Abstract
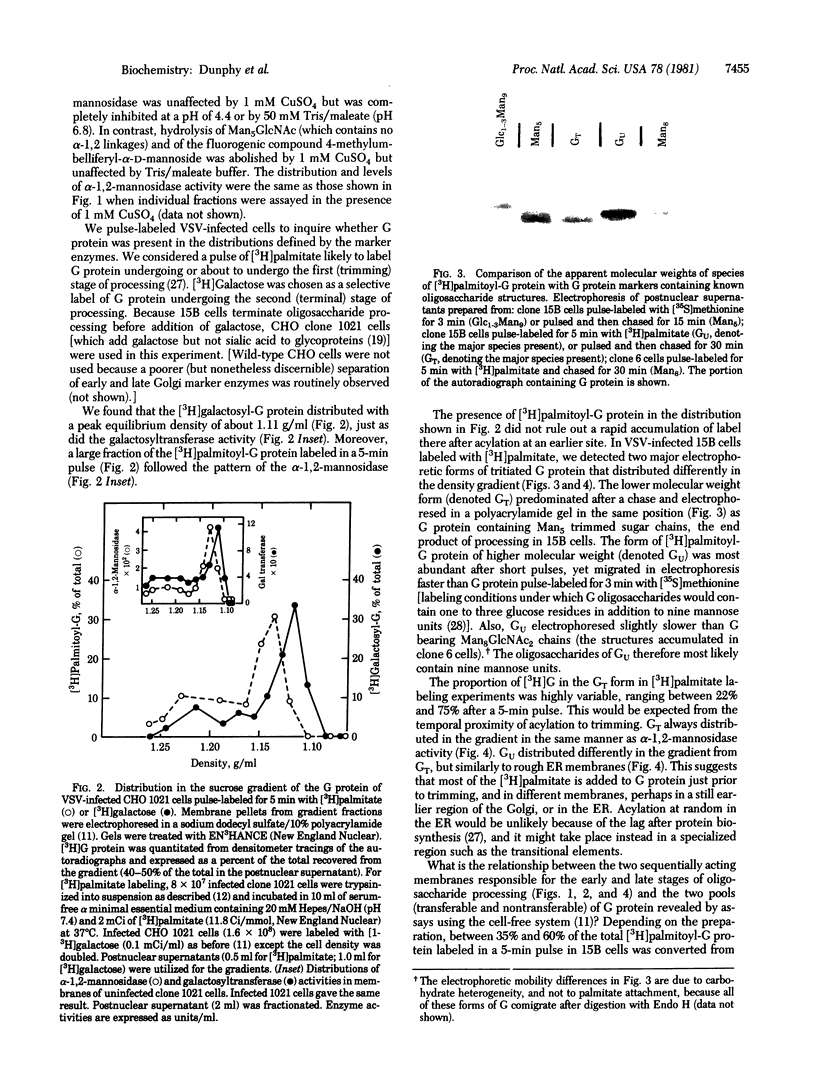
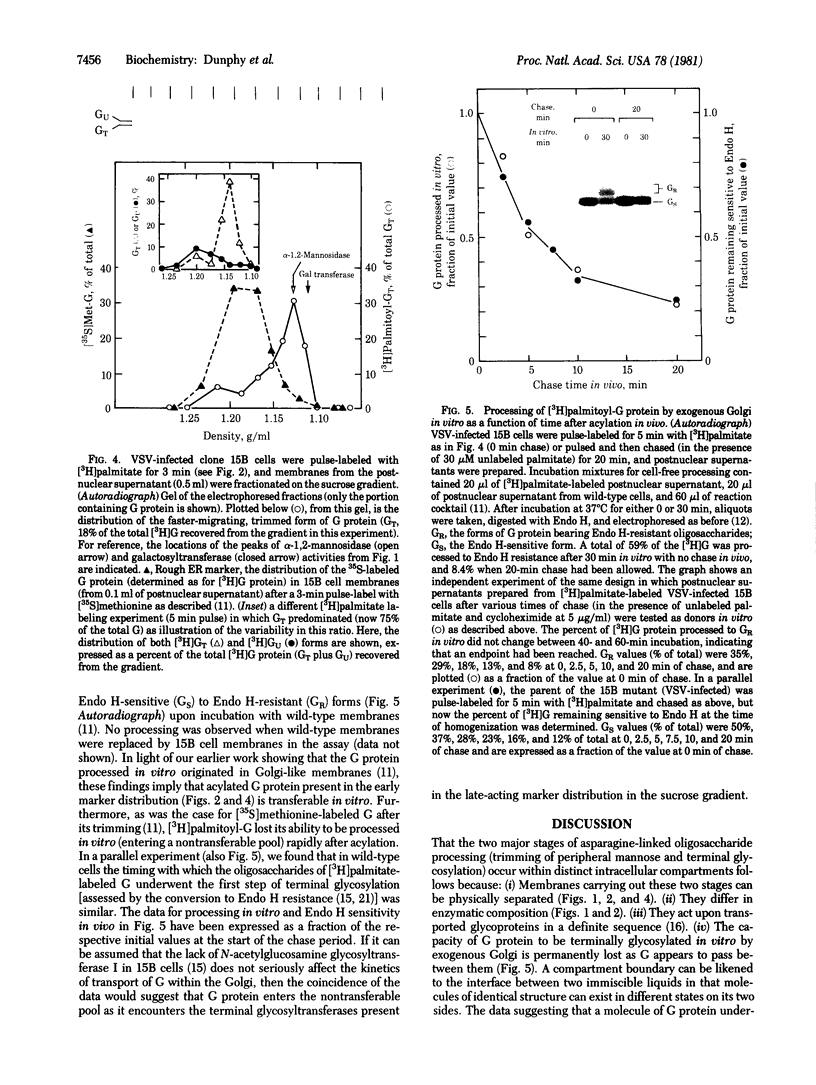
Enzymes that catalyze the two successive stages of Golgi-associated processing of asparagine-linked oligosaccharides distributed differently when membranes from Chinese hamster ovary cells were centrifuged in a sucrose density gradient. A mannosidase that removes only outer, alpha-1,2-linked mannose residues from the precursor oligosaccharides of the vesicular stomatitis viral G protein (to yield a "trimmed" oligosaccharide core) was separated from enzymes (galactosyl- and sialyltransferases) that act in the later, terminal stage of glycosylation. Freshly acylated G protein with newly trimmed oligosaccharides banded in the distribution of early-acting membranes, defined by the mannosidase, whereas G protein pulse-labeled with [3H]galactose distributed in the profile of the late-acting membranes. G protein present in the early-acting membranes in crude fractions could be terminally glycosylated by incubation with exogenous Golgi membranes in vitro; G protein lost its ability to be processed in vitro as it appeared to enter the late-acting membranes in vivo. These experiments reveal the existence of two distinct compartments through which intracellularly transported proteins such as G pass in sequence as Golgi-associated processes are carried out. It is likely that these compartments consist of cisternae on the cis and trans sides of the Golgi stack.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron J. J., Borts D., Cruz J. Passage of serum-destined proteins through the Golgi apparatus of rat liver. An examination of heavy and light Golgi fractions. J Cell Biol. 1978 Jan;76(1):87–97. doi: 10.1083/jcb.76.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Shaper J. H., Olsen K. W., Trayer I. P., Hill R. L. Cross-linking of the components of lactose synthetase with dimethylpimelimidate. J Biol Chem. 1975 Feb 25;250(4):1434–1444. [PubMed] [Google Scholar]
- Briles E. B., Li E., Kornfeld S. Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J Biol Chem. 1977 Feb 10;252(3):1107–1116. [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Bergeron J. J., Palade G. E. Cytochemistry of Golgi fractions prepared from rat liver. J Cell Biol. 1974 Jan;60(1):8–25. doi: 10.1083/jcb.60.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Recovery of surface membrane in anterior pituitary cells. Variations in traffic detected with anionic and cationic ferritin. J Cell Biol. 1978 Jun;77(3):R35–R42. doi: 10.1083/jcb.77.3.r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Rothman J. E. Transient activity of Golgi-like membranes as donors of vesicular stomatitis viral glycoprotein in vitro. J Cell Biol. 1981 Sep;90(3):697–704. doi: 10.1083/jcb.90.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Rothman J. E. Transport of vesicular stomatitis virus glycoprotein in a cell-free extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3870–3874. doi: 10.1073/pnas.77.7.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Gottlieb C., Kornfeld S. Isolation and characterization of two mouse L cell lines resistant to the toxic lectin ricin. J Biol Chem. 1976 Dec 25;251(24):7761–7768. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Liu T., Stetson B., Turco S. J., Hubbard S. C., Robbins P. W. Arrangement of glucose residues in the lipid-linked oligosaccharide precursor of asparaginyl oligosaccharides. J Biol Chem. 1979 Jun 10;254(11):4554–4559. [PubMed] [Google Scholar]
- Novikoff A. B. The endoplasmic reticulum: a cytochemist's view (a review). Proc Natl Acad Sci U S A. 1976 Aug;73(8):2781–2787. doi: 10.1073/pnas.73.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Montesano R., Meda P., Malaisse-Lagae F., Brown D., Perrelet A., Vassalli P. Heterogeneous distribution of filipin--cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):293–297. doi: 10.1073/pnas.78.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen K. Acid hydrolases as models of genetic control. Annu Rev Genet. 1979;13:417–466. doi: 10.1146/annurev.ge.13.120179.002221. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Fries E. Transport of newly synthesized vesicular stomatitis viral glycoprotein to purified Golgi membranes. J Cell Biol. 1981 Apr;89(1):162–168. doi: 10.1083/jcb.89.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. The golgi apparatus: two organelles in tandem. Science. 1981 Sep 11;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- Suzuki K. Enzymic diagnosis of sphingolipidoses. Methods Enzymol. 1978;50:456–488. doi: 10.1016/0076-6879(78)50049-9. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tartakoff A. M. The Golgi complex: crossroads for vesicular traffic. Int Rev Exp Pathol. 1980;22:227–251. [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]