DK-46204).
DISCLOSURES
J. Ng, K. Azuma, C. Kelley, Z. Radikova, C. Laymon, J. Price, and B. H. Goodpaster have no conflicts of interests to declare. R. Pencek is currently employed by Amylin Pharmaceuticals. D. E. Kelley is currently employed by Merck, Sharp, and Dohme Corp.
AUTHOR CONTRIBUTIONS
J.N., K.A., C.K., R.P., and Z.R. performed the experiments; J.N., K.A., C.L., and J.P. analyzed the data; J.N., K.A., B.H.G., and D.E.K. interpreted the results of the experiments; J.N. and K.A. prepared the figures; J.N. drafted the manuscript; J.N., J.P., and B.H.G. edited and revised the manuscript; J.N., B.H.G., and D.E.K. approved the final version of the manuscript; J.P. and D.E.K. did the conception and design of the research.
ACKNOWLEDGMENTS
We gratefully acknowledge the efforts and cooperation of the research volunteers and the support from the staffs of the University of Pittsburgh General Clinical Research Center and the PET Center.
Present address of K. Azuma: Institute for Integrated Sports Medicine, School of Medicine, Keio University, Tokyo, Japan.
Present address of R. Pencek: Amylin Pharmaceuticals, San Diego, CA.
Present address of Z. Radikova: Institute of Experimental Endocrinology, Slovak Academy of Sciences, Bratislava, Slovakia.
Present address of D. E. Kelley: Merck Sharp and Dohme Corp., Rahway, NJ.
REFERENCES
- 1.Amati F, Pennant M, Azuma K, Dubé JJ, Toledo FG, Rossi AP, Kelley DE, Goodpaster BH. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity (Silver Spring) 20: 1115–1117, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Bertoldo A, Peltoniemi P, Oikonen V, Knuuti J, Nuutila P, Cobelli C. Kinetic modeling of [(18)F]FDG in skeletal muscle by PET: a four-compartment five-rate-constant model. Am J Physiol Endocrinol Metab 281: E524–E536, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bier DM, Brosnan JT, Flatt JP, Hanson RW, Heird W, Hellerstein MK, Jéquier E, Kalhan S, Koletzko B, Macdonald I, Owen O, Uauy R. Report of the IDECG Working Group on lower and upper limits of carbohydrate and fat intake. International Dietary Energy Consultative Group. Eur J Clin Nutr 53, Suppl 1: S177–S178, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bingley CA, Gitau R, Lovegrove JA. Impact of menstrual cycle phase on insulin sensitivity measures and fasting lipids. Horm Metab Res 40: 901–906, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Blaak E. Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 4: 499–502, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46: 459–469, 2003 [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 8.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 444: 881–887, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Despres JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 10: 497–511, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Golan R, Shelef I, Rudich A, Gepner Y, Shemesh E, Chassidim Y, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Ben Avraham S, Witkow S, Liberty IF, Tangi-Rosental O, Sarusi B, Stampfer MJ, Shai I. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 35: 640–647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 71: 885–892, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46: 1579–1585, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Graham TE, Yang Q, Blüher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 354: 2552–2563, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Hamacher K, Coenen HH, Stöcklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 27: 235–238, 1986 [PubMed] [Google Scholar]
- 15.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Huang SC, Williams BA, Barrio JR, Krivokapich J, Nissenson C, Hoffman EJ, Phelps ME. Measurement of glucose and 2-deoxy-2-[18F]fluoro-d-glucose transport and phosphorylation rates in myocardium using dual-tracer kinetic experiments. FEBS Lett 216: 128–132, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 96: 2297–2303, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61: 274–278, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60: 2441–2449, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 278: E941–E948, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Kelley DE, Williams KV, Price JC, Goodpaster B. Determination of the lumped constant for [18F] fluorodeoxyglucose in human skeletal muscle. J Nucl Med 40: 1798–1804, 1999 [PubMed] [Google Scholar]
- 23.Koutsari C, Snozek CL, Jensen MD. Plasma NEFA storage in adipose tissue in the postprandial state: sex-related and regional differences. Diabetologia 51: 2041–2048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 72: 1150–1162, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemieux S, Despres JP, Moorjani S, Nadeau A, Theriault G, Prud'homme D, Tremblay A, Bouchard C, Lupien PJ. Are gender differences in cardiovascular disease risk factors explained by the level of visceral adipose tissue? Diabetologia 37: 757–764, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol 27: 661–670, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 6: 180–185, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Matute ML, Kalkhoff RK. Sex steroid influence on hepatic gluconeogenesis and glucogen formation. Endocrinology 92: 762–768, 1973 [DOI] [PubMed] [Google Scholar]
- 29.Minoshima S, Berger KL, Lee KS, Mintun MA. An automated method for rotational correction and centering of three-dimensional functional brain images. J Nucl Med: 1579–1585, 1992 [PubMed] [Google Scholar]
- 30.Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 111: 981–988, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proctor DN, O'Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol Endocrinol Metab 277: E489–E495, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Rebrin K, Steil GM, Mittelman SD, Bergman RN. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J Clin Invest 98: 741–749, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rincon J, Holmäng A, Wahlström EO, Lönnroth P, Björntorp P, Zierath JR, Wallberg-Henriksson H. Mechanisms behind insulin resistance in rat skeletal muscle after oophorectomy and additional testosterone treatment. Diabetes 45: 615–621, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 279: E455–E462, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, Jensen MD, Parati G, Lopez-Jimenez F. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31: 737–746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidell JC, Han TS, Feskens EJ, Lean ME. Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med 242: 401–406, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC; Hoorn study. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 27: 372–377, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Snijder MB, Zimmet PZ, Visser M, Dekker JM, Seidell JC, Shaw JE. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: the AusDiab Study. Int J Obes Relat Metab Disord 28: 402–409, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Stumvoll M, Jacob S, Wahl HG, Hauer B, Löblein K, Grauer P, Becker R, Nielsen M, Renn W, Häring H. Suppression of systemic, intramuscular, and subcutaneous adipose tissue lipolysis by insulin in humans. J Clin Endocrinol Metab 85: 3740–3745, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Vague J. Sexual differentiation, a factor affecting the forms of obesity. Presse Med 55: 339–340, 1947 [PubMed] [Google Scholar]
- 41.Virtanen KA, Iozzo P, Hällsten K, Huupponen R, Parkkola R, Janatuinen T, Lönnqvist F, Viljanen T, Rönnemaa T, Lönnroth P, Knuuti J, Ferrannini E, Nuutila P. Increased fat mass compensates for insulin resistance in abdominal obesity and type 2 diabetes: a positron-emitting tomography study. Diabetes 54: 2720–2726, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, Tolvanen T, Knuuti J, Rönnemaa T, Huupponen R, Nuutila P. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 87: 3902–3910, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Virtanen KA, Peltoniemi P, Marjamäki P, Asola M, Strindberg L, Parkkola R, Huupponen R, Knuuti J, Lönnroth P, Nuutila P. Human adipose tissue glucose uptake determined using [(18)F]-fluoro-deoxy-glucose {[(18)F]FDG} and PET in combination with microdialysis. Diabetologia 44: 2171–2179, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Votruba SB, Jensen MD. Short-term regional meal fat storage in nonobese humans is not a predictor of long-term regional fat gain. Am J Physiol Endocrinol Metab 302: E1078–E1083, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436: 356–362, 2005 [DOI] [PubMed] [Google Scholar]