Abstract
The mouse mammary epithelial cell hierarchy contains both multipotent stem cell as well as lineage-limited duct and lobular progenitor cell functions. The latter—also termed parity-identified mammary epithelial cells (PI-MECs)—are marked by beta-galactosidase (β Gal) expression following pregnancy and involution in whey acidic protein promoter (WAP)-Cre/Rosa26-flox-stop-flox-lacZ (WC/R26) mice, and are the targets of tumorigenic transformation in mouse mammary tumor virus-erbB2 transgenic mice. In this study, we demonstrate that an epithelial population distinct from PI-MECs is transformed during WAP-Int3 tumorigenesis. As expected, WAP-Int3/WC/R26 triple-transgenic mice failed to undergo secretory alveolar development, failed to lactate and developed mammary tumors. Following pregnancy and involution, β Gal+ mammary epithelial cells were found in the normal mammary tissue, but the resulting mammary tumors were all β Gal−. WAP-Int3/WC/R26 mammary glands contained ample estrogen receptor alpha (ERα)+ MECs, but only rare (<1%) progesterone receptor (PR)+ and RANKL+ cells. In addition, dissociated MECs from WAP-Int3/WC/R26 glands failed to regenerate a mammary tree upon transplantation into a cleared fat-pad of a nu/nu recipient mouse. However, when mixed with normal MECs, PI-MECs from WAP-Int3/WC/R26 mice contributed progeny to the resulting functional outgrowth. The WAP-Int3/WC/R26-derived PI-MECs displayed all of the properties of fully functional lobular progenitors including giving rise to ERα+, PR+, smooth muscle actin+ and RANKL+ epithelial progeny. These results demonstrate that WAP-Int3 has no oncogenic effect upon PI-MECs and that the expansion of functional lobular progenitors is required for secretory alveolar development and lactation. Furthermore, lobular progenitor function is ultimately controlled by signals within its microenvironment.
Keywords: lobular progenitor, PI-MEC, Int3, Notch4, mammary development
Introduction
Transplantation studies have revealed three stem/progenitor cellular functions in the mouse mammary gland: lobule-limited, ductal-limited and multi-potent stem cells (reviewed in Smith and Medina, 2008; Bruno and Smith, 2010). The whey acidic protein promoter (WAP)-Cre/Rosa-26-flox-stop(neo)-flox-lacZ (WC/R26) reporter mouse strain has previously been shown to identify a mouse mammary-cell population that displays all of the functional characteristics of lobule-limited progenitors (Wagner et al., 2002). In this model, the transient expression of Cre recombinase (Cre) driven from the WAP during mid to late pregnancy permanently activates the ubiquitously expressing transgene, Rosa26-lacZ, and a small population of β Gal+ epithelial cells survive involution (Figures 1a and b). These cells, termed parity-identified mammary epithelial cells (PI-MECs), were found to be multi-potent, self-renewing and capable of retaining their activity through serial transplantations (Boulanger and Smith, 2001; Boulanger et al., 2005). In subsequent pregnancies, PI-MECs proliferated to produce luminal progeny that were positive for estrogen receptor alpha (ERα) or progesterone receptor (PR), as well as luminal cells that did not express either steroid receptor. In addition, β Gal+ myoepithelial cells (marked by smooth muscle actin (SMA)) were seen in secretory acini, demonstrating that the PI-MECs were capable of producing both luminal and myoepithelial progeny.
Figure 1.
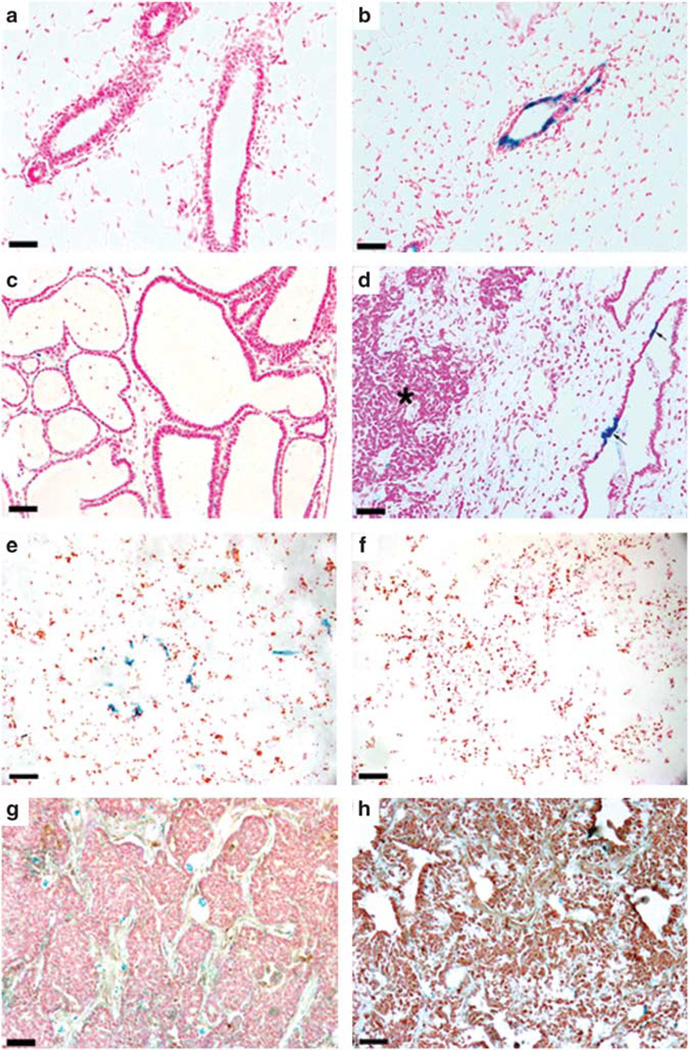
PI-MECs are present in WAP-Int3/WC/R26 mammary glands but do not form tumors. Nulliparous (a) and primiparous (b) WC/R26 control glands demonstrate the marking of PI-MECs with β Gal following pregnancy and involution. (c) A cross-section of a 3-day post-partum WAP-Int3/WC/R26 mammary gland demonstrating lack of secretory lobular development and the presence of cystic ducts. Note structures lack any milk fat in their lumens. (d) Following pregnancy and involution, β Gal+ cells were found in normal mammary epithelium (arrows) but not in tumors (asterisk). (e) Cultures of mammary epithelial cells taken from parous non-pregnant WAP-Int3/WC/R26 mice contain a small population (6%) of β Gal+ cells (blue). (e) Cultures of mammary tumors taken from parous non-pregnant WAP-Int3/WC/R26 mice contain no β Gal+ cells. (g, h) WAP-Int3/WC/R26 tumors stained negative for Cre expression during pregnancy (g) and positive for Neo expression following pregnancy (h). Scale Bars: a, b, e and f = 100 µm; c, d = 250 µm.
PI-MECs (that is, lobule progenitors) were previously described as the targets of transformation in mouse mammary tumor virus (MMTV)-Neu/WC/R26 triple-transgenic mice (Henry et al., 2004; Jeselsohn et al., 2010). Furthermore, when tumors from these mice were dissociated and transplanted along with normal mammary epithelium, their tumorigenic potential was suppressed and they contributed progeny to the resulting normal mammary outgrowth (Booth et al., 2011). However, the role of lobule-limited progenitors in tumorigenesis in other transgenic mouse mammary tumor models has not been explored.
Notch signaling is an evolutionarily conserved pathway involved in the cell-fate determination in a wide variety of cell types (reviewed in Callahan and Egan, 2004). The first link between Notch signaling and tumorigenesis came from the identification of a common integration site of MMTV in the Notch4 gene of MMTV-induced mammary tumors in mice (Gallahan and Callahan, 1987; Gallahan et al., 1987). The integration resulted in the expression of a 2.4-kb truncated mRNA encoding the intracellular domain of Notch4 (Int3). As Int3 does not require cleavage from the transmembrane domain for activation, its expression results in constitutive Notch signaling. Transgenic mice expressing Int3 from WAP (WAP-Int3) exhibit normal ductal development, but fail to develop functional secretory lobules during pregnancy and develop mammary tumors in both virgin and parous females—albeit at an accelerated rate in breeding females (Gallahan et al., 1996). The effects of Int3 on lobulogenesis and tumorigenesis are disparately regulated, with the former requiring the presence of the nuclear transcription factor Rbpj (Raafat et al., 2009).
Here we cross WC/R26 and WAP-Int3 mice and demonstrate that lobular progenitors are not involved in WAP-Int3-induced tumorigenesis. Lobular progenitors persist in these glands, but are inhibited by the expression of the WAP-Int3 transgene. However, they are functionally restored when mixed with MECs from normal mammary glands and transplanted into the cleared mammary fat pad of a recipient nu/nu female mouse. These results demonstrate that WAP-Int3 disrupts the normal lobular-progenitor niche, but does not target lobular progenitors (PI-MEC) for tumorigenesis. Furthermore, PI-MEC from WAP-Int3/WC/R26 glands can be functionally rescued when placed into a competent mammary niche.
Results
PI-MECs are present in parous WAP-Int3/WC/R26 mammary glands, but not in mammary tumors
WC/R26 females were crossed with WAP-Int3-positive males, and females positive for all three transgenes were collected through F2 crosses. These females (n = 10) as well as WAP-Int3-negative control females (n = 20) were set up as breeders and held for mammary tumor development. All of the WAP-Int3-positive females developed tumors within 10 months, while none of the WAP-Int3 controls developed tumors during the same time period (Table 1). As previously reported in WAP-Int3 mice (Gallahan et al., 1996), the glands from WAP-Int3/WC/R26 triple-transgenic mice failed to undergo normal lobular development or lactation. Often, abnormal cystic ducts formed that did not contain any milk fat (Figure 1c). Following pregnancy and involution, β Gal+ cells (that is, PI-MECs) were found in WAP-Int3/WC/R26 glands and in ex-vivo cell cultures (Figures 1d and e, respectively) and represented ~6% of the total cell population. This number is similar to that reported in wild-type WC/R26 glands, which were found to contain ~7% PI-MECs following the first pregnancy (Wagner et al., 2002). However, these cells failed to expand and form lobules during subsequent pregnancies as glands taken from late pregnant or post-partum glands contained a slightly lower percentage of PI-MECs (4.3%). Therefore, the majority of cells that divided during pregnancy were not derived from PI-MECs and did not make functional secretory lobules. Furthermore, none of the tumors observed arose from these β Gal+ cells (Table 1 and Figures 1d and f). This evidence suggests that PI-MECs are present in WAP-Int3/WC/R26 mammary glands, but are not the tumor antecedents. However, to confirm that the lack of β Gal expression in tumors resulted from a lack of Cre-mediated recombination and not from de novo deactivation of the Rosa26 locus, we stained tumors from pregnant mice with an antibody to Cre and found that WAP-Int3/WC/R26 mammary tumors did not express Cre during pregnancy (Figure 1g). Furthermore, Cre-mediated recombination of the Rosa26-flox-stop (neo)-flox-lacZ locus results in the excision of the neomycin phosphotransferase (Neo) gene (Soriano, 1999). As shown in Figure 1h, tumors from parous Wap-Int3/WC/R26 mice expressed Neo, demonstrating that the Rosa26-flox-stop(neo)-flox-lacZ remained intact. These results suggest that the targets of WAP-Int3 tumorigenesis never activated the WAP-Cre locus in their lifespan, and therefore are a distinct population from the PI-MECs.
Table 1.
Results of crosses between WC/R26 and WAP-Int3 mice
| Result of Crosses | # of Mammary Tumors/# females |
β Gal+ tumors |
β Gal+ cells in mammary gland |
|---|---|---|---|
| 10/30 Wap-Int3/WC/R26 | 12/10 | 0/9 | 10/10 |
| 20/30 Wap-Int3-negative | 0/20 | NA | NA |
10 WAP-Int3/WC/R26 triple-transgenic females were identified. All 10 animals developed mammary tumors following pregnancy that were negative for β Gal, but all had β Gal+ cells in their normal mammary tissue.
WAP-Int3/WC/R26 mice do not maintain normal PR+ or RANKL+ epithelial cell populations
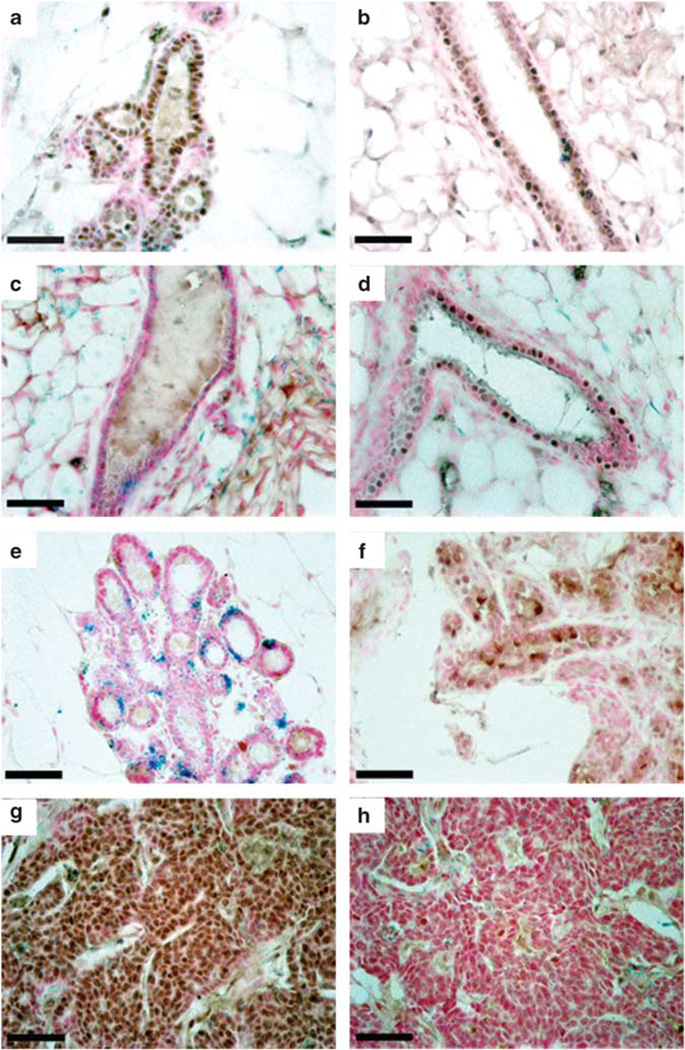
To determine whether hormone receptor status of mammary epithelium was altered by WAP-Int3, we stained parous, non-pregnant mammary glands from WAP-Int3/WC/R26 mice for ERα and PR. WAP-Int3/WC/R26 mammary glands contained 30.4% ERα+ epithelial cells, which did not differ significantly from the percentage observed in wild-type control glands (23.1%; Figures 2a and b). However, the same glands were largely devoid of PR+ epithelial cells, which made up only 0.16% of the total epithelial population, significantly less than the proportion of PR+ cells found in wild-type controls (14.0%; P < 0.05; Figures 3c and d). The receptor of activated nuclear factor κ ligand (RANKL), a major downstream effector of PR (Fata et al., 2000), was also expressed in significantly fewer cells in pregnant WAP-Int3/WC/R26 mice (0.43%) as compared with wild-type controls (13.14%; P < 0.05; Figures 2e and f). Similarly, the resulting tumors were strongly ERα+/PR− (Figures 2g and h). Because PR and RANKL are required for normal secretory lobular development (Brisken et al., 1998; Fata et al., 2000; Mukherjee et al., 2010), their low abundance in WAP-Int3/WC/R26 glands accounts for the perturbed alveolar development in these glands.
Figure 2.
WAP-Int3/WC/R26 mice do not maintain normal PR+ or RANKL+ epithelial cell populations. ERα staining in WAP-Int3/WC/R26 glands (a) and normal glands (b) demonstrates no significant difference between the percentage of ERα+ epithelial cells in transgenic and control glands (30.4 and 23.1%, respectively). PR staining in WAP-Int3/WC/R26 glands (c) and normal mammary glands (d) demonstrates significantly lower percentage of PR+ epithelial cells in transgenic glands (0.16 and 14.0%, respectively; P < 0.05). RANKL staining in staining in WAP-Int3/WC/R26 glands (e) and normal mammary glands (f) demonstrates the transgenic glands also have a significantly lower percentage of RANKL+ cells (0.43 and 13.4%, respectively; P < 0.05). Tumors from WAP-Int3/WC/R26 mice are ERα+ (g) and PR− (h). Scale Bars = 100 µm.
Figure 3.
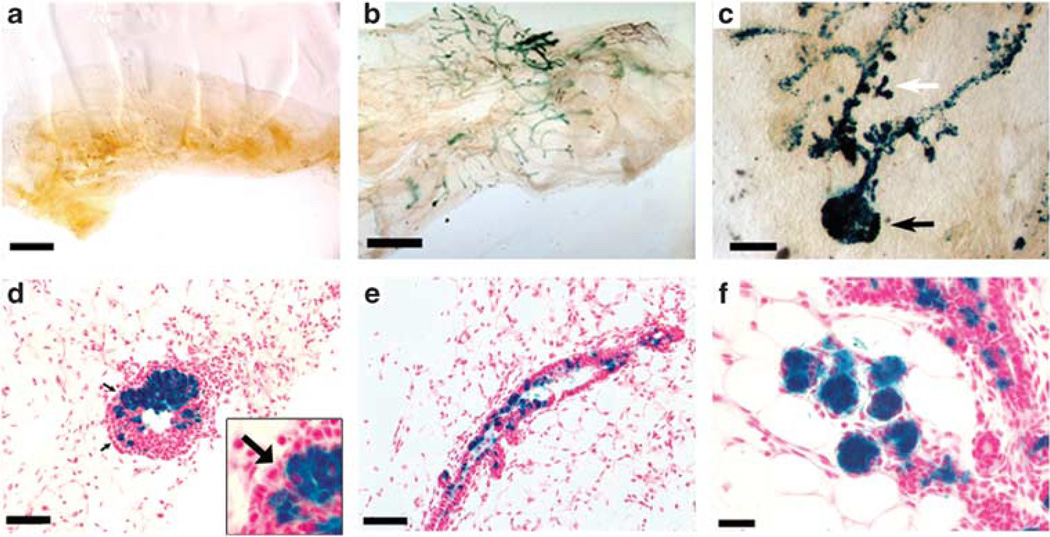
Dissociated WAP-Int3/WC/R26 PI-MECs contribute to mammary gland regeneration when transplanted with normal MECs. (a) A total of 2 × 106 dissociated WAP-Int3/WC/R26 MECs failed to regenerate a gland upon transplantation into a cleared mammary fat pad of a nu/nu female host. (b) When 2 × 105 dissociated WAP-Int3/WC/R26 MECs were mixed with 1 × 105 or 2 × 105 normal MECs, PI-MECs (blue cells) contributed to the resulting complete outgrowths. (c) Higher magnification image of a whole-mounted mammary gland taken from a 8-day pregnant mouse 22 days following inoculation with a mix of 2 × 105 WAP-Int3/WC/R26 and 2 × 105 normal MECs demonstrating contribution of WAP-Int3/WC/R26 PI-MECs to both end buds (black arrow) and developing side branches (white arrow). (d) Cross-section of an end bud in a developing WAP-Int3/WC/R26 and normal MECs chimeric gland demonstrating contribution of the WAP-Int3/WC/R26 PI-MECs to the body of the end bud, but not the cap cells (arrows). Inset is a higher magnification of the cap cells of the same image. (e) Cross section of a duct from a chimeric gland demonstrating presence of WAP-Int3/WC/R26 PI-MECs’ progeny. (f) Cross-section of and 8-day pregnant chimeric gland demonstrating contribution of WAP-Int3/WC/R26 PI-MECs to the developing acinar structures. Scale Bars: a and b = 1000 µm; c = 200 µm; d and e = 150 µm; f = 50 µm.
WAP-Int3/WC/R26 PI-MECs contribute differentiated progeny during gland regeneration upon transplantation with normal MECs
The preceding results led us to the hypothesis that lobule progenitors are present within WAP-Int3/WC/R26 glands, but suppressed because of a dysfunctional niche caused by the expression of Int3 in the surrounding epithelium—evidenced by the lack of PR+ and RANKL+ epithelial cells. Our previous studies have demonstrated that differentiated mammary epithelial cells are a key component of the mammary niche, and that cells from different tissue origins can be reprogrammed to function as normal PI-MECs when mixed with mammary epithelial cells in a cleared mammary fat pad (Boulanger et al., 2007; Booth et al., 2008; Bussard et al., 2010). Therefore, if the β Gal+ cells in WAP-Int3/WC/R26 mammary glands are bonafide lobular progenitors that are suppressed by Int3 expression in their niche, restoring a competent niche would functionally rescue them. To test this, we mixed 2 × 105 MECs isolated from WAP-Int3/WC/R26 glands with 1 × 105 or 2 × 105 normal mouse MECs and inoculated them into the cleared fat pads of 3-weeks-old nu/nu mice (Table 2, Figure 3). To induce simultaneous ductal extension and alveolar development, some mice were mated 2 weeks following implantation, and glands were removed when mice were 8–10 days pregnant (before de novo WAP-Cre activation). Importantly, when dissociated MECs from WAP-Int3/WC/R26 glands were inoculated into cleared fat pads (2 × 106 cells per gland), they were unable to recapitulate a mammary tree on their own (Table 2, Figure 3a). However, when 10-fold fewer cells were mixed with normal MECs, β Gal+ WAP-Int3/WC/R26 cells were able to contribute to gland regeneration, and greater than 50% of the mixed inoculates formed complete mammary ductal trees that filled the entire fat pad (Table 2 and Figure 3b).
Table 2.
Results of cellular transplantations
| Inoculation | Mammary outgrowths |
Tumors | β Gal+ outgrowths/ tumors |
|---|---|---|---|
| 1 × 105 MECs | 4/6 | 0/6 | 0/4 |
| 2 × 105 Int3 : 1 × 105 MECs | 4/7 | 0/7 | 4/4 |
| 2 × 105 Int3 : 2 × 105 MECs | 7/13 | 0/13 | 6/7 |
| 2 × 106 Int3 cells | 0/20 | 0/20 | NA |
| 1 × 105 Int3 tumor cells | 0/4 | 4/4 | 0/4 |
Abbreviation: MECs, mammary epithelial cells.
2 × 105 WAP-Int3/WC/R26 (Int3) MECs were mixed with 1 × 105 or 2 × 105 normal MECs from Balb/c mice and inoculated into cleared mammary fat pads of nu/nu female mice. PI-MECs from WAP-Int3/WC/R26 mice (marked by β Gal) contributed to the resulting outgrowths. WAP-Int3/WC/R26 never regenerated a gland on their own when 2 × 106 cells were inoculated. WAP-Int3/WC/R26 tumor cells only gave rise to β Gal− tumors. There was no significant difference between take rates in either Wap-Int3/WC/R26:wild-type MECs-mixed inoculations and that observed for wild-type MECs alone, but both mixed inoculations and MECs alone had significantly higher take rates than Wap-Int3 cells alone (P = 0.002, 0.0015 and 0.001, respectively).
Lobular progenitors are necessary for gland regeneration in-vivo, but cannot make a complete gland per se, as they are unable to produce cap cells needed for the penetration of end buds through the fat pad during ductal extension (Wagner et al., 2002; Boulanger et al., 2005; Matulka et al., 2007). Therefore, PI-MECs from WC/R26 mice contribute cells along the ducts of regenerated outgrowths, but do not contribute progeny that are capable of differentiation into cap cells. Similarly, PI-MECs from WAP-Int3/WC/R26 mice contributed progeny to both primary and tertiary ducts (Figures 3c and e) but although they were present in the bodies of the end buds, they did not contribute to the formation of cap cells (Figures 3c and d). However, they did proliferate to form developing secretory alveolar structures during pregnancy (Figures 3c and f).
PI-MECs from WAP-Int3/WC/R26 mice are multipotent bonafide lobular progenitors
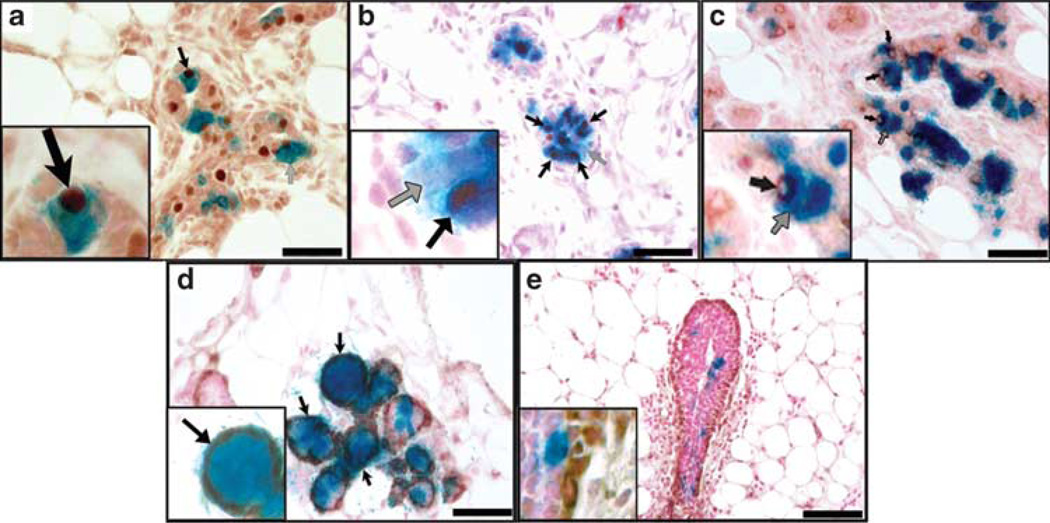
A hallmark of lobular progenitors is their ability to give rise to luminal cells that are ERα+, PR+ or devoid of either receptor, as well as SMA+ myoepithelial cells in lobules but not primary ducts (Wagner et al., 2002; Boulanger et al., 2005). To determine whether the PI-MECs isolated from WAP-Int3/WC/R26 mice were fully functional lobular progenitors, we analyzed the expression of these proteins in chimeric glands formed from WAP-Int3/WC/R26 MECs and normal MECs. As shown in Figure 4, PI-MECs formed ERα+ and ERα− luminal cells (Figure 4a), as well as PR+ and PR− luminal cells (Figure 4b). They also gave rise to cells that were positive and negative for RANKL (Figure 4c). As intact WAP-Int3/WC/R26 glands were largely devoid of PR+ and RANKL+ cells, the fact that PI-MECs from this gland are capable of producing them in the correct environment suggests their activity and expansion were suppressed by the presence of Int3 expression in the epithelial cells neighboring their niche. Furthermore, they contributed SMA+ myoepithelial cells in developing acinar structures (Figure 4c), but did not contribute to the myoepithelial cells lining the ducts, which are derived from the cap cells (Figure 4d). These results demonstrate that PI-MECs from WAP-Int3/WC/R26 mice have all the seminal characteristics of lobular progenitors previously described in wild-type glands and can function normally when placed in the correct environment.
Figure 4.
WAP-Int3/WC/R26 PI-MECs are multipotent and fully functional lobular progenitors when mixed with normal MECs. (a–c) Cross-sections of 8-day pregnant chimeric gland demonstrating WAP-Int3/WC/R26 PI-MECs give rise to (a) PR+ (black arrow) and PR− (gray arrow) progeny; (b) ERα+ (black arrow) and ERα− (gray arrow) progeny; (c) RANKL+ (black arrow) and RANKL− (gray arrow) progeny. (d and e) SMA staining of cross-sections of 8-day pregnant chimeric gland demonstrating WAP-Int3/WC/R26 PI-MECs give rise to myoepithelial cells of developing acinar structures (d; black arrows) but not of primary ducts (e). Insets represent higher magnifications of the same image. Scale Bars: a–d = 100 µm; e = 200 µm.
Discussion
Identification of cellular targets of transformation remains a major focus of research in mammary gland biology. Previously, lobular progenitors were identified as the targets of erbB2/neu tumorigenesis in MMTV-neu/WC/R26 triple-transgenic mice (Henry et al., 2004). Here, we once again used the WC/R26 mouse model to ask whether lobular progenitors are also involved in a Notch-mediated tumorigenic model. As opposed to MMTV-neu, our results demonstrate that lobular progenitors are not transformed in WAP-Int3 mice. Lobular development is instead inhibited by disruptions in the normal epithelial cellular environment, which in turn inhibits lobular-progenitor function. However, lobular progenitors persist in WAP-Int3/WC/R26 glands and are fully functional when placed in a competent mammary niche.
Our results are consistent with those presented in a recent publication by Raafat et al. (2009) that demonstrates that conditional knockout of Rbpj (using a WAP-Cre/Rbpjflox/flox system) in WAP-Int3 female mice results in a restoration of full secretory lobule development and lactation. However, tumorigenesis was not affected by Rbpj ablation, suggesting that failure of secretory lobular development was mediated by Notch4/Int3 signaling through Rbpj. Our results indicate that lobular progenitors (PI-MECs) are unaffected by WAP-Int3 expression and are fully functional when mixed with normal mammary epithelial cells before transplantation. The presence of fully functional PI-MECs is thus required for lobular development to occur. In addition, PI-MECs must receive the appropriate signals from the mammary microenvironment to carry out their function. This is not provided in the mammary microenvironment present in WAP-Int3/WC/R26 mice.
The lack of involvement of PI-MECs in WAP-Int3 tumorigenesis is supported by their presence following pregnancy and involution in WAP-Int3/WC/R26 mice and their absence from all of the resulting tumors (12/12). We confirmed that these tumors did not express Cre during pregnancy and had not removed the flox-neo(stop)-flox locus from the Rosa26-lacZ transgene, as Neo protein was detected in the tumors. This rules out the possibility that the transformed cells had silenced the Rosa26 locus and thus ‘shut off’ β Gal expression following transformation. It should be noted that expression of Int3 from the WAP-Int3 transgene is regulated temporally in the same manner as the endogenous WAP gene, that is, briefly during estrous, during late pregnancy and during lactation within the secretory mammary epithelium. However, expression of Int3 from the WAP-Int3 transgene perturbs normal development of secretory epithelium, and thus very little expression of whey acidic protein is detectable in day 14 pregnant and lactating mammary glands in WAP-Int3 mice (See Gallahan et al. 1996, Figures 1, 3, 4 and 5). Expression of WAP-Cre during lactation marks differentiated secretory epithelium (which do not survive involution) and PI-MECs by activating the R26-LacZ locus. Therefore, it is not surprising that we see so few β Gal-positive cells in the post-partum WC/R26/Wap-Int3 mammary glands (Figure 1), as secretory epithelial differentiation is inhibited by the presence of the WAP-Int3 transgene. Furthermore, the WAP-Int3 construct and the WAP-Cre construct exist on different chromosomes and as such are subjected to regulation by nuclear variegation in the respective cells where they reside. The promoter sequence is identical for each transgene—and thus their ‘hormonal’ regulation is identical—however, their expression will depend on the nuclear context of each transgene in any mammary epithelial cell. We conclude that Notch signaling in these glands does not transform PI-MECs, and this is consistent with the demonstration that removal of Rbpj results in the restoration of full lactation in WAP-Int3 mice. It should be noted that the pattern of WAP-Cre expression (marked by β Gal expression) observed in WAP-Int3/WC/R26 mice was identical to that observed in the parental WC/R26 mice.
The detrimental effect of Int3 expression on the lobular-progenitor niche is most likely mediated by the paucity of PR+ and RANKL+ MEC in WAP-Int3/WC/R26 glands. Progesterone and RANKL are required for side branching and lobulogenesis to occur in wild-type mammary tissue, and thus are important signaling components of the lobular-progenitor niche.
The inability of cells taken from WAP-Int3/WC/R26 glands to recapitulate a mammary tree on their own upon transplantation into a mammary fat pad suggests that this population lacks some necessary signal(s) required for gland regeneration. However, when placed with wild-type MECs, PI-MECs from WAP-Int3/WC/R26 glands were able to contribute to the resulting outgrowth (it is unknown whether other cells from WAP-Int3/WC/R26 glands contributed to the chimeric gland as well as they are unmarked). This is analogous to our recent work in reprogramming cells of non-mammary origin to take on a MECs fate—this includes cells of testicular, neuronal, MMTV-neu tumor and even cancer cells of human origin (Boulanger et al., 2007; Booth et al., 2008; Bussard et al., 2010). These results underscore the importance of signals from mammary epithelial cells to the mammary gland stem/progenitor cellular niche(s).
In summary, we have presented evidence that PI-MECs remain fully functional in WAP-Int3 mammary tissue but are unable to carry out their normal functions because of Notch signaling through Rbpj. Lobular progenitors persist in WAP-Int3 glands, but do not expand during pregnancy to form fully functional secretory lobules presumably because of a lack of PR+ and RANKL+ epithelial cells in the gland. Dissociated cells taken from WAP-Int3/WC/R26 glands are unable to recapitulate a mammary gland upon transplantation per se, but PI-MECs from the glands contribute progeny to functional outgrowths when mixed with normal mammary epithelial cells and exhibit all of the properties of lobular progenitors in the resulting outgrowths. These studies demonstrate that full secretory lobular development and lactation cannot occur without the expansion and function of lobular progenitors, and that the signals from the epithelial cellular niche are required for the manifestation of lobule-limited epithelial progenitor function.
Materials and methods
Mice
WAP-Int3/WC/R26 mice were generated by cross breeding male WAP-Int3 mice and female WC/R26 mice. The presence of all three transgenes was confirmed as previously described for each parental strain (Jhappan et al., 1992; Wagner et al., 2002). Female nu/nu mice were used as hosts for the transplantation studies. All mice were housed in Association and Accreditation of Laboratory Animal Care-accredited facilities in accordance with the NIH Guide for the Care and Use of Laboratory Animals. The National Cancer Institute Animal Care and Use Committee approved all experimental procedure.
Mammary epithelial cell isolation
Mammary glands were dissociated with 0.1% collagenase overnight at 37 °C. The resulting organoids were cultured on plastic culture flasks in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, insulin (1.0 µg/ml), and epidermal growth factor (10 ng/ml). MECs were collected after 4–7 days—fibroblasts were reduced before collection of the epithelial cells by differential trypsinization.
Cell and tissue transplantation
The surgical techniques used to clear the mammary epithelium from the fat pads of 3-weeks-old host mice and the subsequent transplantation of tissue fragments or cell suspensions have been described in detail previously (Boulanger et al., 2007; Booth et al., 2008). In brief, the mice were anesthetized, and the clearing procedure was performed immediately before the insertion of transplanted tissue fragments or cell suspensions. Cell suspensions were implanted in 10 µl volumes of phosphate-buffered saline with a Hamilton syringe equipped with a 30-gauge needle. The implanted females were placed with males 12 days after implantation to initiate pregnancy and secretory development.
X-Gal and immunohistochemical staining of mammary tissues
Briefly, glands were spread on glass slides, fixed in paraformaldehyde (4.0%) for 1–2 h, permeabilized in 0.02% Nonidet P-40, 0.01% sodium deoxycholate and 0.002 m MgCl2 in phosphate-buffered saline overnight at 4 °C, and then processed for X-gal as described previously (Wagner et al., 1997). Cells were processed in the same way except they were fixed for 15 min, and permeabilized for 1 h at room temperature. For histological examinations, X-gal-stained whole mounts were embedded in paraffin, sectioned at 6.0 µm, and counterstained with nuclear fast red (Sigma-Aldrich, St Louis, MO, USA). Immunohistochemistry was performed on deparaffinized sections. Primary antibodies used were rabbit anti-ERα MC-20 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-human PR (1:75; Dako, Carpinteria, CA, USA), mouse anti-SMA 1A4 (1:100; Zymed/Invitrogen, Carlsbad, CA, USA), goat anti-RANKL (1 µg/ml; R&D Systems, Minneapolis, MN, USA), rabbit anti-Cre (1:10 000; Novagen/EMD, Gibbstown, NJ, USA) and rabbit anti-Neo N2008-05 (1:200; US Biological, Swampscott, MA, USA). Antigen retrieval was performed by heating sections in autoclave at 121 °C for 10 min in pH 8.0 EDTA (for ERα, PR, SMA, Cre and Neo) or pH 6.0 citrate buffer (for RANKL). IHC staining procedure was carried out using the TRU Vectastain Kit (Vector Laboratories, Burlingame, CA, USA) per the manufacturers protocol. For RANKL staining, biotinylated rabbit anti-Goat secondary antibody (20 µg/ml; Vector) was used. DAB peroxidase substrate kit (Vector) was used for staining according to manufacturer’s recommended protocol. All sections were counter-stained with nuclear fast red.
Quantification and statistics
For quantification of stained cells, percentages were calculated by counting positively and negatively stained epithelial cells in 10 fields under 400× magnification. Data presented represent the mean percentage calculated in 3–5 glands. Significance was determined by comparing the average proportion of stained cells using a Mann–Whitney U test. For transplantation studies, significant differences in the proportional take rates were determined with a Fisher’s Exact Test.
Acknowledgements
The NCI’s Center for Cancer Research Intramural Research Program funded this work.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Booth BW, Boulanger C, Anderson L, Smith G. The mammary microenvironment restricts the tumorigenic phenotype of MMTV-neu-transformed tumor cells. Oncogene. 2011;30:679–689. doi: 10.1038/onc.2010.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A. 2008;105:14891–14896. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci USA. 2007;104:3871–3876. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CA, Smith GH. Reducing mammary cancer risk through premature stem cell senescence. Oncogene. 2001;20:2264–2272. doi: 10.1038/sj.onc.1204312. [DOI] [PubMed] [Google Scholar]
- Boulanger CA, Wagner KU, Smith GH. Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene. 2005;24:552–560. doi: 10.1038/sj.onc.1208185. [DOI] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RD, Smith GH. Functional characterization of stem cell activity in the mouse mammary gland. Stem Cell Rev. 2010;7:238–247. doi: 10.1007/s12015-010-9191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussard KM, Boulanger CA, Booth BW, Bruno RD, Smith GH. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010;70:6336–6343. doi: 10.1158/0008-5472.CAN-10-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R, Egan SE. Notch signaling in mammary development and oncogenesis. J Mammary Gland Biol Neoplasia. 2004;9:145–163. doi: 10.1023/B:JOMG.0000037159.63644.81. [DOI] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Gallahan D, Callahan R. Mammary tumorigenesis in feral mice: identification of a new int locus in mouse mammary tumor virus (Czech II)-induced mammary tumors. J Virol. 1987;61:66–74. doi: 10.1128/jvi.61.1.66-74.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallahan D, Jhappan C, Robinson G, Hennighausen L, Sharp R, Kordon E, et al. Expression of a truncated Int3 gene in developing secretory mammary epithelium specifically retards lobular differentiation resulting in tumorigenesis. Cancer Res. 1996;56:1775–1785. [PubMed] [Google Scholar]
- Gallahan D, Kozak C, Callahan R. A new common integration region (int-3) for mouse mammary tumor virus on mouse chromosome 17. J Virol. 1987;61:218–220. doi: 10.1128/jvi.61.1.218-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MD, Triplett AA, Oh KB, Smith GH, Wagner KU. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene. 2004;23:6980–6985. doi: 10.1038/sj.onc.1207827. [DOI] [PubMed] [Google Scholar]
- Jeselsohn R, Brown NE, Arendt L, Klebba I, Hu MG, Kuperwasser C, et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17:65–76. doi: 10.1016/j.ccr.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C, Gallahan D, Stahle C, Chu E, Smith GH, Merlino G, et al. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6:345–355. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- Matulka LA, Triplett AA, Wagner KU. Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol. 2007;303:29–44. doi: 10.1016/j.ydbio.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Soyal SM, Li J, Ying Y, He B, DeMayo FJ, et al. Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J. 2010;24:4408–4419. doi: 10.1096/fj.10-157982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raafat A, Lawson S, Bargo S, Klauzinska M, Strizzi L, Goldhar AS, et al. Rbpj conditional knockout reveals distinct functions of Notch4/Int3 in mammary gland development and tumorigenesis. Oncogene. 2009;28:219–230. doi: 10.1038/onc.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GH, Medina D. Re-evaluation of mammary stem cell biology based on in vivo transplantation. Breast Cancer Res. 2008;10:203. doi: 10.1186/bcr1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Boulanger CA, Henry MD, Sgagias M, Hennighausen L, Smith GH. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development. 2002;129:1377–1386. doi: 10.1242/dev.129.6.1377. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, et al. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]