Abstract
Objective
Anticipated pain with intrauterine device (IUD) insertion may be a barrier to widespread use. Our objective was to evaluate the efficacy of intracervical 2% lidocaine gel for pain relief with IUD insertion.
Study Design
We performed a double-blind, randomized controlled trial of women undergoing IUD insertion. Participants were randomly assigned to 2% lidocaine or placebo gel. Study gel (3ccs) wase placed 3 minutes prior to IUD insertion. Pain scores were measured at various time points using a 10-point visual analog scale.
Results
Of the 200 participants randomized, 199 completed the study. Pain scores among lidocaine and placebo arms were similar at tenaculum placement (lidocaine and placebo; median 4, range 0–10 p=0.15) as well as with insertion (lidocaine: median 5 range 1–10, placebo: median 6 range 0–10 p=0.16). These results did not differ by parity.
Conclusions
Topical or intracervical 2% lidocaine gel prior to IUD insertion does not decrease pain scores.
Keywords: IUD, lidocaine gel, pain
INTRODUCTION
Unintended pregnancy accounts for approximately 50% of pregnancies in the United States with 40% of those ending in induced abortion.1 Long-acting reversible contraception (LARC), including intrauterine devices (IUDs) and implants, is associated with higher contraceptive effectiveness and lower rates of discontinuation when compared to other reversible methods.2,3 IUDs remain an underutilized method of contraception in the United States despite their well established safety and efficacy.4–6 Evidence supports the use of IUDs as a first-line contraceptive option.2,7,8 The low rates of IUD use in the United States may be in part influenced by provider misconception about appropriate candidates.9 However, there is also concern that some women may not tolerate the pain or discomfort of the IUD insertion procedure. Qualitative studies have identified fear about the insertion procedure as a barrier to IUD use.10,11
Several steps of the IUD insertion have the potential to cause pain or discomfort to the patient: speculum insertion, tenaculum application, manipulation of the cervix, and passage of the uterine sound or IUD through the endocervical canal. Additionally, some studies suggest parity may play an important role in the difficulty of insertion.12,13 and the risk for severe pain with insertion.14,15 Pain management techniques during IUD insertion have included nonsteroidal anti-inflammatory medications (NSAIDs),15–17 paracervical administration of local anesthetic,18 and pre-procedural administration of misoprostol.19–21 Studies are limited and results have been conflicting. Massey et al performed one of the first randomized controlled trials evaluating the effect of naproxen on pain with insertion of the Dalkon Shield. All women received a paracervical block, and all but one woman had the IUD inserted at the time of menses.17 This study failed to find a reduction in pain in the treatment arm with a mean pain score of 2.46 (on a 5-point scale) compared to 2.54 in the control group. More recently Hubacher published a large trial of 2019 women randomized to placebo or 400mg of ibuprofen prior to copper T380A insertion. This study also failed to show a reduction in pain with preinsertion administration of ibuprofen.15 Overall, NSAIDs have been shown to have some benefit for post-procedure pain, but have not shown improvements in peri-insertional pain.15–17 Misoprostol has been investigated using different doses and routes of administration. Providers report increased ease of insertion, but have not demonstrated a reduction in pain scores.19–21 A recent Cochrane review of interventions for pain with IUD insertion22 evaluated randomized clinical trials and concluded that there was no benefit to either NSAID use or misoprostol on peri-insertional pain. The review also commented on a study published in 1996 in the British Journal of Family Planning that evaluated topical lidocaine as a possible intervention.23 Despite producing promising results, the study was noted to have methodologic flaws. Nonetheless this study has led to widespread use of topical lidocaine in the United Kingdom.24 Findings from a recent trial assessing lidocaine gel applied intracervically with cotton tip application did not find the intervention decreased pain scores.25
Lidocaine gel is routinely used in the distal urethra prior to Foley catheter placement, as well as in the nasal canal prior to nasogastric or nasotracheal tube placement. In each of these cases lidocaine gel has been shown to decrease pain scores associated with insertion.26–28 The endocervical canal is lined with columnar epithelium as is the nasal canal. The ecto-cervix and distal urethra share stratified squamous epithelium lending biologic plausibility to this intervention for both tenaculum placement to the ectocervix as well as IUD insertion. The purpose of our study was to evaluate whether intracervical lidocaine gel improved pain scores compared to placebo. We hypothesized that 2% lidocaine gel would reduce the insertional pain.
Materials and Methods
Approval was obtained by the Human Research Protection Office and the Institutional Review Board at Washington University in St Louis. We performed a single-site, double-blind, randomized controlled trial of women undergoing IUD insertion between August 1st and December 1st 2011 at Washington University in St Louis. Women aged 18–45 years presenting to the Contraceptive CHOICE Project were approached for participation. The Contraceptive CHOICE Project is a prospective cohort study of 9,256 women designed to promote the use of long-acting, reversible methods of contraception (LARC), remove financial barriers to contraception, and evaluate continuation and satisfaction for reversible methods.29 We provide each participant with the reversible contraceptive method of her choice at no cost to her.
Additional inclusion criteria for this study were: 1) ability to give written informed consent in English; 2) willingness to be randomized and complete study questionnaires; and 3) no contraindication to or history of allergic reaction to lidocaine. Participants in each of these groups were randomly assigned by random number generator to either 2% lidocaine or water-based lubricant. Randomization was stratified by parity and an equal number of nulliparous (defined as no pregnancy beyond 20 weeks gestation) and parous women were enrolled. Participants and the clinician placing the IUD were blinded to allocation.
After informed consent was provided and prior to IUD placement, we gave participants a 10-point visual analog scale (VAS) and asked them to indicate their current pain level and anticipated pain level with insertion. The clinician then performed a bimanual exam, placed the speculum, and cleansed the cervix with either Betadine or Hibiclens according to usual protocol. One half to 1 cc of study gel was applied to the ectocervix at the planned tenaculum site. Two to three ccs of study gel was then inserted via 20G angio-catheter into the endocervical canal. After a three minute waiting period, the intrauterine device was inserted in the standard fashion. Participants were asked to rate their pain immediately following tenaculum placement and immediately following device insertion using the same 10-point visual scale. All participants received ibuprofen approximately ten minutes prior to their procedure to minimize post-procedure cramping.
All participants scheduled a follow-up visit for a string check at the time of their insertion. Those who did not complete this follow-up visit were contacted by telephone to assess for any complications related to their insertion. We performed a weekly review of participant telephone calls to the CHOICE clinic to evaluate for potential complications (i.e. perforation, infection, expulsion) for six months following completion of enrollment.
Preliminary data from CHOICE of 250 women undergoing IUD insertion found a mean pain score of 4 (SD = 2.5) on a 10-point scale. We considered a 50% reduction in the mean score as a clinically important difference. Forty-three women per arm were required to reach 90% power with an alpha (type I) error of 0.05. Anticipating the possibility that parity may alter an effect, we planned to stratify by parity and enroll a total 100 nulliparous and 100 parous women, with 50 in each arm. This allowed for a 15% loss in case a woman changed her mind about IUD insertion or there was a failed IUD insertion after randomization.
Using a permuted varying block size randomization scheme with nQuery software (Statistical Solutions Software, Saugus, MA) a predetermined randomization scheme was established. Random assignments were concealed in labeled opaque envelopes until the time of enrollment. The two study gels were labeled “A” and “B”, and only 2 study coordinators who were responsible for filling the study gel syringes immediately preceding IUD insertion were aware of the contents of each syringe. Gels were indistinguishable in appearance by color and consistency. Both the participant and the clinician were blinded to the identity of the gel.
Statistical analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary NC, USA) and significance was set at p<.05. We performed descriptive analyses comparing the baseline characteristics of participants in each treatment arm. Continuous variables were summarized using means, medians, ranges, and standard deviations. Categorical variables were presented as frequencies. Normally distributed continuous variables were analyzed with Student’s t-test; otherwise, nonparametric testing was used. Chi-square and Fisher’s exact tests were used to analyze categorical variables. To account for varying levels of baseline (pre-enrollment pain), tenaculum and insertional pain scores were standardized by subtracting baseline pain levels from the reported procedure pain level. Pain scores were not normally distributed and are therefore presented as median scores. These scores were analyzed both in the entire cohort as well as in parity subgroups using the Wilcoxon rank sum test.
RESULTS
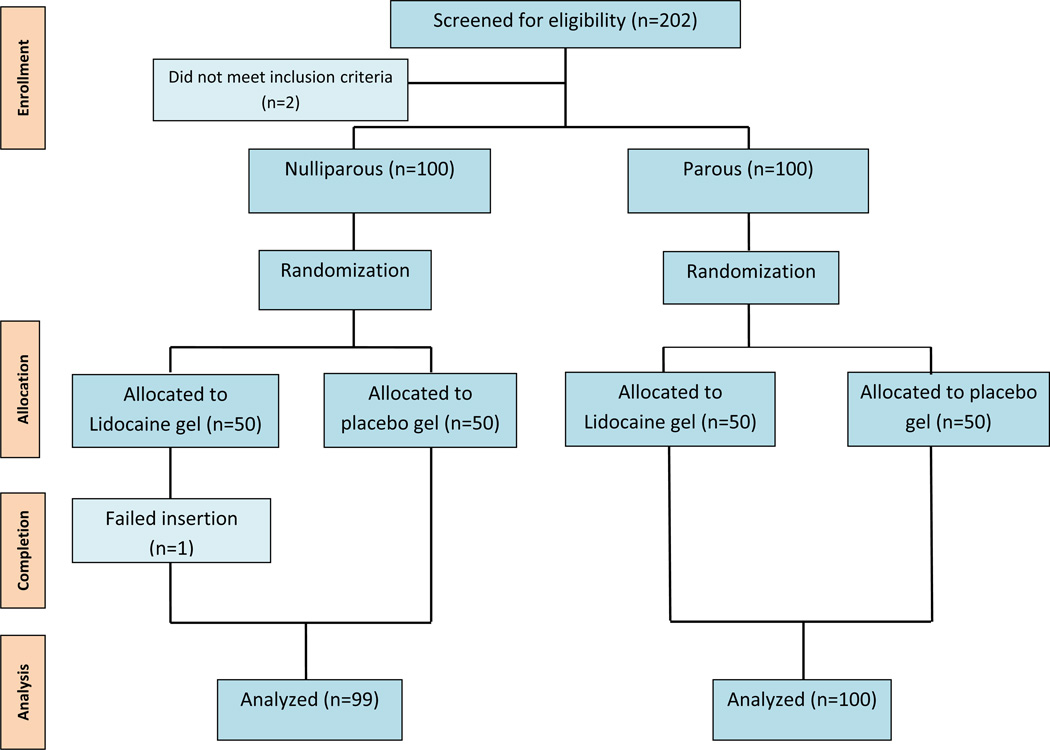
We enrolled and randomized a total of 200 women from Aug 2011 through December 2011. Of the 202 women approached for participation, two did not meet inclusion criteria. There were no women approached that declined participation. There was one failed insertion in the nulliparous group leaving 199 women for analysis (Figure 1). There were no differences in demographic characteristics between the two groups (Table1). Seventy-six percent of participants in each arm chose the levonorgestrel intrauterine system; a rate similar to the overall rates found in the Contraceptive CHOICE Project. The majority of IUDs were inserted by a single physician or single experienced nurse practitioner. A small number (7.5%) were inserted by resident physicians. The proportion of insertions by each of these entities was similar in both intervention arms.
Figure 1.
Table 1.
Baseline Characteristics
| Characteristic | Placebo (n=99) | Lidocaine (n=100) | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years) | |||||
| 18–20 | 10 | 10.1 | 19 | 19.0 | |
| 21–25 | 36 | 36.4 | 30 | 30.0 | |
| 26–45 | 53 | 53.5 | 51 | 51.0 | |
| Race | |||||
| Black | 38 | 38.4 | 37 | 37.0 | |
| White | 51 | 51.5 | 54 | 54.0 | |
| Other | 10 | 10.1 | 9 | 9.0 | |
| BMI | |||||
| ≤18.5 | 2 | 2.0 | 3 | 3.0 | |
| >18.5–25 | 41 | 41.4 | 45 | 45.0 | |
| >25–30 | 28 | 28.3 | 28 | 28.0 | |
| ≥30 | 28 | 28.3 | 24 | 24.0 | |
| Parity | |||||
| 0 | 50 | 50.5 | 50 | 50.0 | |
| 1–2 | 38 | 38.4 | 42 | 42.0 | |
| ≥ 3 | 11 | 11.1 | 8 | 8.0 | |
| Education | |||||
| ≤ high-school | 18 | 18.2 | 15 | 15.0 | |
| some college | 40 | 40.4 | 52 | 52.0 | |
| college/grad | 41 | 41.4 | 33 | 33.0 | |
| Income (monthly) | |||||
| none | 15 | 15.2 | 19 | 19.0 | |
| $1–800 | 21 | 21.2 | 30 | 30.0 | |
| $801–1600 | 29 | 29.3 | 27 | 27.0 | |
| >$1600 | 33 | 33.3 | 24 | 24.0 | |
| History of abortion | |||||
| No | 73 | 73.7 | 75 | 75.0 | |
| Yes | 26 | 26.3 | 25 | 25.0 | |
| History of STI† | |||||
| No | 67 | 67.7 | 59 | 59.0 | |
| Yes | 32 | 32.3 | 41 | 41.0 | |
| IUD Type | |||||
| LNG | 75 | 75.8 | 77 | 77.0 | |
| Copper | 24 | 24.2 | 23 | 23.0 | |
| Clinician inserter | |||||
| MD | 44 | 44.4 | 46 | 46.0 | |
| NP | 45 | 45.5 | 48 | 48.0 | |
| Resident | 9 | 9.1 | 6 | 6.0 | |
| Baseline pain score | |||||
| 0 | 91 | 91.9 | 90 | 90.0 | |
| 1 | 4 | 4.0 | 5 | 5.0 | |
| 2 | 2 | 2.0 | 3 | 3.0 | |
| 3 | 1 | 1.0 | 1 | 1.0 | |
| 4 | 1 | 1.0 | 1 | 1.0 | |
Fischer's exact
Gonorrhea, chlamydia, trichomoniasis, HPV, HSV
Insertional pain scores reported between nulliparous and parous women were significantly different regardless of intervention (Table 2). The median pain score reported by nulliparous women in the placebo group was 7 (range: 2 – 10), and 5 (range: 0 – 9) in parous women (P<0.01). The median pain score reported by nulliparous women in the lidocaine group was 6 (range: 2 – 10) and 4 (range: 1 – 10) in the parous group (P=0.01).
Table 2.
Insertional pain: effect of parity (10-point scale)
| Study gel | Parity | Median | Range | p- Wilcoxon |
|---|---|---|---|---|
| Placebo | Nullip | 7 | 2–10 | < 0.01 |
| Parous | 5 | 0–9 | ||
| Lidocaine | Nullip | 6 | 2–10 | 0.01 |
| Parous | 4 | 1–10 |
We found no difference in the primary outcome of insertional pain between the placebo and lidocaine group (Table3). The median pain score in the placebo arm was 6 (range: 0 – 10) and in the lidocaine arm was 5 (range: 0 – 10). Similarly, we compared median pain scores with placement of tenaculum, and found no difference between the two groups, with a median score of 4 (range: 0 – 10) in both arms. Despite there being an overall difference in reported insertion pain scores between parity groups, we found no difference between intervention arms when stratified by parity (Table 4). Pain scores with insertions among nulliparous participants were the same for both the placebo and lidocaine arms (median=6; range: 2 – 10).
Table 3.
Pain scores by study gel (10-point scale) N=199
| Time point | Study gel | Median | Range | p- Wilcoxon |
|---|---|---|---|---|
| Tenaculum | placebo | 4 | 0–10 | 0.15 |
| lidocaine | 4 | 0–10 | ||
| Insertion | placebo | 6 | 0–10 | 0.16 |
| lidocaine | 5 | 0–10 |
Table 4.
Pain scores by Parity (10-point scale)
| True Nulliparous (n=49) | ||||
| Time point | Study gel | Median | Range | p- Wilcoxon |
| Tenaculum | placebo | 4 | 0–10 | 0.54 |
| lidocaine | 4 | 0–10 | ||
| Insertion | placebo | 6 | 2–10 | 0.18 |
| lidocaine | 6 | 2–10 | ||
| Parous (n=50) | ||||
| Time point | Study gel | Median | Range | p- Wilcoxon |
| Tenaculum | placebo | 4 | 0–10 | 0.23 |
| lidocaine | 3 | 0–9 | ||
| Insertion | placebo | 5 | 0–9 | 0.72 |
| lidocaine | 4 | 1–10 | ||
Baseline enrollment data assessed participants’ obstetric history including mode of delivery and in the case of cesarean section, reason for operative delivery. Using this data we created a third group for analysis, defined as women who were functionally nulliparous. This group contained all true nulliparous participants as well as any participants who had a cesarean delivery performed without labor (N = 4). Again, we found no difference in the insertional pain scores with a median score of 6 (range: 2 – 10) in the placebo arm, and 5 (range: 0 – 10) in the lidocaine arm (p=0.20).
Lastly, we evaluated pain score by IUD type, and again found no differences. The median pain scores in the placebo arm were 6 (range: 2–10) for the LNG-IUD and 5 (range 1–10) for the Cu-IUD (p=0.31). Similarly, pain scores in the lidocaine arm were not different with both groups reporting a median score of 6 (range 0–10; p=0.43).
Cases of expulsion, perforation, and infection were considered adverse events, and were collected for an additional 6 months following the completion of enrollment. There were 5 total expulsions, four in the placebo arm and one in the lidocaine arm. Two of the five expulsions were in nulliparous participants. The overall rate of expulsion was 2.5% which is similar to published data.30 There was 1 perforation in a parous, recently postpartum participant and 1 case of pelvic inflammatory disease (PID) in the study population for an overall rate of 0.5%.
COMMENT
We found no difference in the IUD insertion pain scores in women randomized to preplacement intracervical placebo or lidocaine gel. As suspected, pain scores between nulliparous and parous women significantly differed regardless of study allocation. Despite the finding that nulliparous women had significantly higher pain scores, we did not find that reported pain was different for nulliparous women randomized to intracervical lidocaine. Similarly, we found no difference in pain scores with tenaculum placement between the two arms.
Previous research using lidocaine gel varied widely in the time between anesthetic application and procedure initiation ranging from one to fifteen minutes. We chose three minutes for this study based on previous literature, the pharmacologic properties of the gel, and what we considered a reasonable amount of time to leave the speculum in place. It is possible that with more time, we may have observed an improvement with pain scores in women who received lidocaine. However, this needs to be balanced by how long a patient will tolerate having the speculum in place or having multiple speculum exams in the case that it is removed after anesthetic placement and then replaced for IUD insertion.
The median pain scores were slightly higher than we expected based on the previous data from women undergoing IUD insertion in the CHOICE Project. This can be attributed to several factors. Prior to the initiation of this study, women were asked to rate their pain on a scale of zero to ten, but were not given a standardized scale as a reference. Therefore, each participant based their pain score on an individual interpretation of the scale. Secondly, in an effort to ensure that participants were adequately informed of the study, they were exposed to significantly more discussion about pain with IUD insertion than they normally would have. This may have primed them to perceive a greater amount of pain with the insertion. Comparison of median pain scores found in this study to those of previous studies is difficult as there has not been consistent use of a single pain assessment tool. This study utilized a 10-point visual analog scale, but others have used a 100mm or 5-point scale. Lastly, we did not collect information on the duration of time elapsed since last delivery. As a result, we may have underestimated the number of women considered functionally nulliparous as those with delivery in the distant past may more closely resemble nulliparous women.
This study has several strengths. In addition to the study performed by Maguire et al,25 this study is one of the first randomized trials evaluating lidocaine gel as a potential intervention at the time of IUD insertion since the initial report suggesting efficacy by Oloto in 1996.23We also utilized an angiocatheter as an innovative delivery mechanism allowing for the gel to be placed the entire length of the endocervical canal. Lastly, waiting three minutes before IUD insertion, allowed for physiologically plausible intervention. The use of blinding allowed us to minimize bias both from the provider and participant. We designed our study with an adequate sample size to evaluate both parous and nulliparous women. Lastly, we were able to recruit and enroll our calculated sample size in a relatively short time frame achieving adequate power to detect a clinically significant difference.
Although attempts at providing pain relief with IUD insertion have been disappointing, the benefits of IUDs are profound. Despite our inability to provide pain relief at the time of insertion, IUDs have been shown to be popular among women with high satisfaction and continuation rates.2,3,31 Efforts to expand use of long acting reversible contraception, particularly IUDs, is critical and providers can emphasize these long term outcomes over the acute setting discomfort with placement. Although we had hoped that intracervical or topical lidocaine gel would improve pain scores among women undergoing IUD insertion, the negative findings of our study indicate the need for future research into strategies that decreased pain with IUD insertion. Minimizing discomfort at insertion will continue to reduce barriers and thus expand access to this highly effective method of contraception.
Acknowledgments
This research was supported in part by the National Center for Research Resources (NCRR) grant number UL1 RR024992, and National Institutes of Health (NIH) T32 research training grant number 5T32HD055172-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None of the above authors have a conflict of interest
REFERENCES
- 1.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006 Jun;38(2):90–96. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 2.Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011 May;117(5):1105–1113. doi: 10.1097/AOG.0b013e31821188ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suhonen S, Haukkamaa M, Jakobsson T, Rauramo I. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception. 2004 May;69(5):407–412. doi: 10.1016/j.contraception.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Kulier R, O'Brien PA, Helmerhorst FM, Usher-Patel M, D'Arcangues C. Copper containing, framed intra-uterine devices for contraception. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD005347.pub3. CD005347. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien PA, Kulier R, Helmerhorst FM, Usher-Patel M, d'Arcangues C. Copper-containing, framed intrauterine devices for contraception: a systematic review of randomized controlled trials. Contraception. 2008 May;77(5):318–327. doi: 10.1016/j.contraception.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Rivera R, Best K. Current opinion: consensus statement on intrauterine contraception. Contraception. 2002 Jun;65(6):385–388. doi: 10.1016/s0010-7824(02)00304-9. [DOI] [PubMed] [Google Scholar]
- 7.ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 59, January 2005. Intrauterine device. Obstet Gynecol. 2005 Jan;105(1):223–232. doi: 10.1097/00006250-200501000-00060. [DOI] [PubMed] [Google Scholar]
- 8.ACOG Committee Opinion no. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009 Dec;114(6):1434–1438. doi: 10.1097/AOG.0b013e3181c6f965. [DOI] [PubMed] [Google Scholar]
- 9.Madden T, Allsworth JE, Hladky KJ, Secura GM, Peipert JF. Intrauterine contraception in Saint Louis: a survey of obstetrician and gynecologists' knowledge and attitudes. Contraception. 2010 Feb;81(2):112–116. doi: 10.1016/j.contraception.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weston MR, Martins SL, Neustadt AB, Gilliam ML. Factors influencing uptake of intrauterine devices among postpartum adolescents: a qualitative study. American journal of obstetrics and gynecology. 2012 Jan;206(1):40, e41–e47. doi: 10.1016/j.ajog.2011.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asker C, Stokes-Lampard H, Beavan J, Wilson S. What is it about intrauterine devices that women find unacceptable? Factors that make women non-users: a qualitative study. The journal of family planning and reproductive health care / Faculty of Family Planning & Reproductive Health Care, Royal College of Obstetricians & Gynaecologists. 2006 Apr;32(2):89–94. doi: 10.1783/147118906776276170. [DOI] [PubMed] [Google Scholar]
- 12.Ward K, Jacobson JC, Turok DK, Murphy PA. A survey of provider experience with misoprostol to facilitate intrauterine device insertion in nulliparous women. Contraception. 2011 Dec;84(6):594–599. doi: 10.1016/j.contraception.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Society for Family Planning Clinical Guidelines. Use of the Mirena LNG-IUS and Paragard CuT380A intrauterine devices in nulliparous women. Contraception. 2010;81:367–371. doi: 10.1016/j.contraception.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Chi IC, Galich LF, Tauber PF, et al. Severe pain at interval IUD insertion: a case-control analysis of patient risk factors. Contraception. 1986 Nov;34(5):483–495. doi: 10.1016/0010-7824(86)90057-0. [DOI] [PubMed] [Google Scholar]
- 15.Hubacher D, Reyes V, Lillo S, Zepeda A, Chen PL, Croxatto H. Pain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofen. American journal of obstetrics and gynecology. 2006 Nov;195(5):1272–1277. doi: 10.1016/j.ajog.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Buttram V, Izu A, Henzl MR. Naproxen sodium in uterine pain following intrauterine contraceptive device insertion. American journal of obstetrics and gynecology. 1979 Jul 1;134(5):575–578. doi: 10.1016/0002-9378(79)90844-5. [DOI] [PubMed] [Google Scholar]
- 17.Massey SE, Varady JC, Henzl MR. Pain relief with naproxen following insertion of an intrauterine device. The Journal of reproductive medicine. 1974 Dec;13(6):226–231. [PubMed] [Google Scholar]
- 18.Thiery M. Pain relief at insertion and removal of an IUD: a simplified technique for paracervical block. Advances in contraception : the official journal of the Society for the Advancement of Contraception. 1985 Jun;1(2):167–170. doi: 10.1007/BF01849798. [DOI] [PubMed] [Google Scholar]
- 19.Dijkhuizen K, Dekkers OM, Holleboom CA, et al. Vaginal misoprostol prior to insertion of an intrauterine device: an RCT. Hum Reprod. 2011 Feb;26(2):323–329. doi: 10.1093/humrep/deq348. [DOI] [PubMed] [Google Scholar]
- 20.Li YT, Kuo TC, Kuan LC, Chu YC. Cervical softening with vaginal misoprostol before intrauterine device insertion. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2005 Apr;89(1):67–68. doi: 10.1016/j.ijgo.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Saav I, Aronsson A, Marions L, Stephansson O, Gemzell-Danielsson K. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Hum Reprod. 2007 Oct;22(10):2647–2652. doi: 10.1093/humrep/dem244. [DOI] [PubMed] [Google Scholar]
- 22.Allen RH, Bartz D, Grimes DA, Hubacher D, O'Brien P. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2009;(3) doi: 10.1002/14651858.CD007373.pub2. CD007373. [DOI] [PubMed] [Google Scholar]
- 23.Oloto EJBD, Murty JA. Pain and discomfort perception at IUD insertion: effect of short-duration, low volume, intracervical application of two percent lignocaine (Instillagel), a preliminary study. British Journal of Family Planning. 1996;22:177–180. [Google Scholar]
- 24.Tolcher R. Intrauterine techniques: contentious or consensus opinion? The journal of family planning and reproductive health care / Faculty of Family Planning & Reproductive Health Care, Royal College of Obstetricians & Gynaecologists. 2003 Jan;29(1):21–24. doi: 10.1783/147118903101196846. [DOI] [PubMed] [Google Scholar]
- 25.Maguire K, Davis A, Rosario Tejeda L, Westhoff C. Intracervical lidocaine gel for intrauterine device insertion: a randomized controlled trial. Contraception. 2012 Feb 9; doi: 10.1016/j.contraception.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Siderias J, Guadio F, Singer AJ. Comparison of topical anesthetics and lubricants prior to urethral catheterization in males: a randomized controlled trial. Acad Emerg Med. 2004 Jun;11(6):703–706. [PubMed] [Google Scholar]
- 27.Gross JB, Hartigan ML, Schaffer DW. A suitable substitute for 4% cocaine before blind nasotracheal intubation, 3% lidocaine-0.25% phenylephrine nasal spray. Anesthesia and analgesia. 1984 Oct;63(10):915–918. [PubMed] [Google Scholar]
- 28.Singer AJ, Konia N. Comparison of topical anesthetics and vasoconstrictors vs lubricants prior to nasogastric intubation: a randomized, controlled trial. Acad Emerg Med. 1999 Mar;6(3):184–190. doi: 10.1111/j.1553-2712.1999.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 29.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. American journal of obstetrics and gynecology. 2010 Aug;203(2):115, e111–e117. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatcher RATJ, Nelson A, Cates W, Stewart F, Kowal D. Contraceptive Technology. 19 ed. Ardent Media; 2007. [Google Scholar]
- 31.Trussell J. Contraceptive efficacy. In: Hatcher RATJ, Nelson AL, Cates W, Stewart FH, Kowal D, editors. Contraceptive Technology. 19th revised, ed. New York (NY): Ardent; 2007. p. 759. [Google Scholar]