Abstract
The capacity of any portion of the murine mammary gland to produce a complete functional mammary outgrowth upon transplantation to an epithelium-divested fat pad is unaffected by the age or reproductive history of the donor. Likewise, through serial transplantations, no loss of potency is detected when compared to similar transplantations of the youngest mammary tissue tested. This demonstrates that stem cell activity is maintained intact throughout the lifetime of the animal despite aging and the repeated expansion and depletion of the mammary epithelium through multiple rounds of pregnancy, lactation and involution. These facts support the contention that mammary stem cells reside in protected tissue locales (niches), where their reproductive potency remains essentially unchanged through life. Disruption of the tissue, to produce dispersed cells results in the desecration of the protection afforded by the “niche” and leads to a reduced capacity of dispersed epithelial cells (in terms of the number transplanted) to recapitulate complete functional mammary structures. Our studies demonstrate that during the reformation of mammary stem cell niches by dispersed epithelial cells in the context of the intact epithelium-free mammary stroma, non-mammary cells may be sequestered and reprogrammed to perform mammary epithelial cell functions including those ascribed to mammary stem/progenitor cells.
Keywords: niche, transplantation, stem cells, re-programming, mammary, development
Introduction
Schofield first postulated the idea of a specific location defined by specific cells and cellular signals controlling stem cell function (a stem cell niche) for hematopoietic stem cells.1 This idea was proposed to explain why stem cells from aged mice were as capable of long term engraftment of young recipients as hematopoietic stem cells from young donors when introduced to hosts deficient for stem cell growth factor receptor (ckit), although the older stem cells failed to function in long term reconstitution if isolated as cells functional in spleen colony focus-forming assays. His idea was that stem cells were essentially “immortal” so long as they resided in their niche but when removed from these sites then “immortality” was lost. He went so far as to define this stem cell “niche” as a defined anatomical site where stem cells were sustained and could reproduce; where differentiation of the stem cell was inhibited and most importantly a site where reversion to a stem cell phenotype might be induced in a more (slightly) mature cell type.
Schofield’s concept remained hypothetical and without direct evidence until the late 1990’s when work from Spradling and his colleagues validated each of Schofield’s predictions about a stem cell niche in the ovary of Drosophila melanogaster.2–4 A similar validation shortly followed from the study of the testes in Drosophila4 and later in C. elegans.5 Even the most far-reaching of Schofield’s concept namely that a more mature cell could be induced to acquire stem cell attributes by interaction with the niche was validated in these invertebrate models.
We were inspired to test this final point of Schofield’s niche concept in the regenerating mammary gland because we had successfully rescued mammary stem/progenitor cells from transgenic mammary tissues where regenerative capacity had been obliterated by the ectopic expression of the transgene by simply mixing the incompetent epithelial cells with normal wild type mammary epithelium prior to introduction into the epithelium-free mammary fat pad. In two models, (WAP-Notch4/Int3 X WAP-Cre/Rosa26R and WAP-TGFβ1 X WAP-Cre/Rosa26R), where lacZ-reporter marked cells were present in mammary epithelial populations incapable of growth and reconstitution of mammary epithelium in vivo, we found that interaction with normal wild type epithelial cells allowed them to produce progeny during mammary gland regeneration. These results suggested that the mammary epithelial cells themselves in combination with the mammary fat pad and its stroma were components essential to the mammary stem cell niche. Bolstered by these observations, we set out to determine if cells from non-mammary tissues could be altered from their initial cell fate lineage to adopt mammary epithelial characteristics upon interaction with mammary epithelial cells during reconstitution of mammary epithelium in regenerating mammary tissue in vivo.
A Hierarchy in Mammary Stem/Progenitor Cells
Evidence for lobule-limited and duct-limited pluripotent mammary epithelial cell activities has been established for both rats and mice6–8 by transplantation of limiting dilutions of dispersed mammary epithelial cells into hosts that were subsequently impregnated and/or treated with hormone combinations to produce alveologenesis (Fig. 1A–D). These limited structures contain both luminal epithelial and myoepithelial cells and luminal cells (Fig. 2A–D) positive for ERα (estrogen receptor alpha) and PR (progesterone receptor). Studies with retrovirally-marked clonal mammary populations demonstrated that both of these lineage-limited activities were present within the clonal populations though repeated transplant generations indicating their derivation from a single pluripotent antecedent.7,9 In addition, serial passage of the retrovirally-marked mammary epithelial clones in pregnant hosts showed that the capacity of individual outgrowths to produce lobulogenesis or ductal elongation were independently lost during the acquisition of growth senescence among individual transplants. The distinction between these two progenitor-mediated activities in regenerating mammary tissue is that the lobule-limited progenitor is unable to produce cap cells, which are required for the penetration of the mammary fat pad at the tips of the growing terminal end buds. On the other hand, duct-limited progenitors fail to produce progeny capable of sustaining alveolar development and growth during pregnancy. With the development of the WAP-Cre model used in combination with the Rosa26LacZ reporter mice (R26R), evidence for a LacZ-marked lobular-limited progenitor observable in parous mouse mammary epithelium surfaced.10 These LacZ-positive, parity-identified mammary cells (PI-MEC) were found to be pluripotent, self-renewing and capable of maintaining their lobule-limited progenitor activities following serial transplantation in epithelium-free mammary fat pads when the hosts were subsequently impregnated.11,12 During pregnancy in these hosts, the PI-MEC proliferated and gave rise to LacZ+ luminal progeny that were PR or ERα-positive and luminal progeny that were bereft of these steroid receptors. Further in the developing secretory acini, they contributed not only secretory progeny but also, LacZ-positive myoepithelial cells. Originally, it was proposed that the LacZ+ PI-MEC arose from de-differentiated secretory epithelial cells that had survived involution and remodeling of the mammary tissue however, further study indicated that these cells were present in the mammary tissue of nulliparous females and that they could be detected in explant cultures after treatment of the fragments with growth factors that do not induce lactogenic differentiation.13 These cells were shown to possess all the properties of PI-MEC including self-renewal and pluripotency. These observations support and confirm those reported earlier, which indicated the presence of lobule-limited progenitor activity in limiting dilution transplants of epithelium from nulliparous donors in pregnant transplant hosts. More recent evidence demonstrates that PI-MEC are marked by the expression of GFP in WAP-Cre/Chicken actin gene promoter (CAG)-flox-stop-flox-GFP parous females. In these studies GFP+ PI-MEC were fluorescent activated cell sorted (FACS) and found to be virtually 100% present in the CD49fhi population.14 This population was shown earlier to possess essentially all of the mammary repopulating activity. Subsequent transplantation of GFP+/CD49fhi positive PI-MEC and the GFP−/CD49flo epithelial cells into epithelium-divested mammary fat pads indicated that all the repopulating activity was associated with the GFP+ fraction. The foregoing data and the observations reported earlier suggest strongly that PI-MEC (i.e., lobule-limited progenitors) are indispensable to mammary gland ductal reconstitution in transplanted mammary fat pads.12,14
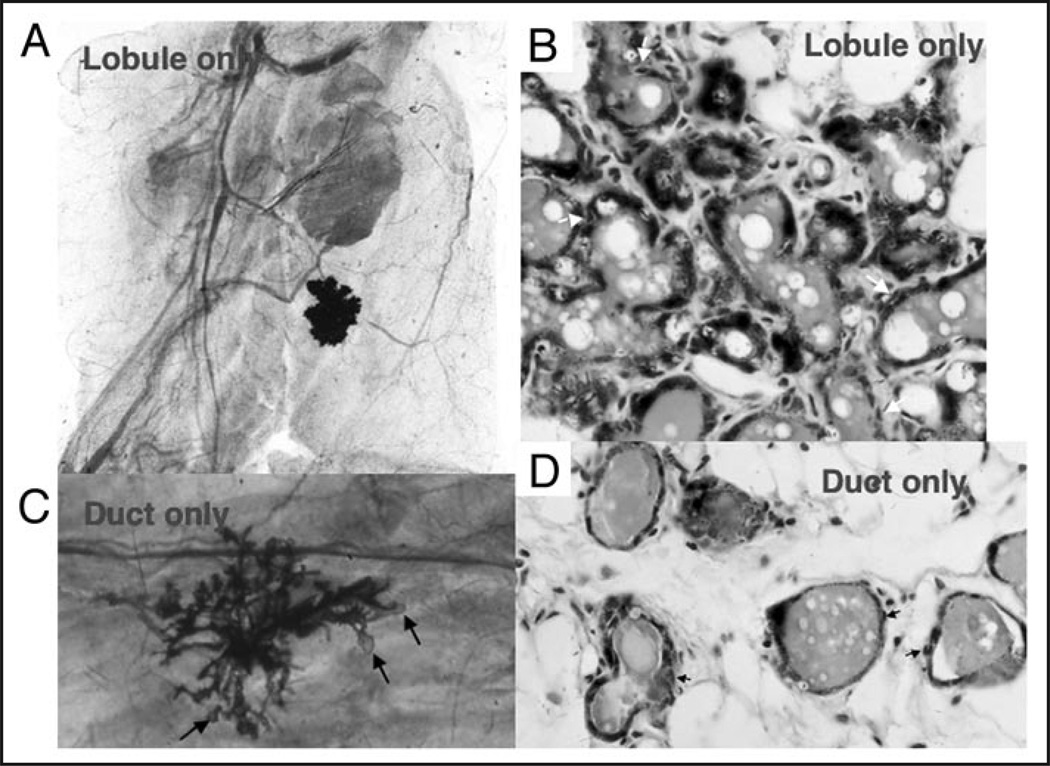
Figure 1.
(A) An X-gal-stained whole mounted mammary fat pad from a full term pregnant host containing a secretory lobule developed from a limiting dilution inoculum of WAP-LacZ expressing mammary cells. (B) Section through X-gal-stained secretory lobule showing the presence of both secretory luminal epithelial cells and myoepithelial cells (white arrows). (C) An X-gal-stained whole mounted mammary fat pad from a full term pregnant host containing a duct-limited outgrowth with no secretory lobule development with attendant terminal end buds (arrows). (D) Section through the duct-limited growth shown in (C) demonstrating the presence of both luminal and myoepithelial cells (small arrows).
Figure 2.
(A) Dark black nuclei (arrows) indicate presence of progesterone receptor (PR)-positive luminal cells in lobule-limited outgrowth. (B) Likewise in duct-limited outgrowth, black nuclei (arrows) represent luminal cells expressing PR. (C) Black nuclei (arrows) indicate black nuclei in luminal epithelium stained positively for estrogen receptor alpha (ERa) in a lobule-limited outgrowth. (D) Similarly, in duct-limited outgrowth, black nuclei (arrows) indicate the presence of ERa in the luminal epithelium.
Non-Mammary Stem/Progenitor Cells Obey Mammary Niche Signals
Armed with this model we set out to determine if cells from organs other than the mammary gland in the WAPCre/Rosa26R mice would be re-directed in vivo to a multipotent mammary epithelial cell fate when interacting with wild type mammary epithelial cells in the context of the regenerating gland in an epithelium-divested mammary fat pad. There were several advantages in this approach. First, a mammary epithelium-specific promoter tightly regulated in its expression by mammotropic hormones. Second, expression from the WAP promoter is the greatest during late pregnancy. Thus non-mammary cells and their progeny co-mingled with wild type epithelium would not express the recombined reporter gene (lacZ) unless pregnancy had ensued. Third, following the extensive remodeling of the lactating gland during post-lactation involution, non-mammary cell progeny would only be evident (lacZ+) if they had adopted the cellular attributes of PI-MEC (lobule-limited progenitors). This provided us with a quick and definite positive answer regarding the successful re-programming of non-mammary cells and their progeny during mammary regeneration and functional differentiation. Our results indicated that cells from adult male seminiferous tubules15 and neural stem cells (NSC) from both embryonic and adult brain were amenable to adopting mammary epithelial cell traits characteristic of PI-MEC,16 but only following interaction with wild type mammary epithelial cells in the context of the mammary fat pad. No mammary growth was observed when themselves introduced seminiferous tubule cells or NSC into the mammary fat pad, a result that supports an essential role of the mammary epithelial cells in the re-programming event.
Potentially Important Mammary Niche Signals
Although it is clear from transplantation studies that the rodent mammary gland contains stem/progenitor cells in all parts of its epithelial tree throughout life, there is little evidence to define or delineate a locale or niche required for the maintenance of this “stem cell” activity. However from various null models it is known that signals from progesterone receptor positive (PR) mammary epithelial cells are essential to secretory alveolar development17 and that signaling from estrogen receptor alpha-positive (ERα) epithelium is needed for ductal growth and expansion.18 The growth factor, Amphiregulin, has been identified as an important mediator of ERα+ signaling for duct elongation and development.19 Gata3 has been shown to be essential for luminal epithelial differentiation20 in the ducts and Beta1 integrin expression for full development of the secretory alveoli.21 Other regulatory factors present or generated in the mammary stroma have also been identified such as transforming growth factor beta (TGFβ), fibroblast growth factor (FGF), heregulin (HGF) and insulin growth factors (IGFs).22–24 Thus the mammary microenvironment that supports and maintains mammary epithelial homeostasis and the capacity for regeneration upon transplantation consists of local signals emanating from both the stroma and the existing epithelium and extra-organic host factors.25,26 Adhesiveness and cell-to-cell contact also plays an important role in mammary structure and function.27
Our experiments show that the mammary microenvironment described above is capable of re-directing somatic stem cells from non-mammary organs to produce progeny destined for mammary epithelial cell fates. This is not accomplished through cell-cell fusion but rather by adoption of the non-mammary cells into the reforming mammary epithelial niche(s) and reformatting their previous cellular repertoire to one shared by other mammary epithelial cells. In our present work we are only able to identify (by lacZ expression) those foreign cell progeny that expressed Cre from the WAP promoter and subsequently survived post lactation remodeling, principally cells with the properties of PI-MEC (Fig. 3A–D). We found lacZ+ progeny that were positive for PR and ERα amongst the luminal epithelium and at basal locations where they expressed the cellular markers associated with myoepithelial cells. Therefore like PI-MEC these reprogrammed cells were multipotent and were able to give rise to diverse mammary epithelial subtypes including cells that were capable of synthesizing milk proteins (Fig. 3B). It is likely that other mammary epithelial subtypes are formed from reprogrammed non-epithelial progeny since ductal elongation proceeds to nearly fill the fat pads prior to the onset of pregnancy and activation of the WAP promoter. We never found lacZ+ cells among the epithelium in chimeras prior to pregnancy and lactation.15 However, during a separate experiment where we excised the implanted glands from nulliparous hosts and placed them in minimal essential medium containing Insulin (1.0 µg/ml), hydrocortisone (1.0 µg/ml) and prolactin (0.1 µg/ml) for 72 hr. to induce lactogenic differentiation. The tissue were then reacted with Xgal and examined for lacZ+ cells within the mammary epithelial population. Sections cut from IHP-treated chimeras from nulliparous mice contained lacZ+ cells among the epithelial structures (Fig. 4) indicating that non-mammary cell progeny were present within the epithelial structures prior to pregnancy and lactation.13 To approach this issue more directly and to provide a means to isolate and characterize the reprogrammed cell, we are using mice, which constitutively express green fluorescent protein (GFP) in their tissues.
Figure 3.
All of these images were made from secondary transplants of neural stem cell (NSC) and mammary epithelial cells chimeras, where NSC progeny are identified by FITC-conjugated antibody against beta galactosidase (beta-gal green). (A) Smooth muscle actin (red) and beta-gal (green) are co-localized in a myoepithelial cell (arrow). (B) Anti-beta casein (red) and beta-gal (green) are shown together (arrow) in a secretory lobule demonstrating that NSC progeny have differentiated into secretory mammary epithelium. (C) ERa (pink) and beta-gal (green) are co-localized in luminal epithelial cell (arrow); nuclei are DAPI (blue). (D) PR (pink) and beta-gal (green) co-localize in luminal cell progeny (arrow) of NSC inn a duct.
Figure 4.
Activation of the beta-gal reporter gene in mammary explant from nulliparous host is accomplished after incubation with insulin, hydrocortisone and prolactin in vitro to induce secretory differentiation. Presence of the blue cells (arrows) after X-gal staining indicates the location of NSC progeny within the epithelium of the ducts prior to pregnancy. Basal (short arrow) and luminal (long arrows) epithelial cells are produced from beta-gal positive NSC progeny.
Cell-Cell Fusion: Is It in Play Here?
In experiments demonstrating the plasticity of “stem/progenitor” cells in transplanted hosts, the question of cell-cell fusion resulting in the apparent but false adoption of a tissue-specific cell fate in the affected tissue has been repeatedly raised and investigated.28,29 In the present studies, there are a number of factors that mitigate against non-mammary-mammary cell fusion as a mechanism for explaining the observed experimental results. The implanted cell mixture (10 µl along a 1.75 cm needle tract) is not held together in vivo by the constraints imposed by Matrigel, collagen or some other non-cellular matrix. Success in producing a positive epithelial outgrowth depends upon the aggregation and reorientation of the inoculated epithelial cells to form a nucleating growth cone. It has been clearly demonstrated that this property is manifest in only a small percentage of the epithelial cells inoculated.6,30 Proliferation subsequent to formation of the growth cone results in the penetration of the fat pad radially from the origination site and results in an expanded epithelial population of approximately two million cells.9 Throughout pregnancy this initial population (comprised of the epithelium in mammary ducts) expands further about twenty fold with the production of secretory alveoli, which branch from the existing ductal tree. It is during this process that activation of the Rosa26/Reporter gene occurs through the massive induction of the WAP promoter around the 15th day of pregnancy. In order for activation of the reporter by Cre-lox recombination, both the WAP-Cre gene and the Rosa26/Reporter gene must reside in the same cell. These two constructs do not share the same chromosome. Thus, each activated lacZ+ epithelial cell must possess each transgene on a separate chromosome. Therefore it is extremely unlikely that the large number of lacZ+ cells observed subsequent to mammary epithelial re-modeling post-lactation (now returning to ~two million epithelial cells after involution), positioned along the length and breadth of the involuted gland result from the proliferation expansion of non-mammary and mammary cells capable of maintaining both genes in the same cell following fusion. Nevertheless, cell-cell fusion was ruled out in the chimeric mammary glands in two different ways. One approach was to carry out X and Y chromosome specific fluorescence in situ hybridization (FISH) upon sections cut from the chimeric mammary outgrowths. The results of this study showed that male and female cells existed side by side in mammary epithelial structures. The second approach was to stain cells with propidium iodide that had been isolated from seminiferous tubules, collected from neural stem cell cultures, primary mammary epithelial cultures and from the mammary chimeras themselves or the un-manipulated glands of the chimeric implant hosts. These stained cells were then analyzed by fluorescence activated cell sorting to determine the ploidy of the cellular nuclei isolated from each tissue. Direct comparison of these scans with one another demonstrated that no aneuploidy or polyploidy was evident in any of the cellular preparations. This provided further evidence that cell-cell fusion had not occurred in the production of the chimeric glands.
Conclusions and Future Directions
The foregoing discussion supports the concept that the tissue microenvironment can affect the cellular repertoire of an adult stem cell. This influence in the murine mammary gland appears to be manifest in signals emanating from the epithelial cells as well as the stromal elements of the mammary fat pad. Several questions remain to be answered. For example, what is the role if any of mammary stem/progenitor cells in this process? Does the mammary fat pad selectively support the reprogramming in conjunction with the mammary epithelial cells or can any fat pad in the female mouse demonstrate this activity? Both testes and neural tissues develop from ectodermal precursors, will cell developing from mesoderm or endoderm precursors respond similarly when mixed with mammary epithelial cells in the context of the mammary fat pad? Finally, what are the cellular, genetic and molecular components that define the mammary epithelial-specific stem cell niche and how can these factors be utilized for developing new paradigms for stem cell control and cancer therapy?
Our preliminary experiments have shed a small amount of light on the questions mentioned above. First, enriching or depleting the mammary epithelial cells for cells expressing the currently accepted cell surface markers for mammary stem/progenitor cells31–33 (CD49f, CD29 or CD24) did not affect the efficiency of reprogramming non-mammary cells. Likewise enriching or depleting for Thy1 a marker associated with mammary myoepithelial cells34 failed to have any significant effect on the redirection of seminiferous tubule cells to mammary epithelial cell fates. Testing mammary epithelial cell populations from various gene knockout models has thus far not revealed any particular gene product that is essential for reprogramming. However our recent findings have delineated at least one essential epithelial cell characteristic necessary for the process of reprogramming.
Serial transplantation of the mammary epithelium inevitably leads to growth senescence, which has clearly been linked to the number of mitotic events required for stem cell activity to reach the outermost periphery of the regenerated gland. We have begun studies designed to determine whether growth senescent mammary epithelial cell populations that are unable to support in vivo mammary epithelial regeneration by themselves may be able to re-program non-mammary stem/progenitor cells. Thus far, those growth-deficient mammary populations that we have tested were able to reprogram non-mammary stem cells and in the process were able to generate full mammary outgrowths in cleared mammary fat pads. These finding have strong implications for recruitment of transformed cells to growth-deficient niches and neoplasia. In addition, our studies have led us to examine the response of cancer cells in this experimental model, as cancer cells show considerable plasticity when placed in developing tissue environments.35,36 Our present work demonstrates that signals from the mammary microenvironment in the context of the regenerating gland are capable of redirecting the repertoire of adult somatic stem cells from at least two non-mammary tissues. Further efforts to extend these initial findings will elucidate at least some of the mechanisms involved.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita YM, Fuller MT. Asymmetric stem cell division and function of the niche in the Drosophila male germ line. Int J Hematol. 2005;82:377–380. doi: 10.1532/IJH97.05097. [DOI] [PubMed] [Google Scholar]
- 5.Kimble J, Crittenden SL. Germline proliferation and its control. WormBook. 2005:1–14. doi: 10.1895/wormbook.1.13.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith GH. Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Res Treat. 1996;39:21–31. doi: 10.1007/BF01806075. [DOI] [PubMed] [Google Scholar]
- 7.Smith GH, Boulanger CA. Mammary stem cell repertoire: new insights in aging epithelial populations. Mech Ageing Dev. 2002;123:1505–1519. doi: 10.1016/s0047-6374(02)00114-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim ND, Oberley TD, Yasukawa-Barnes J, Clifton KH. Stem cell characteristics of transplanted rat mammary clonogens. Exp Cell Res. 2000;260:146–159. doi: 10.1006/excr.2000.5013. [DOI] [PubMed] [Google Scholar]
- 9.Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- 10.Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- 11.Boulanger CA, Smith GH. Reducing mammary cancer risk through premature stem cell senescence. Oncogene. 2001;20:2264–2272. doi: 10.1038/sj.onc.1204312. [DOI] [PubMed] [Google Scholar]
- 12.Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGFbeta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- 13.Booth BW, Boulanger CA, Smith GH. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol. 2007;212:729–736. doi: 10.1002/jcp.21071. [DOI] [PubMed] [Google Scholar]
- 14.Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci USA. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci USA. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:5455–5460. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouros-Mehr H, Kim JW, Bechis SK, Werb Z. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008;20:164–170. doi: 10.1016/j.ceb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu P, Ewald AJ, Martin GR, Werb Z. Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Dev Biol. 2008;321:77–87. doi: 10.1016/j.ydbio.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano JV, Pepper MS, Orci L, Montesano R. Roles of hepatocyte growth factor/scatter factor and transforming growth factor-beta1 in mammary gland ductal morphogenesis. J Mammary Gland Biol Neoplasia. 1998;3:133–150. doi: 10.1023/a:1018790705727. [DOI] [PubMed] [Google Scholar]
- 24.Stull MA, Rowzee AM, Loladze AV, Wood TL. Growth factor regulation of cell cycle progression in mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2004;9:15–26. doi: 10.1023/B:JOMG.0000023585.95430.f4. [DOI] [PubMed] [Google Scholar]
- 25.Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, et al. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz E, Streuli CH. The extracellular matrix as an adhesion checkpoint for mammary epithelial function. Int J Biochem Cell Biol. 2007;39:715–726. doi: 10.1016/j.biocel.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol. 2007;25:903–910. doi: 10.1038/nbt1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajcini KV, Pomerantz JH, Alkan O, Doyonnas R, Blau HM. Myoblasts and macrophages share molecular components that contribute to cell-cell fusion. J Cell Biol. 2008;180:1005–1019. doi: 10.1083/jcb.200707191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith GH, Gallahan D, Zwiebel JA, Freeman SM, Bassin RH, Callahan R. Long-term in vivo expression of genes introduced by retrovirus-mediated transfer into mammary epithelial cells. J Virol. 1991;65:6365–6370. doi: 10.1128/jvi.65.11.6365-6370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 32.Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 34.Kim ND, Clifton KH. Characterization of rat mammary epithelial cell subpopulations by peanut lectin and anti-Thy-1.1 antibody and study of flow-sorted cells in vivo. Exp Cell Res. 1993;207:74–85. doi: 10.1006/excr.1993.1165. [DOI] [PubMed] [Google Scholar]
- 35.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, et al. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA. 2008;105:4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasemeier-Kulesa JC, Teddy JM, Postovit LM, Seftor EA, Seftor RE, Hendrix MJ, et al. Reprogramming multipotent tumor cells with the embryonic neural crest microenvironment. Dev Dyn. 2008;237:2657–2666. doi: 10.1002/dvdy.21613. [DOI] [PMC free article] [PubMed] [Google Scholar]