Abstract
Somatostatin-14 (SST) inhibits insulin and glucagon secretion by activating G protein-coupled somatostatin receptors (SSTRs), of which five isoforms exist (SSTR1–5). In mice, the effects on pancreatic β-cells are mediated by SSTR5, whereas α-cells express SSTR2. In both cell types, SSTR activation results in membrane hyperpolarization and suppression of exocytosis. Here, we examined the mechanisms by which SST inhibits secretion from human β- and α-cells and the SSTR isoforms mediating these effects. Quantitative PCR revealed high expression of SSTR2, with lower levels of SSTR1, SSTR3, and SSTR5, in human islets. Immunohistochemistry showed expression of SSTR2 in both β- and α-cells. SST application hyperpolarized human β-cells and inhibited action potential firing. The membrane hyperpolarization was unaffected by tolbutamide but antagonized by tertiapin-Q, a blocker of G protein-gated inwardly rectifying K+ channels (GIRK). The effect of SST was mimicked by an SSTR2-selective agonist, whereas a SSTR5 agonist was marginally effective. SST strongly (>70%) reduced depolarization-evoked exocytosis in both β- and α-cells. A slightly weaker inhibition was observed in both cell types after SSTR2 activation. SSTR3- and SSTR1-selective agonists moderately reduced the exocytotic responses in β- and α-cells, respectively, whereas SSTR4- and SSTR5-specific agonists were ineffective. SST also reduced voltage-gated P/Q-type Ca2+ currents in β-cells, but normalization of Ca2+ influx to control levels by prolonged depolarizations only partially restored exocytosis. We conclude that SST inhibits secretion from both human β- and α-cells by activating GIRK and suppressing electrical activity, reducing P/Q-type Ca2+ currents, and directly inhibiting exocytosis. These effects are mediated predominantly by SSTR2 in both cell types.
Keywords: somatostatin receptor 2, insulin, glucagon, electrophysiology, exocytosis
the peptide hormone somatostatin (SST) is produced in the central nervous system, notably the hypothalamus, the gastrointestinal tract, and the endocrine pancreas, and acts as a general inhibitor of hormone secretion and gastrointestinal function. It exists in two active forms of 14 and 28 amino acids length (SST-14 and -28). SST exerts its effects by binding to a G protein-coupled receptor [somatostatin receptor (SSTR)], of which five isoforms (SSTR1–5) encoded by five genes (SSTR1–5) exist. All SSTRs are coupled to Gi/o proteins and inhibition of adenylate cyclase, but a variety of additional cellular effects have been described, including inhibition of Ca2+ channels and activation of K+ channels (1, 2, 11).
In pancreatic islets of Langerhans, SST-14 is released from δ-cells in response to glucose and amino acids (4, 23, 47). SST potently inhibits insulin and glucagon secretion in rodents and humans. Several studies indicate that SST is a physiologically important paracrine signal in islets. For example, mice with disruption of the SST gene have increased insulin and glucagon secretion, and the glucose regulation of glucagon release is impaired (19). Furthermore, in rats the incretin glucagon-like peptide-1 (GLP-1) has been postulated to inhibit glucagon secretion by stimulating SST release (14).
Several aspects of human islet architecture are especially favorable for paracrine signaling, including that involving SST. First, the fraction of δ-cells in human islet cells (∼10%) is almost twice as high as in rodent islets (8, 10). Second, 70–80% of human β-cells are in direct physical contact with non-β-cells [compared with 28% in mouse islets (3, 10), although β- to δ-cell contacts have not been analyzed separately]. Third, ultrastructural evidence indicates that the blood flow in human islets is along layers of non-β-cells surrounding the β-cell layers (3) rather than from central β-cells to peripheral non-β-cells, as suggested for rodent islets (36).
Studies in rodents have demonstrated that SST inhibits hormone secretion from β- and α-cells by inducing membrane hyperpolarization and directly suppressing exocytosis at a late stage (17, 29, 45). In mouse islets, the effects on β-cells are mediated predominantly by SSTR5, whereas in α-cells SSTR2 is the prevailing isoform (34, 40, 41). Data obtained from human islets are conflicting. Whereas one study identified SSTR5 as the dominant receptor in β-cells (46), others detected primarily SSTR2 (9, 37). The only investigation of glucagon secretion from human islets found an SSTR2-selective agonist to have the strongest inhibitory effect (37). Immunocytochemical studies have suggested broad expression of all receptor subtypes in human β- and α-cells (39).
The aim of the present study was to functionally identify SSTR isoforms expressed in human islet cell types and to determine their role in SST-14 signaling. To this end, isoform-selective agonists were applied to isolated islet cells and the effects evaluated using the patch clamp recordings of electrical activity, membrane currents, and exocytosis. This approach eliminates paracrine interactions between cell types that may complicate the interpretation of results obtained in intact islets. Furthermore, we have characterized the cellular mechanisms by which SST suppresses hormone secretion from human pancreatic islets.
MATERIALS AND METHODS
Materials.
L-054,264, L-803,087, and L-817,818 and CYN-154,806 were purchased from Tocris Bioscience (Bristol, UK). CH-275 was obtained from Polypeptide Laboratories (Strasbourg, France). L-796,778 was a kind gift from Dr. Susan Rohrer (Merck, Rahway, NJ). ω-Agatoxin IVA and tertiapin-Q were purchased from Alomone Labs (Jerusalem, Israel). A rabbit monoclonal antibody against SSTR2 (clone UMB1) was obtained from LifeSpan Biosciences (Seattle, WA). SST-14, isradipine, tolbutamide, and all other chemicals were from Sigma (Gillingham, UK). SST-14 was used throughout this study and will be referred to as SST.
Cell isolation and culture.
Human pancreases were obtained with ethical approval and clinical consent from heart-beating donors. Pancreatic islets were isolated in the Oxford Diabetes Research & Wellness Foundation Human Islet Isolation Facility or the Alberta Diabetes Institute IsletCore according to published protocols (25, 30). Freshly isolated islets were dispersed into single cells by incubation in enzyme-free cell dissociation buffer (Invitrogen, Paisley, UK), followed by trituration, and plated onto plastic Petri dishes. Cells were cultured in RPMI-1640 medium containing 7.5 mM glucose and 2 mM l-glutamine for >24 h before the experiments.
mRNA expression.
mRNA expression levels of SSTR1–5 and two nonrelated G protein-coupled receptors [GPCRs; gastric inhibitory polypeptide receptor (GIPR) and GLP-1 receptor] were determined in human pancreatic islets (n = 10) using TaqMan quantitative (q)RT-PCR (Applied Biosystems, Warrington, UK). All samples were available through existing collaborations at Oxford University and were collected with full ethical consent. RNA was extracted from all tissues using TRI reagent (Applied Biosystems). Samples were treated with DNase I (Life Technologies, Paisley, UK). cDNA was generated from 1 μg of total RNA by random primed first-strand synthesis (Applied Biosystems) according to the manufacturer's protocol, run at a 1:20 dilution, and amplified in triplicate. Each well contained a multiplexed assay of a gene of interest and, as an internal control, a housekeeping gene [either peptidylprolyl isomerase A (PPIA) or ubiquitin C (UBC)]. Analysis was performed using the ΔCT method (26). Statistical analysis was performed using a two-sided signed-rank test.
For PCR-analysis of Kir3.x subunit expression, total RNA from human pancreatic islets was purified using an RNeasy Mini Kit (Qiagen, Toronto, ON, Canada). DNase I-treated total RNA (2.0 μg) was reverse transcribed (iScript Reverse Transcription Supermix; Bio-Rad) in the presence of an RNase inhibitor. In a negative control, iScript Supermix (minus reverse transcriptase) was used for the cDNA reaction. PCR was performed using Platinum Taq Polymerase (Invitrogen, Burlington, ON, Canada) under the following conditions: 2 min at 95°C followed by 40 cycles of 10 s at 95°C, 20 s at 50°C, and 60 s at 72°C. PCR products were analyzed on a 1% agarose gel. Primers for RT-PCR were designed using the program Primer 3 (University of Massachussetts Medical School; http://biotools.umassmed.edu/bioapps/primer3_www.cgi). For detection of all KCNJ gene transcripts, common regions were selected for insertion into the above program. Expected fragment sizes were between 379 and 410 bp. β-Actin was used as a control.
Electrophysiology.
Patch pipettes were pulled from borosilicate glass, coated with Sylgard (Dow Corning, Wiesbaden, Germany), and fire-polished. Tip resistance was 3–8 MΩ when filled with intracellular solution. Patch clamp experiments were performed in the standard or perforated-patch whole cell configurations using an EPC-10 amplifier and Pulse software (HEKA, Lambrecht, Germany). Cell capacitance was estimated using the Lindau-Neher method as implemented by the LockIn extension of Pulse software. Cells were kept at 32–33°C throughout the experiments by constant superfusion with heated extracellular solution. β-Cells were identified by size [cell capacitance >6 pF, cf. (6)], whereas α-cells were identified by immunocytochemistry after the experiment.
To test effects of inhibitors on depolarization-evoked exocytosis, the duration of the voltage clamp depolarization was initially adjusted to between 200 and 500 ms to obtain capacitance responses >50 fF in β-cells and >20 fF in α-cells. Depolarizing pulses were then applied at 2-min intervals, and inhibitors were added when two sequential stimulations under control conditions yielded similar (± 20%) responses.
Solutions.
The extracellular solution for recording membrane potential and resting currents contained (in mM) 140 NaCl, 3.6 KCl, 0.5 MgSO4, 1.5 CaCl2, 10 HEPES, 0.5 NaH2PO4, 5 NaHCO3, and 6 glucose (pH was adjusted to 7.4 with NaOH). For the membrane capacitance and voltage-gated Ca2+ current measurements, extracellular medium composed of (in mM) 118 NaCl, 20 TEACl, 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2, 5 HEPES, and 5 glucose (pH 7.4, with NaOH) was used. The intracellular solution for capacitance measurements consisted of (in mM) 125 Cs-glutamate, 10 CsCl, 10 NaCl, 1 MgCl2, 5 HEPES, 0.05 EGTA, 3 MgATP, 0.1 NaGTP, and 0.1 cAMP (pH 7.15 with CsOH). The pipette solution for the membrane potential and resting current recordings (conducted using the perforated-patch configuration) contained (in mM) 76 K2SO4, 10 KCl, 10 NaCl, 1 MgCl2, and 5 HEPES (pH 7.35 with KOH) and 0.24 mg/ml amphotericin B. For the Ca2+ current measurements using the perforated-patch configuration, K2SO4 was replaced equimolarly with Cs2SO4.
Immunohistochemistry.
For analysis of SSTR2 expression, deparaffinized human pancreatic tissue sections were heated in a buffer containing 10 mM Tris and 1 mM EDTA (pH 9) for 15 min, allowed to cool in the same buffer for 15 min, and rinsed with PBS. After a 30-min blocking step in 20% goat serum, the sections were incubated with anti-SSTR2 (diluted 1:2,000 in 5% goat serum) and anti-insulin or anti-glucagon antibodies for 1 h at room temperature. The slides were then washed in PBS and incubated with fluorophore-labeled secondary antibodies (diluted in goat serum) for 1 h at room temperature. Fluorescence was visualized using an inverted microscope (Zeiss Apotome).
Immunocytochemical identification of α-cells after patch clamp experiments was performed as described previously (6).
Immunoblotting.
Proteins from human pancreatic islets (∼50 μg) were separated by electrophoresis on an 8% SDS-polyacrylamide gel and transferred by electroblotting onto a nitrocellulose membrane. Molecular weight markers were run alongside samples. Membranes were blocked with 5% milk powder in TBS-T buffer (20 mM Tris·HCl, 140 mM NaCl, and 0.1% Tween-20, pH 7.6) for 1 h at room temperature, washed (3 × 5 min in TBS-T), and incubated overnight at 4°C with anti-SSTR2 (diluted 1:1,000 in TBS-T containing 1% milk). After washing (3 × 5 min in TBS-T), membranes were probed with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:2,000 dilution in TBS-T containing 5% milk powder; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature and washed (3 × 5 min in TBS-T). Hybridized antibody was detected using an enhanced chemiluminescence kit (Western Lightning Plus-ECL; Perkin-Elmer, Waltham, MA).
Data analysis.
Data are expressed as means ± SE. Statistical analysis was performed using Student's t-test. Because of the cell-to-cell variability, capacitance responses are shown as normalized to control values before the addition of test substance(s) in the same cells. In experiments testing effects of Ca2+ channel blockers on Ca2+ currents, the SST-sensitive current in presence of blockers was expressed in percent of the initial current (before addition of blockers).
RESULTS
Expression of SSTR subtypes in human islets.
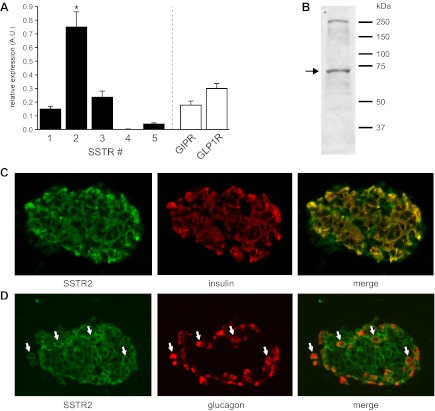
Gene expression of the five SSTR isoforms in human islets was analyzed by relative qRT-PCR (Fig. 1A). Two unrelated GPCRs known to be expressed in human β-cells (GIPR, GLP1R) were analyzed for comparison. SSTR2 showed significantly higher (P < 0.05) relative expression than all other SSTR isoforms as well as the unrelated GPCRs. Corresponding CT values ranged between 27.5 for SSTR2 and 32 for SSTR5. No expression of SSTR4 was detected. Probe affinity for SSTR4 was confirmed in a commercially available human tissue RNA panel (Clontech, Saint-Germain-en-Laye, France).
Fig. 1.
Distribution of somatostatin receptor (SSTR) isoforms in human islets. A: analysis of relative SSTR isoform expression by quantitative RT-PCR in 10 human islet preparations. Data are displayed relative to standard housekeeping genes [peptidylprolyl isomerase A (PPIA) and ubiquitin C (UBC)] using the ΔCT method. SSTR2 relative expression is significantly (P < 0.05) higher than the other SSTR isoforms as well as 2 other islet-expressed G protein-coupled receptors (GPCRs) [gastric inhibitory polypeptide receptor (GIPR) and glucagon-like peptide-1 receptor (GLP-1R)]. B: Western blot analysis of isolated human islets using a SSTR2-specific antibody (expected molecular weight: ∼72 kDa). Positions of molecular weight markers are shown on the right. C and D: coimmunostaining of human pancreatic tissue sections with anti-SSTR2 and anti-insulin (C) or anti-glucagon (D). White arrows indicate cells positive for both SSTR2 and glucagon. AU, arbitrary units.
Expression of SSTR2 protein in human islets was confirmed by Western blotting (Fig. 1B). Immunohistochemical analysis of human pancreatic tissue sections revealed that SSTR2 is present in both β-cells and α-cells (Fig. 1, C and D).
SST hyperpolarizes human β-cells independently of ATP-sensitive K+ channels.
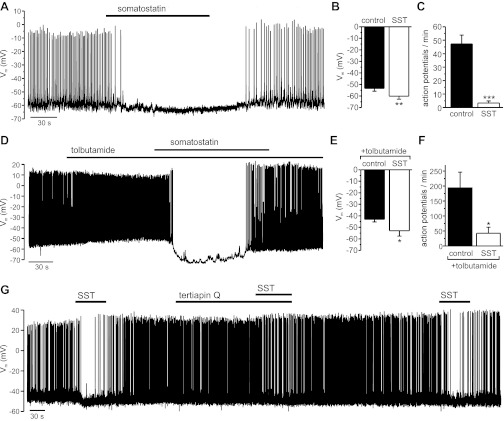
Electrical activity was recorded in isolated human β-cells, using the perforated-patch whole cell configuration of the patch clamp technique. The recordings were performed at 6 mM extracellular glucose, a physiological postprandial concentration that is close to the EC50 of glucose-stimulated insulin secretion (21). Application of SST (10–30 nM) to the extracellular solution hyperpolarized the β-cell membrane potential and suppressed action potential firing (Fig. 2A). The effect was reversible upon washout of the hormone. In a series of seven experiments, SST hyperpolarized β-cells by 7 ± 1.6 mV (Fig. 2B) and reduced the action potential frequency by 91 ± 6% (Fig. 2C).
Fig. 2.
Effect of somatostatin (SST) on the β-cell membrane potential (Vm). All experiments were performed in the presence of 6 mM glucose. A: SST (20 nM) was applied to an electrically active β-cell as indicated. B and C: average effect of SST (10–30 nM) on Vm (n = 7; **P < 0.01) and action potential frequency (n = 6; ***P < 0.001). D: SST (30 nM) was applied in the presence of tolbutamide (200 μM), as indicated by the bars. E and F: average effect of SST (30 nM) on Vm (n = 6) and action potential frequency (n = 4) when applied in the presence of tolbutamide (200 μM). *P < 0.05. G: SST (30 nM) was applied in the absence or presence of tertiapin-Q (100 nM), as indicated by bars.
SST hyperpolarized β-cells to a similar extent when applied in the presence of the ATP-sensitive K+ channel (KATP) channel blocker tolbutamide (Fig. 2D). Under these conditions, the membrane potential was lowered by 9.9 ± 3.7 mV (Fig. 2E) and the action potential frequency reduced by 72 ± 11% (Fig. 2F). By contrast, the SST-induced membrane hyperpolarization was diminished in the presence of tertiapin-Q, an inhibitor of G protein-gated inwardly rectifying K+ (GIRK) channels (Fig. 2G). In this series of experiments, SST hyperpolarized the cells by 9.8 ± 1.5 mV under control conditions, which was reduced to 4.3 ± 1 mV in the presence of tertiapin-Q (100–200 nM) in the same cells (n = 6, P < 0.01). It is notable that in human β-cells, as observed previously in mouse β-cells (29), action potential firing sometimes resumed in the continued presence of SST (Fig. 2, D and G).
Effects of SST on β-cell resting membrane currents.
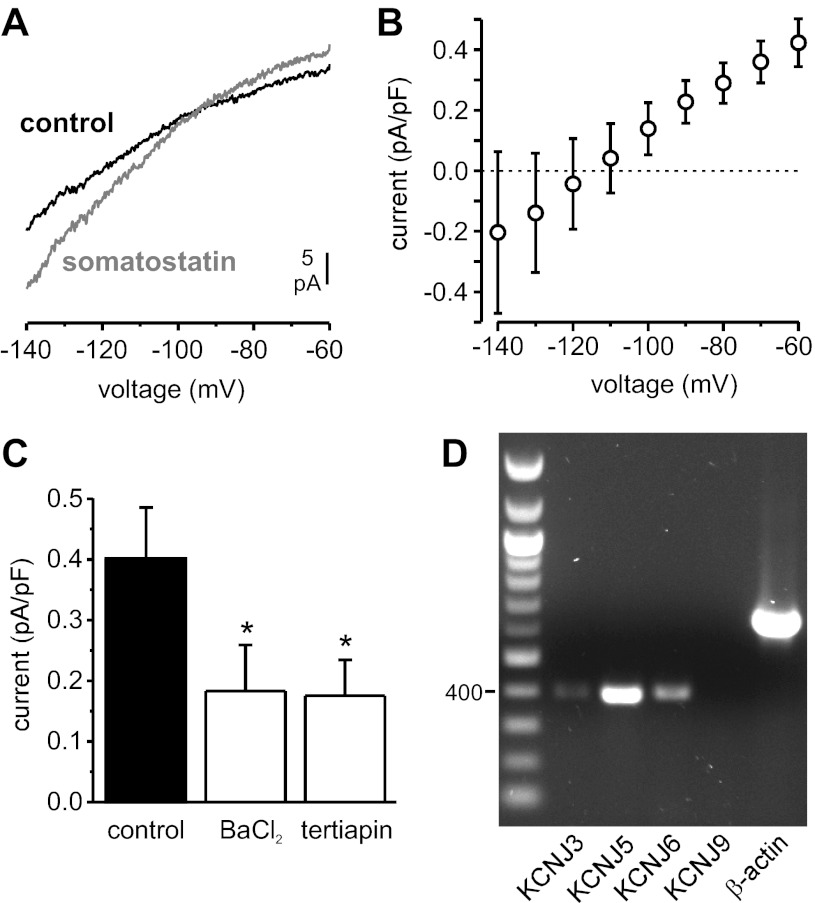
The membrane current underlying the SST-induced membrane hyperpolarization was recorded using the perforated-patch whole cell configuration. The β-cells were clamped at −70 mV (i.e., below the threshold for activation of voltage-gated channels), and voltage ramps from −140 to −60 mV were applied at 10-s intervals (Fig. 3A). Application of SST (30 nM) induced an outward current at −70 mV that averaged 4.4 ± 0.7 pA (0.3 ± 0.05 pA/pF; n = 12). The current-voltage relationship of the SST-activated current is shown in Fig. 3B. The current reversed at approximately −110 mV, slightly more negative than expected for a K+ selective current (−95 mV), and displayed weak inward rectification. The SST-activated current was insensitive to the KATP channel blocker tolbutamide (not shown) but reduced by ∼55% in the presence of Ba2+, a blocker of inwardly rectifying K+ channels, or the GIRK antagonist tertiapin-Q (Fig. 3C). Tertiapin-Q had no effect on the resting membrane conductance in the absence of SST (not shown). RT-PCR confirmed expression of the GIRK channel subunits Kir3.1 (KCNJ3), Kir3.2 (KCNJ6), and Kir3.4 (KCNJ5) in human islets (Fig. 3D).
Fig. 3.
Effect of SST on the β-cell resting membrane current (in the presence of 6 mM extracellular glucose). A: representative recording of membrane currents elicited by voltage ramps from −140 to −60 mV (speed: 0.2 V/s) before (black trace) and during application of 30 nM SST (gray trace). B: current-voltage relationship of the SST-activated current (obtained by subtracting traces recorded before SST application from traces recorded during SST application and normalized to cell size; n = 5). C: amplitude of the outward current at −70 mV (normalized to cell size) activated by SST application in the absence (control) or presence of Ba2+ (1 mM) or tertiapin-Q (0.3 μM; n = 5 in each group). *P < 0.05 vs control. D: RT-PCR analysis of expression of G protein-gated inwardly rectifying K+ channel subunits in human islets (the 400-bp marker is indicated on the left). KCNJ3, Kir3.1; KCNJ5, Kir3.4; KCNJ6, Kir3.2; KCNJ9, Kir3.3.
SST inhibits Ca2+ influx through P/Q-type Ca2+ channels in β-cells.
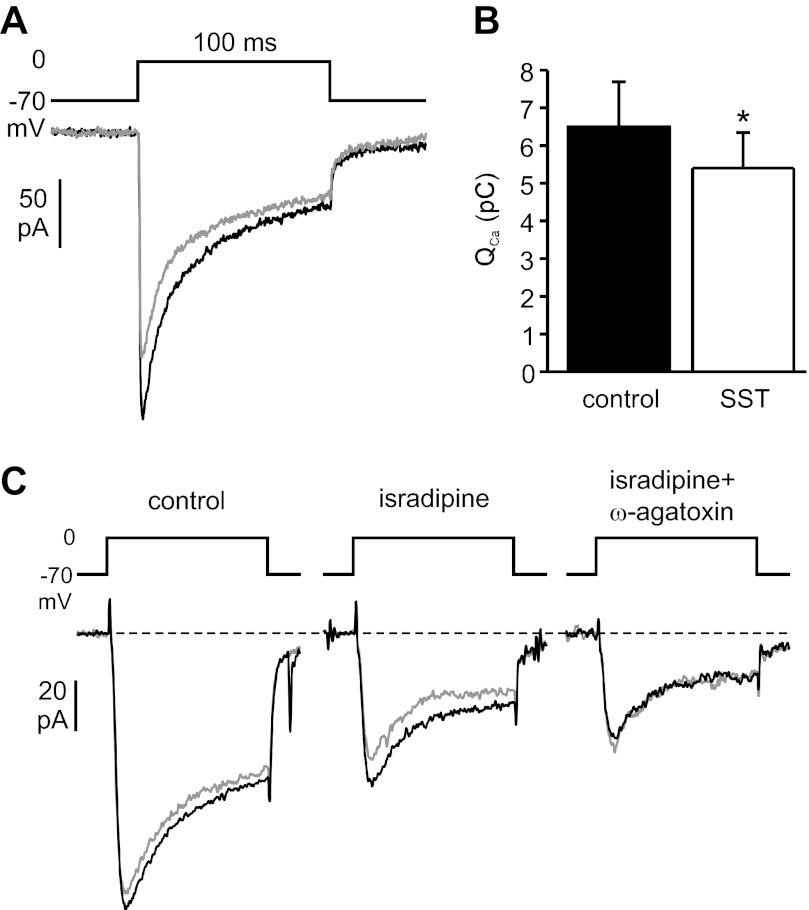
The effect of SST on voltage-gated Ca2+ currents was measured in the perforated-patch whole cell configuration. To block voltage-gated K+ currents, K+ in the pipette solution was replaced by Cs+, and the broad-spectrum K+ channel inhibitor TEA was added to the extracellular solution. Ca2+ currents were measured during voltage clamp depolarizations from −70 to 0 mV. Addition of SST (30 nM) to the bath solution inhibited depolarization-evoked Ca2+ influx (measured as integrated current) by 16 ± 3% (Fig. 4, A and B). The effect was reversible after removal of the hormone (not shown).
Fig. 4.
Effect of SST on β-cell voltage-gated Ca2+ currents. A: representative recording showing Ca2+ currents evoked by 100-ms depolarizations from −70 to 0 mV before (black trace) and during application of 30 nM SST (gray trace) in the same cell. B: the average effect on the integrated Ca2+ current (QCa) from 6 experiments is shown (*P < 0.05). C: SST (30 nM) was applied to the same cell under control conditions (left), in the presence of 10 μM isradipine (middle), and in the combined presence of isradipine and 200 nM ω-agatoxin IVA (right). Black traces are before and gray traces during SST application (50-ms depolarizations). The effect of SST was reversible after each application (not shown).
SST inhibits Ca2+ influx through P/Q type Ca2+ channels in β-cells.
Human β-cells express two types of high voltage-activated (HVA) Ca2+ channels, namely L-type and P/Q-type channels (6). To establish which channel subtype is inhibited by SST, the effect of the hormone on Ca2+ currents was studied in the presence of selective Ca2+ channel blockers (Fig. 4C). When SST was applied in the presence of the L-type channel blocker isradipine, Ca2+ influx was reduced to the same extent as under control conditions (by 17 ± 4% of the control current before application of isradipine; n = 3). By contrast, in the presence of the P/Q-type channel antagonist ω-agatoxin IVA, the effect of SST was essentially abolished (2 ± 3% inhibition, normalized to the control current before application of Ca2+ channel blockers; n = 4). This argues that SST principally inhibits P/Q-type Ca2+ channels in human β-cells.
SST inhibits exocytosis in β-cells downstream of Ca2+ influx.
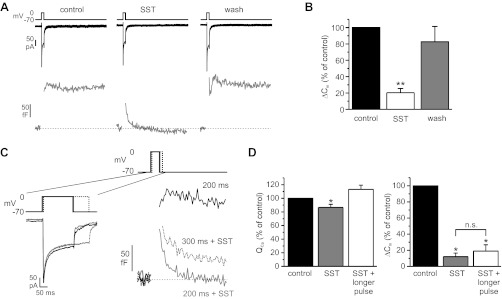
Exocytosis in single β-cells was monitored by capacitance measurements. This method detects increases in the cell surface area due to fusion of secretory granules with the plasma membrane. Exocytosis was evoked in the standard whole cell configuration by applying voltage clamp depolarizations from −70 to 0 mV. This maneuver leads to the opening of voltage-gated Ca2+ channels in the plasma membrane and elicits secretion independently of glucose-induced electrical activity. Addition of SST (30 nM) to the extracellular solution reduced exocytosis evoked by 200- to 500-ms depolarizations by 80 ± 5% (Fig. 5, A and B). The inhibition was stable for the duration of SST application (78 ± 6 and 81 ± 7% inhibition after 1 and 3 min, respectively) and reversible upon removal of the hormone (Fig. 5, A and B). In the same experiments, SST also decreased the depolarization-evoked Ca2+ influx by 11 ± 3% (n = 11, P < 0.05).
Fig. 5.
Effect of SST on β-cell exocytosis and involvement of Ca2+ current. A: inward Ca2+ current (black trace) and capacitance response (gray trace) evoked by 300-ms depolarizations from −70 to 0 mV before (left) and during exposure to 30 nM SST (middle) and after removal of the hormone (right). B: summary of capacitance responses (ΔCm) from 13 cells (**P < 0.01). C: Ca2+ currents (left) and capacitance responses (right) evoked by a 200-ms depolarization under control conditions (black traces), by a 200-ms depolarization in the presence of 30 nM SST (gray traces), and by a 300-ms depolarization in the continued presence of SST (dotted lines) in the same cell. D: integrated Ca2+ currents (QCa) and exocytotic responses (ΔCm) from 4 experiments performed similarly as described in C (i.e., the Ca2+ influx in the presence of SST was increased above pre-SST levels by prolongation of the depolarizing pulse). *P < 0.05.
To test whether the reduction of exocytosis can be explained by the inhibition of the Ca2+ current, the duration of depolarization pulse was prolonged in the continued presence of SST to adjust the total Ca2+ influx to the same (or a slightly higher) level as before SST addition (Fig. 5, C and D). In this series of experiments, SST reduced exocytosis evoked by pulses of control duration by 87 ± 4% and Ca2+ influx by 13 ± 5%. With prolonged depolarizing pulses, Ca2+ influx was restored to 113 ± 6% of the control value (i.e., before addition of SST), but exocytosis remained strongly suppressed (by 80 ± 8%). This suggests that the inhibitory effect of SST on β-cell exocytosis is principally exerted downstream of Ca2+ influx.
SST exerts its effects on β-cells mainly via SSTR2.
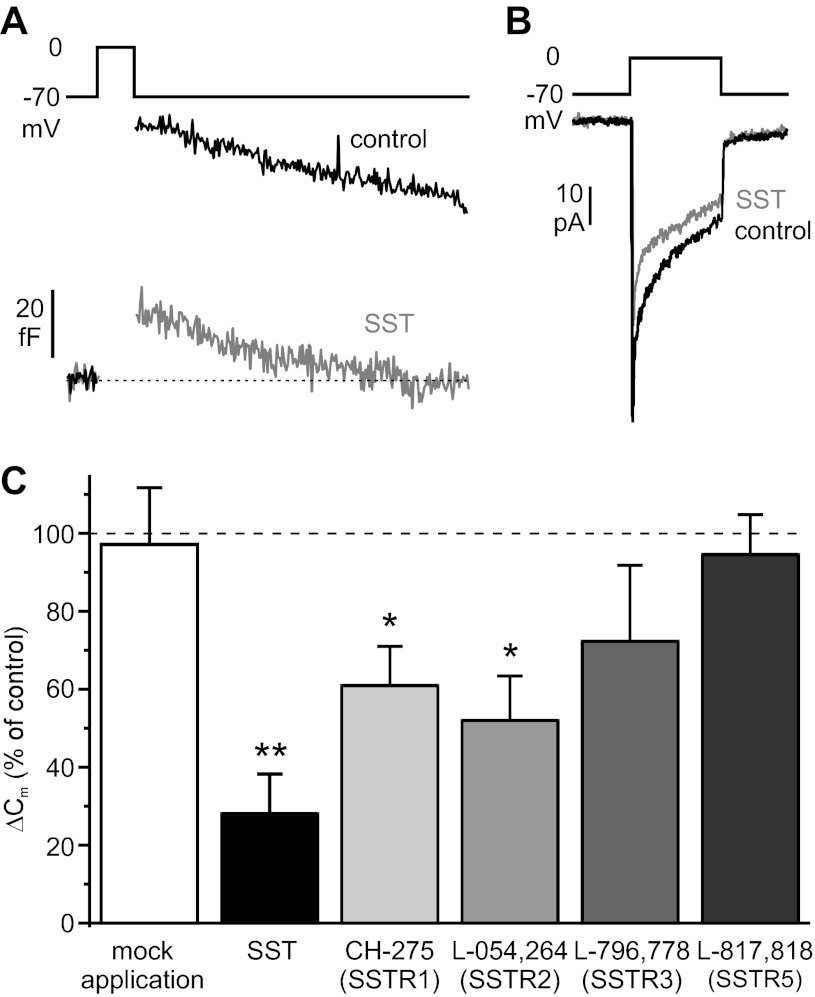
To identify the SSTR isoforms mediating SST effects in human β-cells, isoform-specific SSTR agonists were employed. To ensure maximal inhibition of the targeted receptor, the agonist concentrations used were ∼20-fold higher than their respective Ki values for the most sensitive isoform (Table 1). First, the effects of these agonists on depolarization-evoked exocytosis were examined (Fig. 6). The SSTR2-specific agonist L-054,264 inhibited β-cell exocytosis significantly, albeit to a lesser extent than SST itself. The exocytotic response in the presence of the agonist amounted to 44 ± 11% of control values. A weaker inhibitory effect was obtained with the SSTR3-specific agonist L-796,778 (by 24 ± 6%). The SSTR1 agonist CH-275, the SSTR4 agonist L-803,087, and the SSTR5-specific agonist L-817,818 were all without significant effect on the capacitance responses. It was ascertained that the exocytotic responses were stable in the absence of any drug addition (Fig. 6), thus excluding that the observed inhibitory effects reflect spontaneous rundown of exocytosis.
Table 1.
Binding affinities of SSTR agonists for cloned human SSTRs and concentrations used in this study
Fig. 6.
Effects of subtype-selective SSTR agonists on depolarization-evoked exocytosis (ΔCm) in β-cells (n = 7, 6, 10, 8, 5, and 7 in mock application, CH-275, L-054,264, L-796–778, L-803–087, and L-817–818, respectively). *P < 0.05; **P < 0.01.
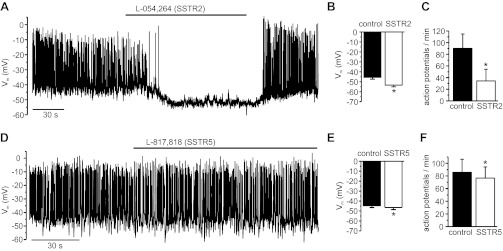
These results were verified in membrane potential measurements. As shown in Fig. 7A, the SSTR2-specific agonist L-054,264 hyperpolarized the membrane potential and inhibited glucose-induced electrical activity of human β-cells, although (similarly as above) to a slightly lesser extent than SST itself. The agonist lowered the membrane potential by 7.8 ± 3.1 mV and reduced the action potential frequency by 57 ± 18% (Fig. 7, B and C). The SSTR5-agonist L-817,818 had marginal effects on β-cell membrane potential (−1.8 ± 0.7 mV) and action potential frequency (10 ± 2% reduction; Fig. 7, D–F).
Fig. 7.
Effects of SSTR2- and SSTR5-selective agonists on β-cell electrical activity. A and D: representative recordings; agonists were applied as indicated by bars. Average effects on Vm (B and E) and action potential frequency (C and F); n = 8 (SSTR2) and 5 (SSTR5). *P < 0.05.
SST inhibits exocytosis from human α-cells.
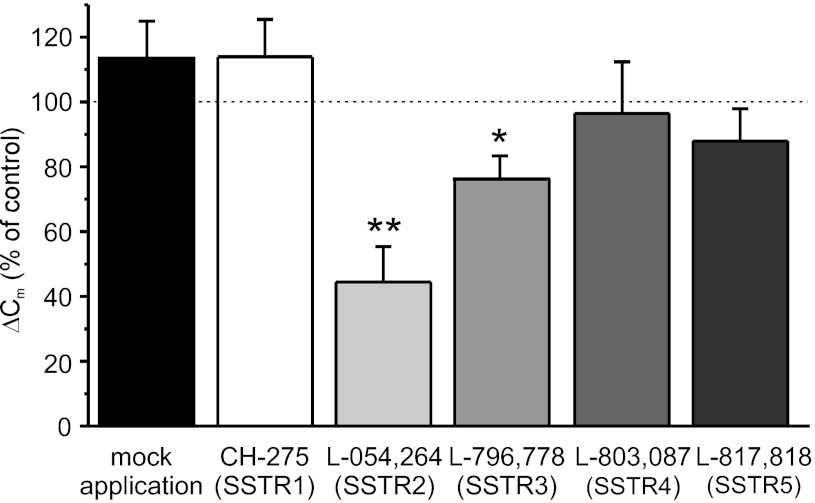
We have shown recently that SST inhibits electrical activity and hyperpolarizes the membrane potential of human α-cells, an effect that is antagonized by an SSTR2-selective blocker (28). We investigated the effects of SST and SSTR subtype-specific agonists on depolarization-evoked exocytosis in human α-cells. Addition of SST (30 nM) reduced exocytosis by 72 ± 10% (Fig. 8, A and C). In the same experiments, SST also decreased the Ca2+ influx by 15 ± 2% (P < 0.05, n = 7; Fig. 8B). The SSTR2-specific agonist L-054,264 likewise strongly inhibited α-cell exocytosis (by 48 ± 11%). A slightly weaker inhibition of exocytosis (by 39 ± 10%) was obtained after application of the SSTR1-selective agonist CH-275. Activation of SSTR3 or SSTR5 failed to affect the exocytotic responses significantly.
Fig. 8.
Effects of SST and SSTR subtype-specific agonists on α-cell exocytosis and Ca2+ current. Representative recordings of capacitance responses (A) and Ca2+ currents (B) evoked by 500-ms depolarizing pulses from −70 to 0 mV under control conditions (black traces) and in the presence of 30 nM SST (gray traces). C: average effects of SST and SSTR agonists on exocytotic responses (ΔCm; n = 6, 8, 8, 5, 8, and 5 in mock application, SST, CH-275, L-054–264, L-796–778, and L-817,818, respectively). *P < 0.05; **P < 0.01.
DISCUSSION
In MIN6 β-cells, KATP channels and non-KATP inwardly rectifying K+ channels contribute equally to the SST-activated K+ current (38). We show here that the KATP channel blocker tolbutamide did not diminish the SST-induced membrane hyperpolarization in human β-cells. This indicates that SST in human β-cells, as suggested previously in mouse β-cells (35), acts principally on conductances distinct from KATP channels. We instead found that tertiapin-Q reduces the SST-induced membrane hyperpolarization and inhibits the SST-activated resting membrane current, suggesting a key role for the Kir3.x/GIRK subfamily of inwardly rectifying K+ channels in the SST response. In support of this conclusion, we were able to confirm a previous report that human islets express Kir3.x subunits (16). GIRK channels have been shown to underlie K+ currents activated by SST in rodent α-cells (45) and by noradrenaline in mouse β-cells (24). Our data suggest that GIRK channels in human islet cells are heteromultimers consisting of Kir3.1 and Kir3.2 or Kir3.4 subunits (22).
Tertiapin-Q did not completely prevent the SST-induced membrane hyperpolarization, and a substantial component (∼45%) of the SST-activated outward leak current was unaffected by either tertiapin-Q or the Kir channel blocker Ba2+. Moreover, the reversal potential of the SST-activated current was more negative than predicted by the Nernst equation for a purely K+-selective conductance. These findings can best be explained if SST, in addition to activating a hyperpolarizing (GIRK-mediated) K+ current, also inhibits a depolarizing leak current. The channel or transporter underlying this depolarizing current remains to be identified.
In contrast to mouse β-cells (29), SST significantly reduced the depolarization-evoked Ca2+ influx in human β-cells. This difference may reflect the different SSTR subtypes expressed. SSTR2 (the major subtype in human β-cells; Fig. 6) but not SSTR5 [the subtype present in mouse β-cells (34)] has been shown to couple to Ca2+ currents (1). The inhibitory effect of SST was unaffected by the L-type Ca2+ channel blocker isradipine but abolished by the P/Q-type channel blocker ω-agatoxin IVA, indicating that SST specifically inhibits the latter type of channel. To our knowledge, this is the first demonstration of G protein regulation of P/Q-type channels in pancreatic islet cells. This result is at variance with previous studies on insulin-secreting cell lines, where L-type and R-type Ca2+ channels were reported to conduct the SST-sensitive Ca2+ current (27), but in accord with findings in neurons (43).
Under the experimental conditions used here, the reduction of Ca2+ influx by SST had only a minor contribution to its inhibitory effect on exocytosis (Fig. 5, C and D). However, this does not exclude that the inhibition of the voltage-gated Ca2+ current is physiologically relevant. Exocytosis in human β-cells is steeply dependent on the Ca2+ influx, and a 16% reduction of the Ca2+ current can consequently result in an ∼40% inhibition of exocytosis. Moreover, there is evidence that P/Q-type channels may be coupled specifically to exocytosis in human β-cells (6, 7).
In line with previous findings in rodents (29), SST inhibited depolarization-evoked exocytosis in human β- and α-cells principally downstream of Ca2+ influx (Fig. 5, C and D). Physiologically, the inhibition of exocytosis may be especially important at high postprandial plasma glucose levels, because SST suppressed action potential firing incompletely when applied in the presence of tolbutamide (which mimics the effects of high glucose concentrations on the membrane potential). The capacitance measurements were performed in the standard whole cell configuration, and the cytosol was dialysed with a saturating concentration of cAMP throughout the experiment. Therefore, although SST may inhibit adenylate cyclase in human islet cells, this is unlikely to contribute to the suppression of exocytosis we describe here. A variety of other pathways have been implicated in SST signaling in neuroendocrine cells, including activation of the protein phosphatase calcineurin in mouse β-cells (29) and rat α-cells (17), activation of protein acylation in rat β-cells (13), and direct effects of G protein βγ-subunits in chromaffin cells (12). The intracellular pathways that link SSTR activation to the functional changes described in this study remain to be identified.
The SST-induced inhibition of electrical activity and membrane hyperpolarization were transient in many cells, and action potential firing resumed in the continued presence of the hormone. By contrast, the inhibitory effect on exocytosis was stable throughout the experiments and may ensure that hormone secretion remains strongly inhibited even when the cells have repolarized. In vitro studies suggest that SST secretion, similar to insulin and glucagon secretion, is pulsatile with a period of 7–8 min (20). It is possible that the troughs of SST secretion allow the effects of SST on membrane potential to reset and that the effect on electrical activity is more significant in vivo than suggested by the transient effect during a sustained application in the present study.
The present study suggests that SSTR2 is the functionally dominant SSTR in human β-cells and that SSTR3 and to a lesser extent SSTR5 also contribute to the SST effects on exocytosis and electrical activity. In α-cells, activation of SSTR2 likewise had the strongest effect, followed by SSTR1. Although the effects of the SSTR3 agonist did not reach overall statistical significance, a clear and reversible inhibition of exocytosis was obtained in some α-cells, which is compatible with heterogeneous receptor expression. A similar order of potency for SSTR1, -2, and -5 agonists (i.e., SSTR2 > SSTR5 > SSTR1 for insulin, SSTR2 > SSTR1 > SSTR5 for glucagon) was observed in hormone secretion measurements from isolated human islets (9, 37), but an SSTR3 agonist was found to be ineffective in these studies. It is likely that the same SSTR3 agonist employed in the present study was also used in the studies of Brunicardi et al. (9) and Singh et al. (37) (based on its Ki values for SSTR isoforms), but it is unclear which concentrations were tested in those studies. The low affinity of the compound for SSTR2 makes it unlikely that the effects observed here were mediated by a different receptor isoform.
Reducing glucagon secretion can improve glucose homeostasis in diabetes and is therefore therapeutically desirable (18). Our data tentatively indicate that, by using SSTR1-specific agonists, this might achievable without affecting insulin secretion. Somatostatin analogs are used in the treatment of various endocrine disorders. For example, SOM230 is employed to inhibit ACTH secretion in Cushing disease, an effect that depends mainly on the activation of SSTR5 (42). A common side effect of the treatment is hyperglycemia. Although SOM230 activates SSTR5 with approximately sixfold higher potency than SSTR2 (2), the present study suggests that this risk might be further reduced by using a more SSTR5-specific agonist.
In summary, we demonstrate that SST inhibits pancreatic hormone secretion by a combination of several effects. First, it hyperpolarizes the membrane potential of human β-cells and inhibits action potential firing. This hyperpolarization is independent of KATP channels and involves GIRK channels. Second, the hormone inhibits Ca2+ influx through voltage-gated P/Q-type Ca2+ channels. Finally, SST directly inhibits Ca2+-dependent exocytosis in β- and α-cells. These effects are mediated predominantly by SSTR2 in both cell types, whereas in mice SSTR2 and SSTR5 are believed to mediate the effects in α- and β-cells, respectively. This is yet another example where observations in rodents cannot be extrapolated to humans (5, 6, 10, 15, 32, 33).
GRANTS
This research was supported by the Canadian Institutes of Health Research (MOP-106435), the Medical Research Council (grants GO801995 and GO700222/81696), the Wellcome Trust (095331/Z/11/Z and 95101/Z/10/Z), and the Department of Health (National Institute for Health Research Biomedical Research Centre's funding scheme). A. L. Gloyn is a Wellcome Trust Senior Fellow in Basic Biomedical Science. M. van de Bunt is a Nuffield Department of Medicine Prize Student.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.K., M.v.d.B., S.C., and M.B. performed the experiments; B.K., M.v.d.B., S.C., and M.B. analyzed the data; B.K., M.v.d.B., S.C., P.R.J., P.E.M., A.L.G., P.R., and M.B. interpreted the results of the experiments; B.K., M.v.d.B., S.C., P.R.J., P.E.M., A.L.G., P.R., and M.B. edited and revised the manuscript; B.K., M.v.d.B., S.C., P.R.J., P.E.M., A.L.G., P.R., and M.B. approved the final version of the manuscript; M.B. did the conception and design of the research; M.B. prepared the figures; M.B. drafted the manuscript.
ACKNOWLEDGMENTS
We thank Sheena Lesyk (Histology Core, Alberta Diabetes Institute) for help with immunohistochemistry and Jamie Boisvenue (Cardiovascular Research Center, University of Alberta) for help with immunoblotting.
REFERENCES
- 1. Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine 20: 255–264, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Ben-Shlomo A, Melmed S. Pituitary somatostatin receptor signaling. Trends Endocrinol Metab 21: 123–133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59: 1202–1210, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braun M, Ramracheya R, Amisten S, Bengtsson M, Moritoh Y, Zhang Q, Johnson PR, Rorsman P. Somatostatin release, electrical activity, membrane currents and exocytosis in human pancreatic delta cells. Diabetologia 52: 1566–1578, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Braun M, Ramracheya R, Bengtsson M, Clark A, Walker JN, Johnson PR, Rorsman P. Gamma-aminobutyric acid (GABA) is an autocrine excitatory transmitter in human pancreatic beta-cells. Diabetes 59: 1694–1701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic beta-cells: Electrophysiological characterization and role in insulin secretion. Diabetes 57: 1618–1628, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Braun M, Ramracheya R, Johnson PR, Rorsman P. Exocytotic properties of human pancreatic beta-cells. Ann NY Acad Sci 1152: 187–193, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53: 1087–1097, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Brunicardi FC, Atiya A, Moldovan S, Lee TC, Fagan SP, Kleinman RM, Adrian TE, Coy DH, Walsh JH, Fisher WE. Activation of somatostatin receptor subtype 2 inhibits insulin secretion in the isolated perfused human pancreas. Pancreas 27: e84–e89, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103: 2334–2339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cervia D, Bagnoli P. An update on somatostatin receptor signaling in native systems and new insights on their pathophysiology. Pharmacol Ther 116: 322–341, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Chen XK, Wang LC, Zhou Y, Cai Q, Prakriya M, Duan KL, Sheng ZH, Lingle C, Zhou Z. Activation of GPCRs modulates quantal size in chromaffin cells through G(betagamma) and PKC. Nat Neurosci 8: 1160–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Cheng H, Straub SG, Sharp GW. Protein acylation in the inhibition of insulin secretion by norepinephrine, somatostatin, galanin, and PGE2. Am J Physiol Endocrinol Metab 285: E287–E294, 2003 [DOI] [PubMed] [Google Scholar]
- 14. de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51: 2263–2270, 2008 [DOI] [PubMed] [Google Scholar]
- 15. De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest 96: 2489–2495, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrer J, Nichols CG, Makhina EN, Salkoff L, Bernstein J, Gerhard D, Wasson J, Ramanadham S, Permutt A. Pancreatic islet cells express a family of inwardly rectifying K+ channel subunits which interact to form G-protein-activated channels. J Biol Chem 270: 26086–26091, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Gromada J, Hoy M, Buschard K, Salehi A, Rorsman P. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G(i2)-dependent activation of calcineurin and depriming of secretory granules. J Physiol 535: 519–532, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hare KJ, Vilsboll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 59: 1765–1770, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson IC, Low MJ, Christie MR, Persaud SJ, Jones PM. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58: 403–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hellman B, Salehi A, Gylfe E, Dansk H, Grapengiesser E. Glucose generates coincident insulin and somatostatin pulses and antisynchronous glucagon pulses from human pancreatic islets. Endocrinology 150: 5334–5340, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 55: 3470–3477, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Ipp E, Dobbs RE, Arimura A, Vale W, Harris V, Unger RH. Release of immunoreactive somatostatin from the pancreas in response to glucose, amino acids, pancreozymin-cholecystokinin, and tolbutamide. J Clin Invest 60: 760–765, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iwanir S, Reuveny E. Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels. Pflugers Arch 456: 1097–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Lake SP, Bassett PD, Larkins A, Revell J, Walczak K, Chamberlain J, Rumford GM, London NJ, Veitch PS, Bell PR, James RF. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes 38, Suppl 1: 143–145, 1989 [DOI] [PubMed] [Google Scholar]
- 26. McCulloch LJ, van de Bunt M, Braun M, Frayn KN, Clark A, Gloyn AL. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: Implications for understanding genetic association signals at this locus. Mol Genet Metab 104: 648–653, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Mergler S, Singh V, Grotzinger C, Kaczmarek P, Wiedenmann B, Strowski MZ. Characterization of voltage operated R-type Ca2+ channels in modulating somatostatin receptor subtype 2- and 3-dependent inhibition of insulin secretion from INS-1 cells. Cell Signal 20: 2286–2295, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, Johnson PR, Rorsman P, Braun M. Membrane potential-dependent inactivation of voltage-gated ion channels in alpha-cells inhibits glucagon secretion from human islets. Diabetes 59: 2198–2208, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Renström E, Ding WG, Bokvist K, Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron 17: 513–522, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 37: 413–420, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Rivier JE, Hoeger C, Erchegyi J, Gulyas J, DeBoard R, Craig AG, Koerber SC, Wenger S, Waser B, Schaer JC, Reubi JC. Potent somatostatin undecapeptide agonists selective for somatostatin receptor 1 (sst1). J Med Chem 44: 2238–2246, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab 14: 45–54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med 17: 888–892, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rohrer SP, Schaeffer JM. Identification and characterization of subtype selective somatostatin receptor agonists. J Physiol Paris 94: 211–215, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Rorsman P, Bokvist K, Ammala C, Arkhammar P, Berggren PO, Larsson O, Wahlander K. Activation by adrenaline of a low-conductance G protein-dependent K+ channel in mouse pancreatic B cells. Nature 349: 77–79, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Samols E, Bonner-Weir S, Weir GC. Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab 15: 33–58, 1986 [DOI] [PubMed] [Google Scholar]
- 37. Singh V, Brendel MD, Zacharias S, Mergler S, Jahr H, Wiedenmann B, Bretzel RG, Plockinger U, Strowski MZ. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. J Clin Endocrinol Metab 92: 673–680, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Smith PA, Sellers LA, Humphrey PP. Somatostatin activates two types of inwardly rectifying K+ channels in MIN-6 cells. J Physiol 532: 127–142, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strowski MZ, Blake AD. Function and expression of somatostatin receptors of the endocrine pancreas. Mol Cell Endocrinol 286: 169–179, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Strowski MZ, Kohler M, Chen HY, Trumbauer ME, Li Z, Szalkowski D, Gopal-Truter S, Fisher JK, Schaeffer JM, Blake AD, Zhang BB, Wilkinson HA. Somatostatin receptor subtype 5 regulates insulin secretion and glucose homeostasis. Mol Endocrinol 17: 93–106, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141: 111–117, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Tritos NA, Biller BM, Swearingen B. Management of Cushing disease. Nat Rev Endocrinol 7: 279–289, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Viana F, Hille B. Modulation of high voltage-activated calcium channels by somatostatin in acutely isolated rat amygdaloid neurons. J Neurosci 16: 6000–6011, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang L, Guo L, Pasternak A, Mosley R, Rohrer S, Birzin E, Foor F, Cheng K, Schaeffer J, Patchett AA. Spiro[1H-indene-1,4′-piperidine] derivatives as potent and selective non-peptide human somatostatin receptor subtype 2 (sst2) agonists. J Med Chem 41: 2175–2179, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Yoshimoto Y, Fukuyama Y, Horio Y, Inanobe A, Gotoh M, Kurachi Y. Somatostatin induces hyperpolarization in pancreatic islet alpha cells by activating a G protein-gated K+ channel. FEBS Lett 444: 265–269, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Zambre Y, Ling Z, Chen MC, Hou X, Woon CW, Culler M, Taylor JE, Coy DH, Van Schravendijk C, Schuit F, Pipeleers DG, Eizirik DL. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochem Pharmacol 57: 1159–1164, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Q, Bengtsson M, Partridge C, Salehi A, Braun M, Cox R, Eliasson L, Johnson PR, Renstrom E, Schneider T, Berggren PO, Gopel S, Ashcroft FM, Rorsman P. R-type Ca(2+)-channel-evoked CICR regulates glucose-induced somatostatin secretion. Nat Cell Biol 9: 453–460, 2007 [DOI] [PubMed] [Google Scholar]