Abstract
Certain single nucleotide polymorphisms (SNPs) in transcription factor 7-like 2 (TCF7L2) are strongly associated with the risk of type 2 diabetes. TCF7L2 and β-catenin (β-cat) form the bipartite transcription factor cat/TCF in stimulating Wnt target gene expression. cat/TCF may also mediate the effect of other signaling cascades, including that of cAMP and insulin in cell-type specific manners. As carriers of TCF7L2 type 2 diabetes risk SNPs demonstrated increased hepatic glucose production, we aimed to determine whether TCF7L2 expression is regulated by nutrient availability and whether TCF7L2 and Wnt regulate hepatic gluconeogenesis. We examined hepatic Wnt activity in the TOPGAL transgenic mouse, assessed hepatic TCF7L2 expression in mice upon feeding, determined the effect of insulin on TCF7L2 expression and β-cat Ser675 phosphorylation, and investigated the effect of Wnt activation and TCF7L2 knockdown on gluconeogenic gene expression and glucose production in hepatocytes. Wnt activity was observed in pericentral hepatocytes in the TOPGAL mouse, whereas TCF7L2 expression was detected in human and mouse hepatocytes. Insulin and feeding stimulated hepatic TCF7L2 expression in vitro and in vivo, respectively. In addition, insulin activated β-cat Ser675 phosphorylation. Wnt activation by intraperitoneal lithium injection repressed hepatic gluconeogenic gene expression in vivo, whereas lithium or Wnt-3a reduced gluconeogenic gene expression and glucose production in hepatic cells in vitro. Small interfering RNA-mediated TCF7L2 knockdown increased glucose production and gluconeogenic gene expression in cultured hepatocytes. These observations suggest that Wnt signaling and TCF7L2 are negative regulators of hepatic gluconeogenesis, and TCF7L2 is among the downstream effectors of insulin in hepatocytes.
Keywords: transcription factor 7-like 2, β-catenin, glucose production, liver, phosphoenolpyruvate carboxykinase, Wnt-3a
transcription factor 7-like 2 (TCF7L2, previously known as TCF-4) belongs to the T cell factor (TCF) family of high-mobility group box transcription factors and is a major effector of the canonical Wnt signaling pathway (defined as the Wnt pathway hereafter unless further clarification is necessary) (18, 24). Upon stimulation by Wnt ligands, a TCF protein binds to nuclear β-catenin (β-cat) to form a bipartite transcription factor cat/TCF, leading to the stimulation of Wnt target gene expression (14). cat/TCF may also serve as an effector of other signaling molecules, including certain peptide hormones that utilize cAMP as a second messenger, IGF-I, insulin, and the lipid metabolite lysophosphatidic acid (LPA) (2, 5, 14, 15, 37). Previous studies have established the fundamental role of TCF7L2 and Wnt in embryogenesis and tumorigenesis. However, the involvement of Wnt signaling and TCF7L2 in hormone gene expression and metabolic homeostasis has been recognized only recently (15, 21, 25, 26, 41).
During the past few years, extensive genome-wide association studies have revealed that certain single nucleotide polymorphisms (SNPs) within intronic regions of TCF7L2 are strongly associated with the risk of type 2 diabetes (9, 10). This finding fueled many efforts to investigate the role of TCF7L2 and Wnt signaling in pancreatic β-cells (22, 33, 34). Several studies have demonstrated the beneficial effect of TCF7L2 on β-cell proliferation, insulin secretion, and the expression of incretin hormone receptors (3, 33, 34). These findings, however, are in contradiction with the suggestion that TCF7L2 exerts deleterious effects on β-cells by other investigations (22, 30). It has also been demonstrated that TCF7L2 type 2 diabetes risk SNPs are associated with elevated hepatic glucose production in individuals even during a hyperinsulinemic euglycemic clamp as well as during hepatic insulin resistance (22, 28, 39). Moreover, hepatic β-cat deficiency in mice resulted in alterations in glucose production and expression of the key rate-limiting gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G-6-Pase) (20). These observations prompted us to assess whether TCF7L2 is regulated by nutrient availability as well as the role of TCF7L2 and Wnt signaling in hepatic glucose production.
Here, we show the detection of hepatic Wnt activity in the TOPGAL Wnt/TCF-reporter transgenic mouse and the expression of TCF7L2 in both human and mouse hepatic cells. We have also found that insulin and feeding stimulated TCF7L2 expression in vitro and in vivo, respectively, and that insulin is able to stimulate the activity of β-cat, the partner of TCF7L2 in mediating Wnt activity. Moreover, we have defined the repressive effect of both the Wnt ligand Wnt-3a and TCF7L2 on hepatic glucose production.
MATERIALS AND METHODS
Cell culture and treatment.
Human HepG2 and mouse Hepa1–6 hepatoma cell lines (ATCC) were cultured in DMEM (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Gibco). Mouse primary hepatocytes were isolated by liver perfusion of 12-wk-old C57BL/6 mice, as described previously (40). Hepatocytes or hepatic cell lines were starved of serum overnight prior to treatment with 100 nM insulin (Eli Lilly), 10 μM forskolin (Sigma), 10 nM glucagon (Sigma), 10 mM lithium chloride (Bioshop), or 2.5 nM Wnt ligand, either Wnt-11 or Wnt-3a (R & D Systems) for the indicated times. The chemical inhibitor Akti1/2 (10 μM) was obtained from Sigma, whereas the inhibitors LY-294002 (50 μM) and PD-98059 (50 μM) were obtained from Calbiochem. Transfection of 5 nM small interfering RNA (siRNA) recognizing either a scrambled sequence or TCF7L2 (Ambion) was achieved using Lipofectamine RNAiMAX (Invitrogen) per the manufacturer's instructions. Transfection of luciferase (LUC) reporter plasmids (2.0 μg/well in a 12-well plate) was achieved using 3 μg of polyethylenimine (Sigma).
Animals.
Twelve-week-old male C57BL/6 mice (Jackson) were fasted overnight prior to refeeding or a defined treatment. Mice were intraperitoneally (ip) injected with lithium chloride (Bioshop) at a final dosage of 3 mmol/kg body wt or PBS as the control 4 h prior to extraction of liver tissue for reverse transcription PCR (RT-PCR) or Western blot analysis. TOPGAL transgenic mice (4) were purchased from Jackson (004623, Tg-Fos-lacZ-34Efu/J). β-Galactosidase (LacZ) staining was performed as described previously (2). All animal procedures were approved by the Animal Resource Centre, Toronto General Research Institute, University Health Network.
RNA isolation, RT-PCR, and real-time RT-PCR.
Total RNA was isolated from HepG2, Hepa1–6, mouse primary hepatocytes, or liver tissue using TRI reagent (Sigma) and reverse transcribed using a complimentary DNA reverse transcription kit (Applied Biosystems), following the instructions of the manufacturers. For RT-PCR detection of TCF7, LEF1, TCF7L1, TCF7L2, and Wnt-3a, specific transcript sequences were amplified by Taq DNA polymerase (New England BioLabs) using the S1000 Thermal Cycler (Bio-Rad) per the instructions of the manufacturers. A reaction lacking any DNA template served as a negative control. For quantitative real-time RT-PCR analysis, specific transcript sequences were amplified using iTaq SYBR Green (Bio-Rad) and the Rotor-Gene 3000 machine (Corbett) per the instructions of the manufacturers. 18S or β-actin genes served as normalizing controls. Relative quantification was calculated using the 2−ΔΔCT method. Each sample was analyzed in triplicate. Primers used for RT-PCR or real-time RT-PCR were synthesized by ACGT and are presented in Table 1.
Table 1.
Primers used for RT-PCR or real-time RT-PCR
| Gene | Sequence | Size, bp |
|---|---|---|
| Human | ||
| TCF7 | ||
| Sense | 5′-AAGGCCTGAAGGCCCCGGAGT-3′ | 173 |
| Antisense | 5′-GAGAGAGAGTTGGGGGACACCGT-3′ | |
| LEF1 | ||
| Sense | 5′-TCACACCCGTCACACATCCCA-3′ | 180 |
| Antisense | 5′-GGGTTGCCTGAATCCACCCGT-3′ | |
| TCF7L1 | ||
| Sense | 5′-AAGCCGCGGGACTATTTCGC-3′ | 196 |
| Antisense | 5′-CGCTGGAGGGGACATCGAGGA-3′ | |
| TCF7L2 | ||
| Sense | 5′-TCGCCTGGCACCGTAGGACA-3′ | 200 |
| Antisense | 5′-GGATGCGGAATGCCCGTCGT-3′ | |
| PEPCK | ||
| Sense | 5′-AGAATAAGCCAGATGTAATCAGGG-3′ | 206 |
| Antisense | 5′-TAGCTACTACCCAGTGTTCTGTGG-3′ | |
| G-6-Pase | ||
| Sense | 5′-AACAACCATGCCAGGGATT-3′ | 201 |
| Antisense | 5′-TACGTGATATGGCACCTCC-3′ | |
| Wnt-3a | ||
| Sense | 5′-TTCTTACTCCTCTGCAGCCTGA-3′ | 201 |
| Antisense | 5′-GCCAATCTTGATGCCCTCGG-3′ | |
| 18S | ||
| Sense | 5′-GTAACCCGTTGAACCCCATT-3′ | 151 |
| Antisense | 5′-CCATCCAATCGGTAGTAGCG-3′ | |
| β-Actin | ||
| Sense | 5′-TCATGAAGTGTGACGTTGACA-3′ | 285 |
| Antisense | 5′-CCTAGAAGCATTTGCGGTG-3′ | |
| Mouse | ||
| TCF7 | ||
| Sense | 5′-AGGTCAGATGGGTTGGACTG-3′ | 412 |
| Antisense | 5′-AGGGTGCACACTGGGTTTAG-3′ | |
| LEF1 | ||
| Sense | 5′-TCCGGGATCCCACCCGTCAC-3′ | 124 |
| Antisense | 5′-GATCTGTCCAACGCCGCCCG-3′ | |
| TCF7L1 | ||
| Sense | 5′-GAGTGCGAAATCCCCAGTTA-3′ | 384 |
| Antisense | 5′-ATGCATGGCTTCTTGCTCTT-3′ | |
| TCF7L2 | ||
| Sense | 5′-GCATCCCTCACCCGGCCATC-3′ | 243 |
| Antisense | 5′-GCCACCTGCGCCCGAGAATC-3′ | |
| PEPCK | ||
| Sense | 5′-CATAACGGTCTGGACTTCTCTGC-3′ | 417 |
| Antisense | 5′-GAATGGGATGACATACATGGTGCG-3′ | |
| G-6-Pase | ||
| Sense | 5′-CTCTGGGTGGCAGTGGTCGG-3′ | 83 |
| Antisense | 5′-AGGACCCACCAATACGGGCGT-3′ | |
| Wnt-3a | ||
| Sense | 5′-GCTGTGGGACCCCAGTACTC-3′ | 191 |
| Antisense | 5′-GTGGTGCAGTTCCAACGCC-3′ | |
| 18S | ||
| Sense | 5′-GTAACCCGTTGAACCCCATT-3′ | 151 |
| Antisense | 5′-CCATCCAATCGGTAGTAGCG-3′ | |
| β-Actin | ||
| Sense | 5′-TCATGAAGTGTGACGTTGACA-3′ | 285 |
| Antisense | 5′-CCTAGAAGCATTTGCGGTG-3′ | |
TCF7, transcription factor 7; TCF7L1 and -2, transcription factor 7-like 1 and 2, respectively; PEPCK, phosphoenolpyruvate carboxykinase; G-6-Pase, glucose-6-phosphatase.
Western blotting and antibodies.
Methods for whole cell lysate preparation and Western blotting have been described previously (42). Protein samples (30 μg/sample) were separated by SDS-PAGE and transferred to nitrocellulose membrane. Antibodies for TCF7L2, phosphorylated GSK-3β (Ser9), and phosphorylated CREB (Ser133) were acquired from Cell Signaling Technology. Antibodies for phosphorylated β-cat (Ser675), β-cat, β-actin, cyclin D1, Akt, and phosphorylated ERK1 (Tyr204) were purchased from Santa Cruz Biotechnology. The antibody for phosphorylated Akt (Ser473) was the product of Signalway Antibody and for PEPCK was that of Abcam.
LUC reporter analysis.
The generation of the TCF7L2 (3.1 kbp)-LUC fusion gene construct was described previously (38). The rat PEPCK (−595 to +67 bp)-LUC construct was generated first by PCR amplification of the PEPCK promoter sequence from rat genomic DNA (forward, 5′-CGGACGCGTTTACAATCACCCCTCCCTCT-3′; reverse, 5′-ACCTTTCTTCCTCCTTTTGG-3′), followed by insertion into the pGL3-basic vector (Promega) via MluI/BglII restriction sites. LUC reporter analyses were performed as described previously (41).
Glucose production assay.
Mouse primary hepatocytes and hepatoma cell lines were cultured in six-well plates (1.0 × 106 cells/well) in DMEM and starved of serum and glucose overnight. Cells were then washed three times with PBS and incubated in glucose production buffer (DMEM without glucose, serum, or phenol red and supplemented with 2 mM sodium pyruvate and 20 mM sodium lactate; Sigma). After 4 h, the medium was collected and assayed for glucose content using a colorimetric glucose assay kit (Sigma). Glucose content was normalized to total protein content measured in whole cell lysates.
Statistical analysis.
All experiments were performed independently as a minimum of three replicates or mice. Quantitative results are expressed as means ± SE relative to the indicated control group. Statistical analyses were performed using the two-tailed Student's t-test or one-way ANOVA as appropriate for single or multiple comparisons, respectively. Differences were considered significant when P < 0.05.
RESULTS
Detection of Wnt signaling and TCF7L2 expression in hepatocytes.
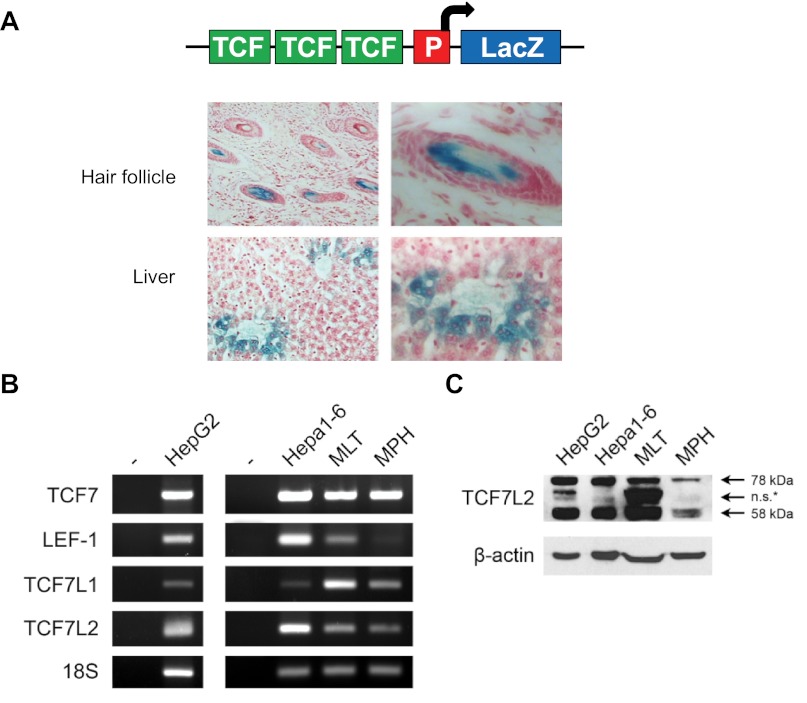
To assess Wnt activity in hepatocytes, we took advantage of the availability of TOPGAL transgenic mice (4). In this mouse model, LacZ expression is driven by the Wnt/TCF-responsive promoter element (Fig. 1A, top). As shown in Fig. 1A, positive LacZ staining was observed in hair follicles (positive control) (4) and adult mouse pericentral hepatocytes. Pericentral hepatocytes are typically associated with reduced levels of gluconeogenesis but elevated levels of lipogenesis (16). We then determined by RT-PCR that both the human HepG2 cell line and mouse hepatocytes [Hepa1–6 cell line, mouse liver tissue (MLT), and mouse primary hepatocytes (MPH)] express TCF7L2 and three other members of the TCF family (Fig. 1B). Western blotting showed that the human HepG2 cell line and mouse hepatic cells express primarily two isoforms of TCF7L2 of ∼78 and 58 kDa in size (Fig. 1C).
Fig. 1.
Active Wnt signaling and transcription factor 7-like 2 (TCF7L2) expression are present in hepatocytes. A, top: depiction of the TOPGAL mouse transgene. A, bottom: β-galactosidase (LacZ) staining showing active Wnt activity in pericentral hepatocytes of the adult TOPGAL mouse liver (n = 3). Hair follicle serves as a positive control. B: detection of transcription factor (TCF) 7, LEF1, TCF7L1, and TCF7L2 by RT-PCR in human HepG2 and mouse Hepa1–6 hepatic cell lines, C57BL/6 mouse liver tissue (MLT), and mouse primary hepatocytes (MPH). C: detection of TCF7L2 by Western blotting in human HepG2 and mouse Hepa1–6 hepatic cell lines, MLT, and MPH. ns*, Nonspecific or the identity is not yet known. P, promoter.
Feeding stimulates TCF7L2 expression.
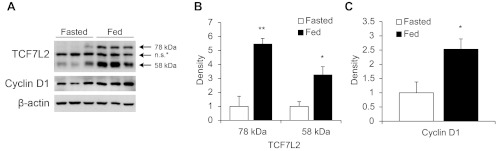
To determine whether hepatic TCF7L2 expression levels can be modulated by nutritional availability, we compared TCF7L2 expression levels in the livers of fasted or refed C57BL/6 mice. As shown in Fig. 2, A and B, refed animals showed substantially increased hepatic TCF7L2 protein levels (both 78- and 58-kDa isoforms) compared with fasted mice. Increased TCF7L2 expression was associated with elevated expression of cyclin D1, a known downstream target of the Wnt signaling pathway (Fig. 2, A and C).
Fig. 2.
Feeding stimulates TCF7L2 expression. A: effect of fasting and refeeding (4 h) on hepatic TCF7L2 expression in C57BL/6 mice by Western blotting. Cyclin D1, a known downstream target of the Wnt signaling pathway. Representative blot (n = 6/group). B and C: densitometric analysis of A. Values are presented as means ± SE. *P < 0.05 and **P < 0.01 vs. fasted mouse group.
Insulin stimulates TCF7L2 expression and β-cat Ser675 phosphorylation.
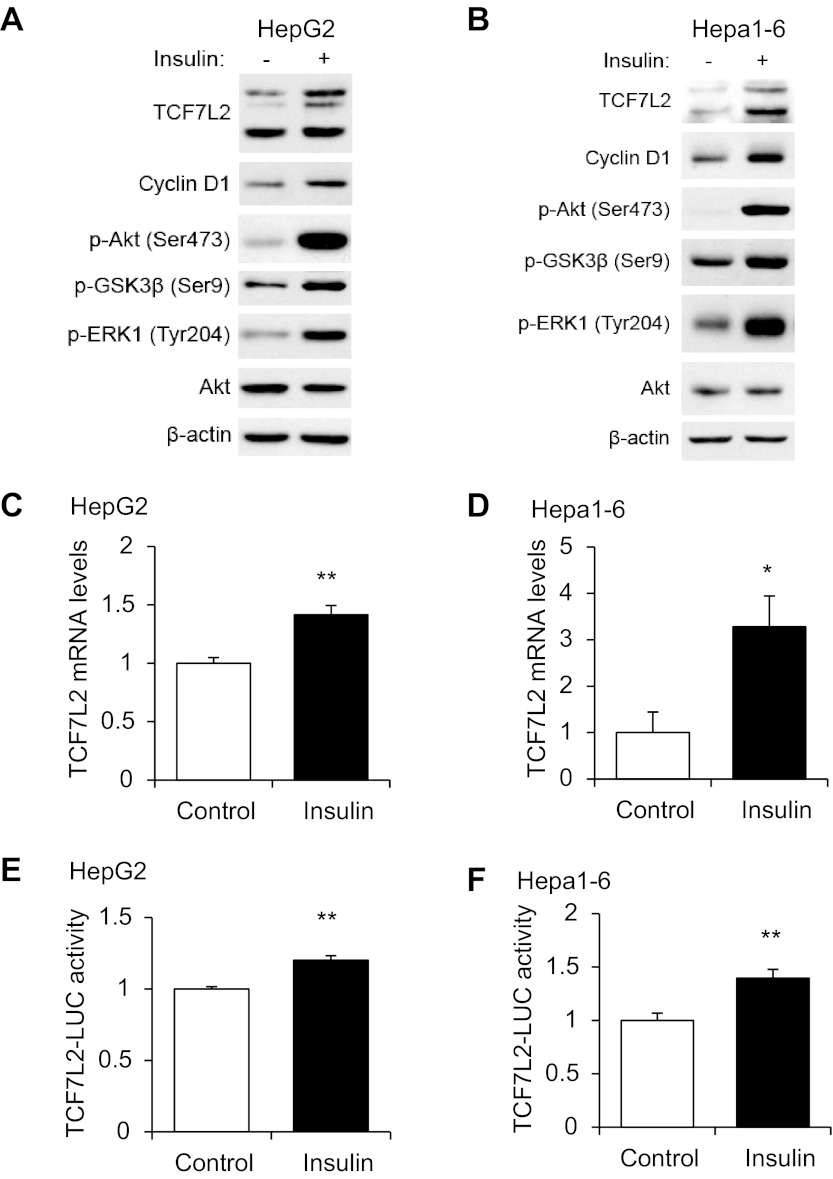
Because feeding increases plasma insulin levels and insulin is the principle hormone involved in repressing gluconeogenesis in response to feeding, we assessed directly the effect of 4-h insulin treatment on TCF7L2 expression in human HepG2 and mouse Hepa1–6 cell lines in vitro. As anticipated, insulin treatment stimulated the phosphorylation of Akt (Ser473), GSK-3β (Ser9), and ERK1 (Tyr204) (Fig. 3, A and B). This treatment also increased TCF7L2 protein levels in both cell lines, which was associated with elevated cyclin D1 levels (Fig. 3, A and B). In addition, 4-h insulin treatment was shown to increase TCF7L2 mRNA levels in these two cell lines detected by real-time RT-PCR (Fig. 3, C and D). Furthermore, when a TCF7L2-LUC fusion gene plasmid (38) was transfected into either HepG2 or Hepa1–6 cells, 4-h insulin treatment moderately but significantly increased LUC reporter activity (Fig. 3, E and F).
Fig. 3.
Insulin stimulates TCF7L2 expression in 2 hepatic cell lines. Effect of 100 nM insulin (4 h) on TCF7L2 expression assessed by Western blotting (A and B) and quantitative RT-PCR (C and D) in HepG2 and Hepa1–6 cell lines. E and F: effect of 100 nM insulin (4 h) on TCF7L2 (3.1kb)-luciferase (LUC) reporter expression in HepG2 (E) and Hepa1–6 (F) cell lines. Blots shown are representative of at least 3 independent experiments. In C–F, values in each independent experiment were normalized to the control group (defined as 1-fold) and then presented as means ± SE (n ≥ 3). *P < 0.05 and **P < 0.01 vs. control.
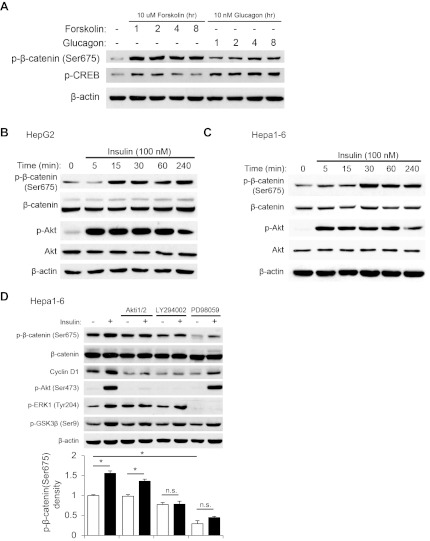
β-Cat is the essential partner of TCF members in stimulating Wnt downstream target gene expression. β-Cat Ser675 phosphorylation in response to PKA activation was demonstrated in other cell lineages to be positively correlated with the transcriptional activity of cat/TCF (12). We have also shown previously the activation of β-cat Ser675 phosphorylation by insulin in gut cells (37, 42). In the current study, we have first of all determined that in the mouse Hepa1–6 cell line both cAMP/PKA activators forskolin and glucagon were able to stimulate CREB phosphorylation, which was associated with β-cat Ser675 phosphorylation (Fig. 4A). As anticipated, these two agents also increased de novo glucose synthesis in this cell line (data not shown). We then assessed the effect of insulin on β-cat Ser675 phosphorylation in the two hepatic cell lines. As shown in Fig. 4, B and C, insulin was able to stimulate β-cat Ser675 phosphorylation. In the Hepa1–6 cell line, this stimulation could be attenuated by inhibition of either phosphoinositide 3-kinase (PI3K) or mitogen-activated extracellular signal-regulated kinase (MEK) but not by the inhibition of protein kinase B (PKB/Akt) (Fig. 4D). MEK inhibition also markedly repressed basal levels of Ser675 β-cat but did not affect insulin-stimulated Akt Ser473 phosphorylation. Inhibition of PI3K or Akt led to the loss of Akt phosphorylation at Ser473 as expected, although phosphorylation of GSK-3β at Ser9 could still be observed likely due to the involvement of multiple kinases (36). Insulin-stimulated expression of cyclin D1 was attenuated by either Akt or PI3K inhibition (Fig. 4D). This is likely due to the involvement of Akt in regulating protein translation via mTOR activation (37). These observations collectively suggest that in hepatocytes insulin is able to cross-talk with the Wnt signaling pathway by both increasing TCF7L2 expression levels and stimulating β-cat Ser675 phosphorylation. The activation of β-cat Ser675 phosphorylation by insulin appears to be an Akt-independent phenomenon, as we have observed previously in gut endocrine L cells (42).
Fig. 4.
Insulin stimulates β-catenin (β-cat) Ser675 phosphorylation in 2 hepatic cell lines. A: effect of forskolin (10 μM) or glucagon (10 nM) treatment on β-cat Ser675 phosphorylation in Hepa1–6. B and C: effect of 100 nM insulin for the indicated time points on β-cat Ser675 phosphorylation in HepG2 (B) and Hepa1–6 (C) cell lines. D: effect of protein kinase B (10 μM Akti1/2), phosphoinositide 3-kinase (50 μM LY-294002), and mitogen-activated extracellular signal-regulated kinase (50 μM PD-98059) inhibition on insulin-stimulated (1 h) β-cat Ser675 phosphorylation (top) with accompanying densitometric analysis (bottom). Treatment with inhibitors was performed 45 min prior to treatment with insulin. Blots shown are representative of at least 3 independent experiments. D: values in each independent experiment were normalized to the control untreated group (defined as 1-fold) then presented as means ± SE (n ≥ 3). *P < 0.05 between indicated groups.
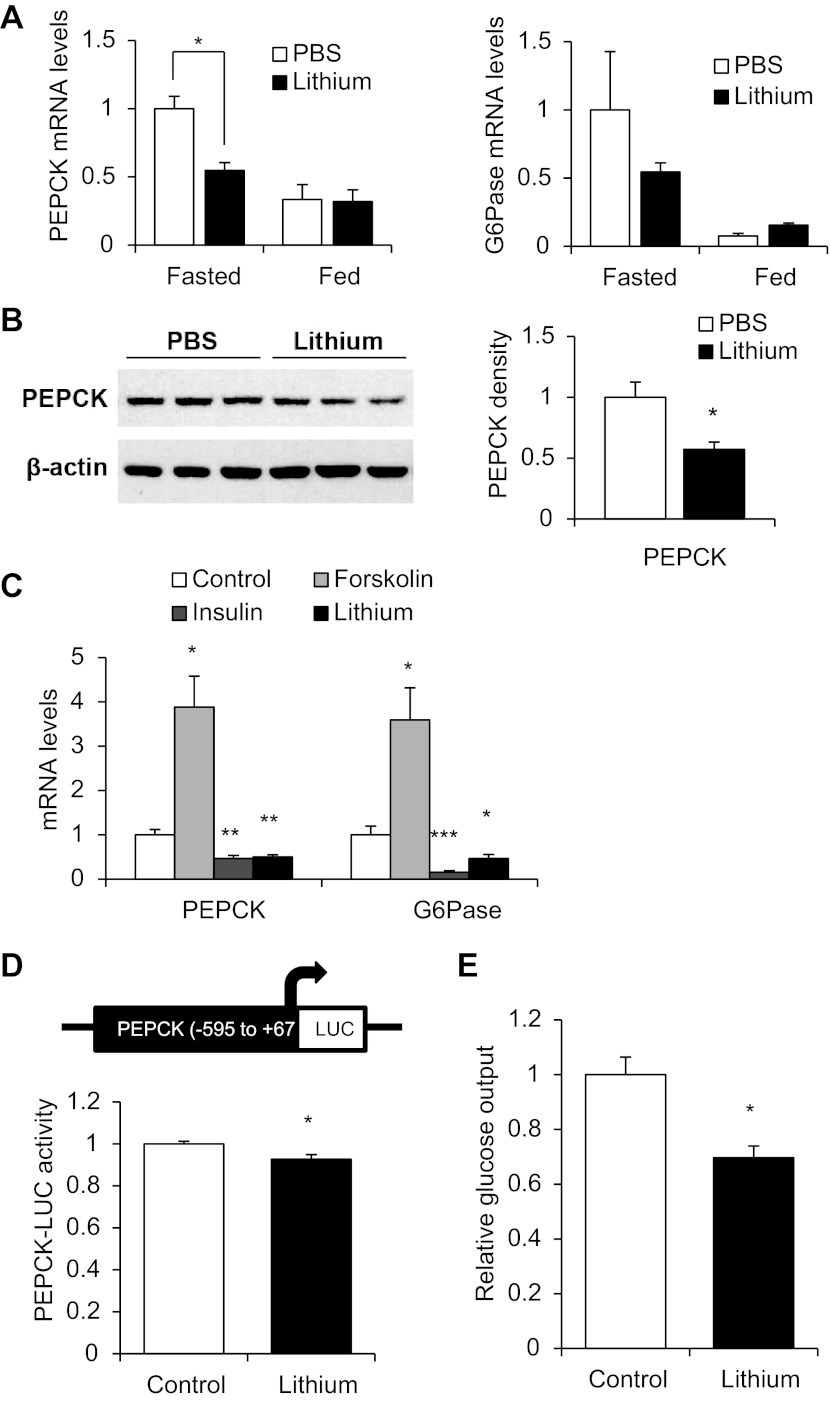
Wnt activation by lithium reduces gluconeogenesis.
We then assessed the contribution of Wnt activation on gluconeogenesis in vivo. As an inhibitor of GSK-3, lithium has been widely utilized to mimic the activation of Wnt via increasing nuclear levels of β-cat (35, 41). We found that it has no appreciable effect on TCF7L2 expression or β-cat Ser675 phosphorylation in hepatocytes in vitro (data not shown). Male C57BL/6 mice at the age of 12 wk were injected ip with lithium chloride (3 mmol/kg body wt). Four hours after injection, mice were euthanized, followed by extraction of liver tissue for RNA as well as protein isolation. In fasted animals, this short-term Wnt activation by lithium injection reduced hepatic expression of PEPCK mRNA significantly (Fig. 5A, left) and generated a trend of reducing the level of G-6-Pase mRNA (Fig. 5A, right), although the effect did not reach statistical significance. As expected, feeding reduced hepatic PEPCK and G-6-Pase mRNA levels (17), although lithium injection in fed animals generated no additive reduction (Fig. 5A). As shown in Fig. 5B, lithium injection also reduced hepatic PEPCK protein level in animals during fasting. Feeding reduced PEPCK protein level, and lithium injection generated no further reduction (data not shown).
Fig. 5.
Wnt activation represses hepatic gluconeogenesis. A: effect of Wnt activation by intraperitoneal (ip) lithium injection (3 mmol/kg body wt) on hepatic phosphoenolpyruvate (PEPCK; left) and glucose-6-phosphatase (G-6-Pase; right) mRNA levels assessed 4 h following injection by real-time RT-PCR (n = 3/each of 4 groups of animals). Levels in the fasted PBS-treated mouse were defined as 1-fold. B: effect of ip lithium injection on hepatic PEPCK protein levels in fasted animals assessed 4 h following injection by Western blotting (left) with accompanying densitometric analysis (right). C: effect of 4-h lithium treatment (10 mM) on PEPCK and G-6-Pase mRNA levels in HepG2 cells. D: effect of 4-h lithium treatment (10 mM) on the expression of a rat PEPCK-LUC fusion gene construct in HepG2 cells. E: effect of 4-h lithium treatment on glucose output in MPH. In C–E, values in each independent experiment were normalized to the control group (defined as 1-fold) and then presented as means ± SE (n ≥ 3). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control group or fasted PBS control mouse.
We then assessed the effect of lithium in the HepG2 cell line in vitro. Forskolin (which mimics the effect of glucagon on cAMP elevation) treatment significantly stimulated PEPCK and G-6-Pase mRNA levels, whereas insulin or lithium treatment significantly reduced the expression of these two gluconeogenic genes (Fig. 5C). To assess whether the repressive effect of lithium on PEPCK expression occurs at the transcriptional level, we obtained the PEPCK promoter sequence (from −595 to +67 bp) by PCR and constructed a LUC reporter. As anticipated, this fusion gene construct can be positively regulated by PKA activation (data not shown). When this construct was transfected into the HepG2 cell line, lithium treatment was shown to modestly but reproducibly reduce its expression (Fig. 5D). Finally, lithium treatment was found to significantly reduce relative glucose output in mouse primary hepatocytes (Fig. 5E). Although the in vitro glucose production assay utilized here alone cannot eliminate the potential involvement of glycogenolysis, our observation that lithium represses gluconeogenic gene expression (Fig. 5, A–C) indicates that the Wnt pathway activator lithium represses gluconeogenesis.
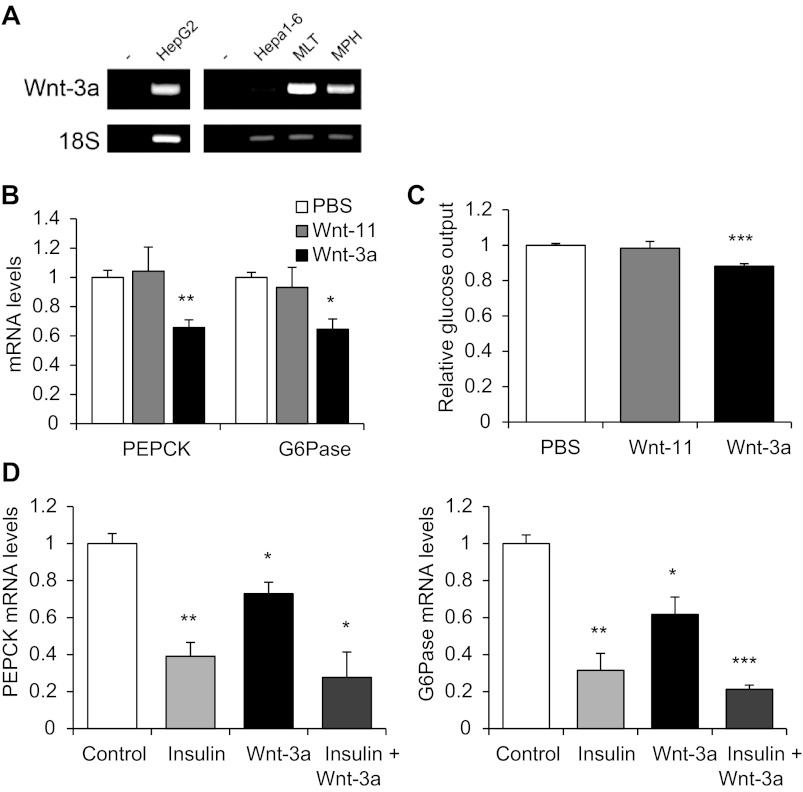
Wnt-3a reduces gluconeogenesis.
To verify the repressive effect of Wnt activation on gluconeogenesis in vitro, we directly utilized the canonical Wnt ligand Wnt-3a along with the noncanonical Wnt ligand Wnt-11 as a negative control. Wnt-3a is known to be differentially expressed at critical stages of human liver development in vivo (11) and is commonly used as a canonical Wnt ligand. In addition, we detected the expression of Wnt-3a in human and mouse hepatocytes via RT-PCR (Fig. 6A), consistent with a previous study demonstrating its expression in the mouse liver (20). As shown in Fig. 6B, treatment of HepG2 cells with Wnt-3a, but not with Wnt-11, reduced the levels of PEPCK and G-6-Pase mRNA significantly. Wnt-3a, but not Wnt-11, was also found to reduce glucose output in mouse primary hepatocytes (Fig. 6C). Wnt-3a, however, could not further reduce gluconeogenic gene expression upon insulin treatment (Fig. 6D). These observations suggest that Wnt signaling is a negative regulator of hepatic gluconeogenic gene expression and glucose production.
Fig. 6.
Wnt ligand Wnt-3a represses hepatic gluconeogenesis. A: detection of Wnt-3a in the human HepG2 and the mouse Hepa1–6 cell lines, MLT, and MPH by RT-PCR. B and C: effect of 2.5 nM Wnt-11 or Wnt-3a treatment (4 h) on PEPCK and G-6-Pase mRNA expression in HepG2 (B) and glucose production in MPH (C). D: effect of 4-h treatment of 100 nM insulin, 2.5 nM Wnt-3a, or both insulin and Wnt-3a on PEPCK and G-6-Pase mRNA levels in HepG2 compared with a vehicle control. Values in each independent experiment were normalized to the control group (defined as 1-fold) and then presented as means ± SE relative to the control group (n ≥ 3). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control.
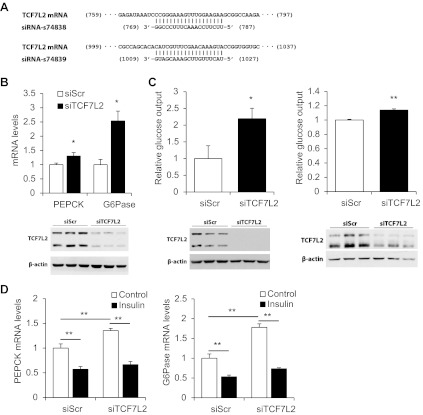
TCF7L2 negatively regulates gluconeogenesis.
Norton et al. (26) have shown that knockdown of TCF7L2 in the rat hepatoma cell line H4IIE increased glucose production significantly in the presence or absence of insulin or metformin treatment. Here, we have established the methodology to knockdown TCF7L2 in both human and mouse hepatocytes using siRNA and directly assessed the contribution of TCF7L2 in regulating gluconeogenic gene expression and glucose output. Figure 7A shows the RNA sequence of two TCF7L2 siRNA oligonucleotides used in this study. These two siRNA sequences would collectively recognize and knock down major isoforms of TCF7L2 in both human and mouse. Knockdown of TCF7L2 resulted in a significant elevation of PEPCK and G-6-Pase mRNA levels (Fig. 7B), which was associated with increased glucose output in the mouse Hepa1–6 cell line and mouse primary hepatocytes (Fig. 7C). However, the repressive effect of insulin on gluconeogenic gene expression was still present after the silencing of TCF7L2 (Fig. 7D), consistent with the report by Norton et al. (26) in the rat H4IIE hepatoma cell line.
Fig. 7.
Knockdown of TCF7L2 upregulates hepatic gluconeogenesis. A: RNA sequences of TCF7L2 small interfering RNA (siRNA) oligonucleotides utilized in this study. The human TCF7L2 sequence is recognized by siRNA-s74839, whereas the mouse sequence is recognized by both siRNA-s74838 and s74839. Knockdown was confirmed by Western blotting in B and C. B: effect of TCF7L2 silencing on PEPCK and G-6-Pase mRNA levels in the HepG2 cell line. C: effect of TCF7L2 silencing on glucose output in the Hepa1–6 cell line (left) and mouse primary hepatocytes (right). D: effect of TCF7L2 silencing and 100 nM insulin treatment (4 h) on PEPCK and G-6-Pase levels in the HepG2 cell line. Values in each independent experiment were normalized to the control group (defined as 1-fold) and then presented as means ± SE (n ≥ 3). *P < 0.05 and **P < 0.01 between indicated groups.
DISCUSSION
TCF7L2 has been recognized as the most prominent type 2 diabetes risk gene due to the robust association between its selected SNPs and the risk of type 2 diabetes (9, 10, 31). However, the functional role of TCF7L2 in pancreatic islets remains unclear and controversial (3, 19, 22, 30, 33, 34). Because TCF7L2 is also expressed in organs other than pancreatic islets, including liver, brain, muscle, and fat tissues, which are likewise important in metabolic homeostasis, it is necessary to define the function of TCF7L2 and further study the metabolic function of Wnt signaling in each of those organs. Although it is well known that Wnt signaling is important for the development and zonation of the embryonic liver (8), little effort has been made to explore the potential hepatic role of TCF7L2 and Wnt signaling in mediating glucose homeostasis in adulthood until recently. Liu et al. (20) found that starvation induced the expression of mRNAs encoding different Wnt isoforms. They have also demonstrated with gain- and loss-of-function models the role of β-cat in hepatic glucose production. This group, however, did not directly assess the contribution of TCF7L2 in gluconeogenesis. Norton et al. (26) demonstrated that TCF7L2 silencing led to increased basal levels of hepatic glucose production in the rat hepatic cell line H4IIE, which is associated with the overexpression of gluconeogenic genes, including PEPCK and G-6-Pase. Utilizing chromatin immunoprecipitaiton (ChIP) combined with massively parallel DNA sequencing (ChIP-Seq), Norton et al. (26) detected more than 2,000 potential binding events across the genome. They suggested that TCF7L2 may affect fasting and postprandial hyperglycemia in carriers of type 2 diabetes risk SNPs of TCF7L2 (26). This group, however, did not assess whether TCF7L2 expression can be regulated by nutrient availability or a metabolic hormone. In our study, we present direct evidence for the first time that hepatic TCF7L2 expression can be stimulated by feeding in vivo and that insulin is able to stimulate cat/TCF activity by increasing TCF7L2 expression as well as facilitating β-cat Ser675 phosphorylation. We then presented novel evidence of the involvement of both TCF7L2 and the canonical Wnt ligand (Wnt-3a) in repressing gluconeogenesis. Both lithium and Wnt-3a were shown to repress whereas TCF7L2 knockdown was shown to increase gluconeogenic gene expression and glucose output. Taken together, our results led us to suggest that Wnt signaling and TCF7L2 negatively regulate gluconeogenesis and that TCF7L2 and β-cat are downstream effectors of insulin in hepatocytes.
It has been well established that, within the liver lobule, a functional gradient lies along the axis between the portal triad and the central vein (16). On the one hand, periportal hepatocytes, which receive incoming more aerobic blood, are specialized for oxidative functions such as gluconeogenesis and express higher amounts of gluconeogenic genes (16). On the other hand, in pericentral hepatocytes, which typically receive outgoing oxygen-depleted blood, processes that include gluconeogenesis are downregulated. The strong LacZ staining that we have observed in pericentral hepatocytes in the TOPGAL transgenic mice is consistent with a previous report from a different research angle (13). This observation further supports our suggestion that Wnt signaling is a negative regulator of gluconeogenesis. It will be interesting to assess whether the expression of TCF7L2 or Wnt ligands varies across the liver lobule gradient. Since pericentral hepatocytes are also specialized in lipogenesis (16), whether Wnt signaling functions as a positive regulator of lipogenesis deserves a systematic further examination.
In many other cell lineages, cat/TCF has been shown to serve as an effector for a variety of signaling molecules, including insulin, IGF-I, several peptide hormones that use cAMP as a second messenger, and the lipid metabolite LPA (2, 5, 14, 15, 37). Insulin and Wnt inhibit GSK-3 activity via different mechanisms to carry out distinct downstream signaling events such that stimulation by insulin may not result in free β-cat accumulation, whereas stimulation by Wnt does (6). We show in this study that in hepatocytes insulin is able to stimulate TCF7L2 transcription and activate β-cat Ser675 phosphorylation, which may account for the observed stimulation of Wnt reporter activity by insulin in other systems (5). In addition, feeding substantially increased hepatic TCF7L2 protein level. These observations, along with the fact that insulin is a strong inhibitor of hepatic gluconeogenesis in response to food intake, revealed a novel physiological application of the cross-talk between insulin and the Wnt signaling pathways. The metabolic hormone insulin, in response to food intake, may utilize the Wnt signaling effector cat/TCF7L2 as a mediator in repressing gluconeogenic gene expression and glucose production. Knockdown of TCF7L2, however, did not diminish the repressive effect of insulin on gluconeogenic gene expression, which was assessed by Norton et al. (26) in a rat cell line and by this study in a human cell line (Fig. 7). A potential explanation is that insulin can also repress gluconeogenesis via attenuating the effect of the transcription factor forkhead box O (FoxO), a known positive regulator of gluconeogenesis (23). This effect is presumably independent of the stimulatory effect of insulin on TCF7L2 expression.
The regulation of TCF7L2 expression by insulin has been recognized in several investigations across various tissues. Whereas it has been demonstrated that insulin upregulated TCF7L2 expression in intestinal cells, other studies have shown that insulin can downregulate the expression of TCF7L2 in adipocytes or pancreatic cells (1, 2, 38, 42). These seemingly controversial occurrences could manifest as a result of tissue-specific applications of the cross-talk between insulin and TCF7L2. In intestinal endocrine L cells, insulin stimulates the expression of the proglucagon gene (gcg) and production of the incretin hormone glucagon-like peptide-1 via TCF7L2 and β-cat (42). However, insulin is known to be a potent inducer of adipocyte differentiation, whereas Wnt signaling inhibits adipogenesis (1, 29, 32). Downregulation of TCF7L2 by insulin in adipocytes may mediate the stimulation of adipogenesis by insulin. Indeed, insulin has been shown to up- and downregulate the expression of specific genes in cell type-specific manners. For example, insulin is known to repress pancreatic gcg expression (27) in contrast to its stimulatory effect on gcg expression in the gut (42). Understanding the detailed molecular mechanisms underlying the cell type-specific regulation of TCF7L2 expression by insulin requires further investigation.
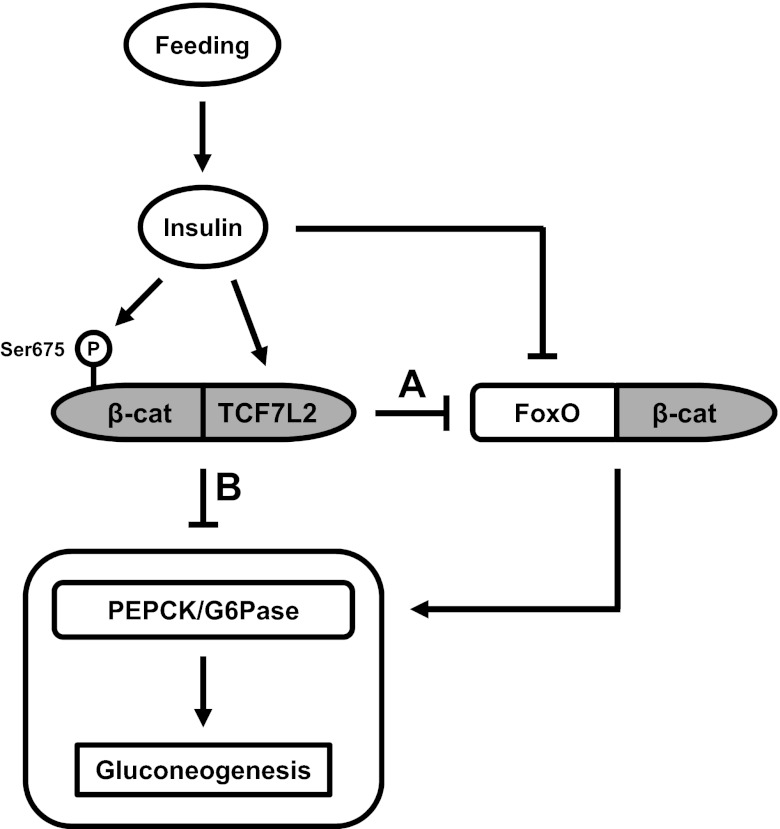
Liu et al. (20) found that β-cat depletion led to reduced hepatic glucose production. However, observations made by Norton et al. (26) and by the current study have indicated that TCF7L2 and Wnt are negative regulators of gluconeogenesis. An explanation for these seemingly contradictory findings may rely on the fact that β-cat also serves as a cofactor for FoxO transcription factors (7). Figure 8 is a simplified illustration that summarizes our current understanding of the role of signaling pathways in regulating hepatic gluconeogenesis. FoxO1 is a positive regulator of gluconeogenic gene expression, whereas its activity is positively and negatively regulated by glucagon and insulin, respectively. Glucagon-stimulated cAMP signaling leads to FoxO1-mediated increases in gluconeogenic gene expression, whereas insulin-stimulated PI3K/Akt signaling leads to the phosphorylation and nuclear exclusion of FoxO1, thereby attenuating gluconeogenic gene expression. The observations made in our current study suggest that elevated insulin in response to feeding can also upregulate Wnt activity via increasing TCF7L2 expression and β-cat Ser675 phosphorylation. Increased levels of TCF7L2 may compete with FoxO1 for β-cat and hence, attenuate gluconeogenesis. However, insulin also increases the levels of active β-cat (Ser675; Fig. 4, B–D). This led us to speculate that cat/TCF7L2 may possess an intrinsic repressive effect on gluconeogenic gene expression. This speculation is further supported by our observation that both Wnt-3a and lithium can repress gluconeogenesis independently of the insulin-signaling pathway (Figs. 5–7).
Fig. 8.
A schematic presentation of the role of Wnt signaling and TCF7L2 in hepatic gluconeogenesis. Feeding upregulates plasma insulin levels, which leads to β-cat Ser675 phosphorylation and TCF7L2 expression. A: increased levels of TCF7L2 may repress gluconeogenesis via competing with forkhead box O (FoxO) for β-cat. B: in addition, cat/TCF7L2, as the effector of the Wnt signaling pathway, may possess an intrinsic repressive effect on gluconeogenesis. P, Ser675 phosphorylation.
In conclusion, we have obtained evidence that insulin cross-talks with the Wnt signaling pathway via upregulating TCF7L2 expression and β-cat Ser675 phosphorylation in hepatocytes, that the extracellular canonical Wnt ligand Wnt-3a downregulates gluconeogenesis, and that TCF7L2 knockdown upregulates gluconeogenesis in primary hepatocytes. We speculate that insulin may partially utilize the Wnt signaling pathway effector cat/TCF7L2 in negatively regulating gluconeogenesis. How TCF7L2 and Wnt signaling downregulate hepatic gluconeogenesis via modulating the expression of a network of Wnt downstream target genes deserves further investigation.
GRANTS
This study was supported by operating grants from the Canadian Institutes of Health Research to T. Jin (MOP-89987 and MOP-97790). W. Ip is supported by graduate scholarships from the Canadian Institutes of Health Research and the Banting and Best Diabetes Centre/University Health Network. W. Shao is supported by a fellowship from the Banting and Best Diabetes Centre/University Health Network.
DISCLOSURES
The authors declare that there are no conflicts of interest, financial or otherwise, associated with this article.
AUTHOR CONTRIBUTIONS
W.I. and T.J. did the conception and design of the research; W.I., W.S., and Y.-T.A.C. performed the experiments; W.I., W.S., Y.-T.A.C., and T.J. analyzed the data; W.I. and T.J. interpreted the results of the experiments; W.I. prepared the figures; W.I. drafted the manuscript; W.I., W.S., Y.-T.A.C., and T.J. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Khosrow Adeli and his laboratory for providing guidance in isolating mouse primary hepatocytes.
REFERENCES
- 1. Ahlzen M, Johansson LE, Cervin C, Tornqvist H, Groop L, Ridderstrale M. Expression of the transcription factor 7-like 2 gene (TCF7L2) in human adipocytes is down regulated by insulin. Biochem Biophys Res Commun 370: 49–52, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Columbus J, Chiang Y, Shao W, Zhang N, Wang D, Gaisano HY, Wang Q, Irwin DM, Jin T. Insulin treatment and high-fat diet feeding reduces the expression of three Tcf genes in rodent pancreas. J Endocrinol 207: 77–86, 2010 [DOI] [PubMed] [Google Scholar]
- 3. da Silva Xavier G, Loder MK, McDonald A, Tarasov AI, Carzaniga R, Kronenberger K, Barg S, Rutter GA. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes 58: 894–905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557–4568, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoël MJ, Bertrand F, Cherqui G, Perret C, Capeau J. Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 20: 252–259, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem 275: 32475–32481, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 308: 1181–1184, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Gebhardt R, Hovhannisyan A. Organ patterning in the adult stage: the role of Wnt/beta-catenin signaling in liver zonation and beyond. Dev Dyn 239: 45–55, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Grant SF, Hakonarson H, Schwartz S. Can the genetics of type 1 and type 2 diabetes shed light on the genetics of latent autoimmune diabetes in adults? Endocr Rev 31: 183–193, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38: 320–323, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z, Pryde A, Filippi C, Currie IS, Forbes SJ, Ross JA, Newsome PN, Iredale JP. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci USA 105: 12301–12306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol 25: 9063–9072, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, Sylvester KG. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology 133: 1579–1591, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Jin T, George Fantus I, Sun J. Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal 20: 1697–1704, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Jin T, Liu L. The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol Endocrinol 22: 2383–2392, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Jungermann K, Katz N. Functional specialization of different hepatocyte populations. Physiol Rev 69: 708–764, 1989 [DOI] [PubMed] [Google Scholar]
- 17. Kahn CR, Lauris V, Koch S, Crettaz M, Granner DK. Acute and chronic regulation of phosphoenolpyruvate carboxykinase mRNA by insulin and glucose. Mol Endocrinol 3: 840–845, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19: 379–383, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Krutzfeldt J, Stoffel M. Regulation of wingless-type MMTV integration site family (WNT) signalling in pancreatic islets from wild-type and obese mice. Diabetologia 53: 123–127, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Liu H, Fergusson MM, Wu JJ, Rovira II, Liu J, Gavrilova O, Lu T, Bao J, Han D, Sack MN, Finkel T. Wnt signaling regulates hepatic metabolism. Sci Signal 4: ra6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Locke JM, Da Silva Xavier G, Rutter GA, Harries LW. An alternative polyadenylation signal in TCF7L2 generates isoforms that inhibit T cell factor/lymphoid-enhancer factor (TCF/LEF)-dependent target genes. Diabetologia 54: 3078–3082, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyssenko V, Lupi R, Marchetti P, Del Guerra S, Orho-Melander M, Almgren P, Sjögren M, Ling C, Eriksson KF, Lethagen AL, Mancarella R, Berglund G, Tuomi T, Nilsson P, Del Prato S, Groop L. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J Clin Invest 117: 2155–2163, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab 6: 208–216, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275: 1787–1790, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Ni Z, Anini Y, Fang X, Mills G, Brubaker PL, Jin T. Transcriptional activation of the proglucagon gene by lithium and beta-catenin in intestinal endocrine L cells. J Biol Chem 278: 1380–1387, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Norton L, Fourcaudot M, Abdul-Ghani MA, Winnier D, Mehta FF, Jenkinson CP, Defronzo RA. Chromatin occupancy of transcription factor 7-like 2 (TCF7L2) and its role in hepatic glucose metabolism. Diabetologia 54: 3132–3142, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Philippe J. Insulin regulation of the glucagon gene is mediated by an insulin-responsive DNA element. Proc Natl Acad Sci USA 88: 7224–7227, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pilgaard K, Jensen CB, Schou JH, Lyssenko V, Wegner L, Brons C, Vilsboll T, Hansen T, Madsbad S, Holst JJ, Volund A, Poulsen P, Groop L, Pedersen O, Vaag AA. The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia 52: 1298–1307, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science 289: 950–953, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Savic D, Ye H, Aneas I, Park SY, Bell GI, Nobrega MA. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res 21: 1417–1425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schäfer SA, Tschritter O, Machicao F, Thamer C, Stefan N, Gallwitz B, Holst JJ, Dekker JM, 't Hart LM, Nijpels G, van Haeften TW, Häring HU, Fritsche A. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 50: 2443–2450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schinner S. Wnt-signalling and the metabolic syndrome. Horm Metab Res 41: 159–163, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet 18: 2388–2399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shu L, Sauter NS, Schulthess FT, Matveyenko AV, Oberholzer J, Maedler K. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes 57: 645–653, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6: 1664–1668, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol 153, Suppl 1: S137–S153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun J, Khalid S, Rozakis-Adcock M, Fantus IG, Jin T. P-21-activated protein kinase-1 functions as a linker between insulin and Wnt signaling pathways in the intestine. Oncogene 28: 3132–3144, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Sun J, Wang D, Jin T. Insulin alters the expression of components of the Wnt signaling pathway including TCF-4 in the intestinal cells. Biochim Biophys Acta 1800: 344–351, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Wegner L, Hussain MS, Pilgaard K, Hansen T, Pedersen O, Vaag A, Poulsen P. Impact of TCF7L2 rs7903146 on insulin secretion and action in young and elderly Danish twins. J Clin Endocrinol Metab 93: 4013–4019, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Xi L, Xiao C, Bandsma RH, Naples M, Adeli K, Lewis GF. C-reactive protein impairs hepatic insulin sensitivity and insulin signaling in rats: role of mitogen-activated protein kinases. Hepatology 53: 127–135, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 280: 1457–1464, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Yi F, Sun J, Lim GE, Fantus IG, Brubaker PL, Jin T. Cross talk between the insulin and Wnt signaling pathways: evidence from intestinal endocrine L cells. Endocrinology 149: 2341–2351, 2008 [DOI] [PubMed] [Google Scholar]