Abstract
The serine proteases released by activated polymorphonuclear neutrophils [NSPs (neutrophil serine proteases)] contribute to a variety of inflammatory lung diseases, including CF (cystic fibrosis). They are therefore key targets for the development of efficient inhibitors. Although rodent models have contributed to our understanding of several diseases, we have previously shown that they are not appropriate for testing anti-NSP therapeutic strategies [Kalupov, Brillard-Bourdet, Dade, Serrano, Wartelle, Guyot, Juliano, Moreau, Belaaouaj and Gauthier (2009) J. Biol. Chem. 284, 34084–34091). Thus NSPs must be characterized in an animal model that is much more likely to predict how therapies will act in humans in order to develop protease inhibitors as drugs. The recently developed CFTR−/− (CFTR is CF transmembrane conductance regulator) pig model is a promising alternative to the mouse model of CF [Rogers, Stoltz, Meyerholz, Ostedgaard, Rokhlina, Taft, Rogan, Pezzulo, Karp, Itani et al. (2008) Science 321, 1837–1841]. We have isolated blood neutrophils from healthy pigs and determined their responses to the bacterial pathogens Pseudomonas aeruginosa and Staphylococcus aureus, and the biochemical properties of their NSPs. We used confocal microscopy and antibodies directed against their human homologues to show that the three NSPs (elastase, protease 3 and cathepsin G) are enzymatically active and present on the surface of triggered neutrophils and NETs (neutrophil extracellular traps). All of the porcine NSPs are effectively inhibited by human NSP inhibitors. We conclude that there is a close functional resemblance between porcine and human NSPs. The pig is therefore a suitable animal model for testing new NSP inhibitors as anti-inflammatory agents in neutrophil-associated diseases such as CF.
Keywords: lung inflammation, neutrophil, neutrophil extracellular trap, neutrophil serine protease, pathogen, pig model
Abbreviations: Abz, o-aminobenzoic acid; ACT, α1-antichymotrypsin; α1-Pi, α1-proteinase inhibitor; cat G, cathepsin G; CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; dsDNA, double-stranded DNA; ECL, enhanced chemiluminescence; EDDnp, N-(2,4-dinitrophenyl) ethylenediamine; FRET, fluorescence resonance energy transfer; HNE, human neutrophil elastase; NE, neutrophil elastase; NET, neutrophil extracellular trap; NSP, neutrophil serine protease; PE, phycoerythrin; PEM, protein epitope mimetic; PMN, polymorphonuclear neutrophil; Pr3, protease 3; SEM, scanning electron microscopy; SLPI, secretory leucocyte protease inhibitor
INTRODUCTION
PMNs (polymorphonuclear neutrophils) or neutrophils are the main acute innately specialized phagocytes that are recruited early to inflammatory sites to provide a first line of defence against bacteria and fungi. They kill pathogens both intracellularly and extracellularly through oxidative and proteolytic pathways and via antimicrobial peptides and proteins [1,2]. Neutrophils also kill pathogens through the active release of NETs (neutrophil extracellular traps), fibrous structures composed of chromatin fibres covered with granular and cytoplasmic proteins [3,4].
Neutrophils are also potent inflammatory cells that cause massive damage to host tissues once they are activated. They secrete huge quantities of NSPs (neutrophil serine proteases), NE (neutrophil elastase), Pr3 (protease 3) and cat G (cathepsin G), that overwhelm the endogenous antiproteases, resulting in the uncontrolled proteolysis observed in emphysema, chronic obstructive pulmonary disease and CF (cystic fibrosis) [5–7]. Strategies have been developed to restore the protease–antiprotease balance using exogenous protease inhibitors [6,8], but the results of clinical trials have not yet been convincing [6]. This could be explained, in part, by the impaired access of therapeutic inhibitors to cationic target proteases when they are bound to DNA and other anionic compounds in bronchial secretions [9]. NETs contain much of the extracellular DNA in CF secretions and are a reservoir of active NSPs. These enzymes are released by DNase, which increases the proteolytic potential in the extracellular environment [9], but also greatly improves inhibition of NSPs in CF sputum [9]. Thus NETs probably have functions other than that of antimicrobial weapons as initially described [3]. Their putative deleterious role has been emphasized in chronic inflammatory diseases [10,11]. It is essential to know how NETs regulate the activities of extracellular NSPs, especially in CF. But this requires a pertinent animal model that reproduces the clinical features of the disease. CFTR−/− (CFTR is CF transmembrane conductance regulator) animal models have been developed in the mouse (for a review see [12,13]) and the ferret [14], but they do not display all the main features of CF. The CFTR-deficient pigs that have been obtained show all the main clinical features of CF, with intestinal lesions, destruction of the exocrine pancreas, gallbladder abnormalities and early focal biliary cirrhosis [15,16]. Unlike mice, CFTR−/− pigs spontaneously develop the typical symptoms of CF lung disease, airway inflammation, remodelling, mucus accumulation and bacterial infection [17]. Furthermore, the lungs of pigs and humans have many anatomical, histological, physiological and biochemical features in common [18,19]. But the similarities between the spatiotemporal distributions and activities of porcine NSPs and those of their human homologues and their responses to anti-inflammatory inhibitors directed against human NSPs are not known.
In the present study we have investigated the fate of porcine NSPs and the secretion of NETs by chemically and/or bacterially activated purified porcine neutrophils. We have also measured the proteolytic activities of porcine NSPs in suspensions of NET-containing neutrophils and in the supernatants of neutrophils degranulated with the calcium ionophore A23187. We then compared these results with those obtained with purified human neutrophils in the same experimental conditions. Lastly, we have shown that physiological and chemical inhibitors of human NSPs also inhibit porcine NSPs in the same way.
EXPERIMENTAL
Materials
Percoll™ was from GE Healthcare. PBS and RPMI 1640 medium were from Invitrogen. The FRET (fluorescence resonance energy transfer) substrates were synthesized by Genecust. The human proteases NE (EC 3.4.21.37) and cat G (EC 3.4.21.20), and protease inhibitors, α1-Pi (α1-proteinase inhibitor) and ACT (α1-antichymotrypsin), were from BioCentrum, and human Pr3 (EC 3.4.21.76) was from Athens Research. The recombinant inhibitor EPI-hNE4 was from Debiopharm, and recombinant human SLPI (secretory leucocyte protease inhibitor) was from R&D Systems. The PEM (protein epitope mimetic) cyclic peptide HNE (human NE) inhibitor P0005259 was provided by Dr E. Chevalier (Polyphor Ltd, Allschwil, Switzerland) [20]. The chemical inhibitor of human Pr3, azapro-3, was prepared as described by Epinette et al. [21]. DNase was from Roche. The low-binding polypropylene 96-well plates were from Corning.
EvaGreen™ FluoProbes®, DRAQ5™ and anti-IgG conjugated to FluoProbes®-488 used for confocal microscopy were from Interchim. Superfrost slides were from CML.
The primary antibodies used for flow cytometry, mouse anti-SWC8 and anti-SWC1 IgMs, were from Biosource, and mouse anti-CD45Ra IgM was from Gentaur. Rabbit anti-mouse IgM–PE (phycoerythrin) was from Biosource. An IgM isotype control was from Dako.
Vivaspin membranes were from Sartorius. The DNA extraction kit was from Stratagene. The ECL (enhanced chemiluminescence) chemiluminescence detection kit was from GE Healthcare. All other reagents were from Sigma–Aldrich.
Human samples
Samples of human blood were collected from healthy volunteers at the EFS (Etablissement Français du Sang) of the Centre Hospitalier Régional Universitaire, Tours, France. This research was carried out in accordance with the Helsinki Declaration (2000) of the World Medical Association and was approved by the local Ethical Committee (#2007-R17). Written informed consent was obtained from all subjects.
Porcine samples
Porcine blood was obtained from healthy large white pigs kept at the INRA (Institut National de la Recherche Agronomique), Nouzilly, France. The pigs were cared for in accordance with the guidelines of the Institutional Animal Care and Use committee at INRA.
Isolation of human and porcine blood neutrophils
Human blood neutrophils were purified as described previously [9]. Blood porcine neutrophils were purified using this protocol with some modifications. Blood was collected over 0.2% (final concentration) EDTA (pH 8.0), and processed within 1 h of collection. An aliquot of blood (10 ml) was incubated, with shaking, with 40 ml of lysis buffer [0.1 mM EDTA, 10 mM potassium bicarbonate and 150 mM ammonium chloride (pH 7.4)] for 15 min at room temperature (22°C) and then centrifuged at 500 g at 20°C for 5 min. The resulting pellet was washed with RPMI 1640 medium and a sample was removed for flow cytometric analysis; the remaining cells were suspended in RPMI 1640 medium (2×107 cells in 6 ml) and gently layered on to 6 ml of a discontinuous Percoll™ density gradient prepared with 1.2 ml layers of the following densities: 1.105, 1.100, 1.093, 1.087 and 1.081 g/ml. After centrifugation at 800 g for 30 min at 20°C, the neutrophil band sedimented at the interface of the 1.087 and 1.081 g/ml layer, whereas monocytes were found between 1.105 and 1.100 g/ml density. The PMNs were gently recovered and washed with PBS (without calcium or magnesium), suspended in PBS (2×106 cells/ml), counted and their viability was determined by Trypan Blue exclusion.
Flow cytometry
The white blood cell populations and purified neutrophils were characterized by the specific markers on their surface [22]. Purified cells (106 in 400 μl of PBS) were incubated with primary antibody (mouse IgM), diluted 1:50 (anti-SWC8, anti-SWC1, anti-CD45Ra or IgM control), for 30 min at room temperature. Samples were then incubated with an anti-IgM coupled to PE (1:100 dilution) for 1 h. Cells were collected by centrifugation at 500 g for 5 min at 20°C, suspended in 1 ml of fixative solution [4% (v/v) formaldehyde and 0.01% glutaraldehyde] and stored at 4°C. Before analysis, cells were centrifuged at 500 g for 5 min at 20°C and suspended in PBS. Fixed cells were analysed with a Coulter® Epics XL-MCL™ flow cytometer (Beckman Coulter); data for at least 10 000 events were recorded.
Bacterial strains and growth conditions
One colony of Pseudomonas aeruginosa strain PAO1 and one colony of Staphylococcus aureus aureus strain CIP 103-811 isolated on blood agar were grown in brain heart infusion medium overnight at 37°C without agitation. A sample (50 μl) of the overnight culture was placed in fresh brain heart infusion medium with aeration and agitation and grown to the exponential phase. The bacteria were collected by centrifugation at 10000 g for 10 min at 20°C, washed twice with PBS and suspended in 5 ml of PBS. The bacteria concentration was determined by measuring the D600.
Neutrophil activation
Freshly isolated PMNs (107 cells in 700 μl) were placed in low-binding microtubes and activated by incubation with 20 μM A23187, 5 mM MgCl2 and 5 mM CaCl2 for 30 min at 37°C. Cells were also activated by incubation with 500 nM PMA for 1 h at 37°C or with bacteria (P. aeruginosa or S. aureus) at a MOI (multiplicity of infection) of 1:20 for 1 h at room temperature with agitation. Some samples of PMNs were incubated with 100 μg of DNase for 30 min at room temperature with agitation.
DNA quantification
Aliquots (3×105 cells) of the suspensions of activated PMNs were transferred into 150 μl of PBS and extracellular DNA was quantified with 5 μl of the non-cell-permeant fluorochrome EvaGreen™ dsDNA (double-stranded DNA) reagent (λexcitation=488 nm and λemission=520 nm) as described previously [9]. Standard curves were prepared with genomic DNA purified from control blood neutrophils using a DNA extraction kit according to the manufacturer's instructions.
Measurement of serine protease activities
The protease activities in suspensions of quiescent and activated PMNs, and in the supernatants of activated PMNs were measured as described in [23] using the FRET substrates previously optimized for human NSPs: Abz-TPFSGQ-EDDnp [Abz is o-aminobenzoic acid and EDDnp is N-(2,4-dinitrophenyl) ethylenediamine] for cat G [24] and Abz-VADCADYQ-EDDnp for Pr3 [25], and that designed for mouse NE, Abz-QPMAVVQSVPQ-EDDnp [26].
Inhibition of porcine proteases by inhibitors of human NSPs
A23187-activated cells were centrifuged at 2000 g for 10 min at 4°C. The resulting supernatant was recovered and concentrated 10-fold with a Vivaspin membrane (10 000 Da cut-off). Aliquots of concentrated supernatant (107 cells in 150 μl of PBS) were placed in the wells of microplates and the proteases inhibited with 10−7 M (final concentration) of physiological inhibitors of HNE and Pr3, α1-Pi, and human cat G, ACT. NSPs were also inhibited with 10−6 M (final concentration) of reversible recombinant inhibitors (EPI-hNE4 or SLPI) or synthetic inhibitors [10−6 M (final concentration) P0005259 or 5×10−5 M (final concentration) azapro-3]. Residual activities on the selective FRET substrates were measured after incubation for 30 min. The remaining free proteases and the serpin–protease complexes were detected by Western blotting as described below.
Preparation of anti-human NSPs antibodies
The HNE, Pr3 and cat G antisera were raised in rabbits as described previously [27] using 16-mer or 17-mer peptides corresponding to position 88–103 (104) of the pro-sequence of each protease (numbering based on the sequence of pro-chymotrypsinogen [28]): IFENGYDPVNLLNDIV for HNE, VFLNNYDAENKLNDVL for Pr3 and AIRHPQYNQRTIQNDIM for cat G.
Immunoblotting
Aliquots of concentrated supernatant (100 μl corresponding to the degranulation of 1.6×107 cells) were incubated with human α1-Pi or ACT (2.5×10−6 M) for 30 min at room temperature. Human Pr3, NE and cat G (8×10−8 M final concentration) incubated under the same experimental conditions were used as controls. Aliquots of the incubation mixtures were subjected to SDS/PAGE (15% gel), transferred on to nitrocellulose membrane, and free proteases and serpin–protease complexes were detected by immunoblotting. The membranes were incubated with the rabbit polyclonal anti-peptide antibodies specific for each human protease [NE (1:400 dilution), Pr3 (1:600 dilution) and cat G (1:400 dilution)], followed by goat anti-rabbit antibody coupled to horseradish peroxidase. Immunoreactivity was visualized by ECL with an ECL detection kit.
SEM (scanning electron microscopy)
Approximately 106 purified blood neutrophils were settled on polylysine-coated glass slides. Samples were treated as described previously [9] and cells were examined with a FEG-SEM-ZEISS Ultraplus scanning electron microscope (Carl Zeiss).
Confocal microscopy
Approximately 3×105 cells were seeded on to Superfrost slides and activated as described above or left unstimulated. The cells were fixed by incubation with 4% (v/v) formaldehyde in PBS for 30 min at room temperature and washed three times with PBS. Non-specific binding sites were blocked by incubation with 10% (v/v) normal goat serum and 1% (w/v) BSA in PBS for 1 h. Samples were then incubated overnight at 4°C with PBS containing 5% (v/v) normal goat serum, 1% (w/v) BSA and primary rabbit anti-peptide antibodies specific for each human protease diluted 1:200 (anti-NE and anti-cat G antibodies) or 1:300 (anti-Pr3 antibodies). Bound antibodies were detected by incubation for 2 h with an anti-IgG coupled to FluoProbes®-488 (diluted 1:100) in PBS containing 5% (v/v) normal goat serum and 1% (w/v) BSA. DRAQ5™ (10 μM) was used (30 min incubation) to detect dsDNA. Samples were analysed with an Olympus FV 500 confocal microscope.
Statistical analysis
Data were analysed using non-parametric Mann–Whitney U tests (Minitab.16® software) and differences between groups were considered significant when P<0.05.
RESULTS
Purification of porcine blood neutrophils
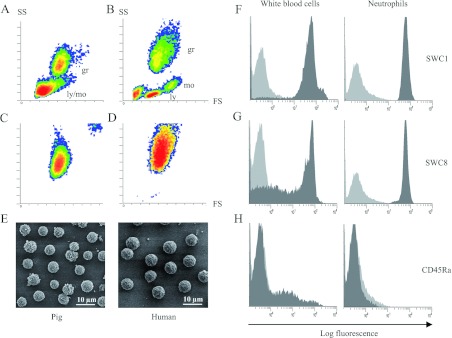
The protocol used to purify pig PMNs was adapted from that used for human PMNs [9] to preserve their NET-secreting capacity [29] and to take into account the higher concentration of erythrocytes in pig blood than in human blood [30]. We purified 6×106 to 2×107 PMNs from 15 ml of blood with a purity greater than 99%, as checked by flow cytometry SS (side scatter)/FS (forward scatter) analysis (Figures 1A–1D) and using antibodies directed against the positive markers for leucocytes (SWC1) and neutrophils (SWC8) (Figures 1F and 1G) and the negative marker CD45Ra that labels only monocytes and lymphocytes (Figure 1H) [22]. Porcine PMNs were smaller than their human counterparts, but had the same morphology; they were round with a rough surface characteristic of quiescent cells (Figures 1C–1E).
Figure 1. Characterization of purified porcine blood neutrophils.
Flow cytometric analysis of pig (A) and human (B) white blood cells and of purified porcine (C) and human (D) neutrophils. The quiescent porcine neutrophils were >99% pure. SEM (E) of purified quiescent pig and human neutrophils. Porcine white blood cells and quiescent neutrophils were characterized using antibodies raised against the surface marker of leucocytes (SWC1) (F), neutrophils (SWC8) (G) and lymphocytes and monocytes (CD45Ra) (H) (grey peaks), with IgM isotype as control (light grey peaks). gr, granulocyte; ly, lymphocyte; mo, monocyte.
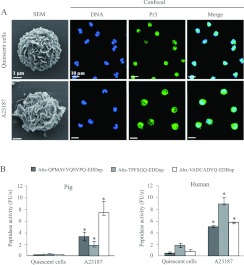
Activation of porcine neutrophils by the calcium ionophore A23187
Purified porcine PMNs were treated with the chemical ionophore A23187, as for human PMNs, to induce degranulation and the release of NSPs [31]. NSPs in porcine neutrophil suspensions were immunodetected by confocal microscopy using anti-peptide antibodies raised in the laboratory against human NSPs. Fractions of the secreted proteases remained bound to the external plasma membrane, as for human neutrophils (results not shown) (Figure 2A). We recorded the proteolytic activities in suspensions of activated pig neutrophils using the specific human Pr3 and cat G substrates and the mouse NE substrate (which is also hydrolysed by HNE) previously developed in the laboratory [23–26] (Figure 2B). We ensured that no other class of proteases cleaved the NSP substrates by adding inhibitors of metalloproteases (10 mM EDTA) and cysteine proteases (100 μM E64) to the suspensions of activated cells (results not shown). This suggests that the specificities of pig NSPs are similar to those of their human homologues. Activation of PMNs with the calcium ionophore A23187 increased the activity of each protease in the cell suspension 6–10-fold (Figure 2). This is similar to the result obtained with human neutrophils based on measuring the extracellular proteolytic activities in suspensions of pig and human activated and quiescent neutrophils (Figure 2B). We measured the capacity of natural or synthetic inhibitors of the human enzymes to inhibit pig NSPs because the specificity of pig NSPs for peptide substrates is similar to that of their human homologues.
Figure 2. Degranulation of pig neutrophils by the calcium ionophore A23187.
SEM and confocal microscopy of quiescent and activated porcine neutrophils (A) showing DNA (blue) and Pr3 (green), chosen here as a representative NSP. Proteolytic activities as measured by the increase in fluorescence units/s of FRET substrates specific for each NSP in suspensions of 1.5×106 /150 μl of pig neutrophils (median±interquartiles, n=10) and human neutrophils (median±interquartiles, n=5) (B). * indicate significant (α=5%) increases over unstimulated cells. FRET substrates were Abz-QPMAVVQSVPQ-EDDnp for NE, Abz-TPFSGQ-EDDnp for cat G and Abz-VADCADYQ-EDDnp for Pr3 [24–26].
Inhibition of pig NSPs by protease inhibitors targeting their human homologues
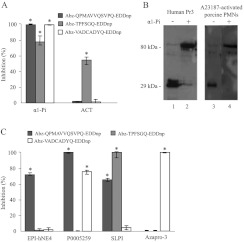
We incubated aliquots of supernatant from degranulated pig PMNs (107 cells) with α1-Pi, the polyvalent circulating inhibitor of human NSPs, and with ACT, the main physiological inhibitor of cat G. The NE and Pr3 substrates were not cleaved in the presence of α1-Pi, and the cat G substrate was not cleaved when ACT was present (Figure 3A). Our Western blotting studies also showed that human serpins form irreversible complexes with porcine proteases (Figure 3B). This indicates that the mechanism by which pig NSPs are inhibited is the same as that reported for human proteases.
Figure 3. Inhibition of porcine NSPs by human NSP inhibitors.
(A) Percentage inhibition of porcine NSPs in supernatants of A23187-activated neutrophil suspensions (106 cells in 150 μl) by human α1-Pi (10−7 M final concetration) and human ACT (10−7 M final concentration) showing that the specificity of human inhibitors for porcine proteases is the same as that for human NSPs (median±interquartiles, n=4). * indicate significant (α=5%) inhibition of NSPs. (B) Irreversible complexes formed between human α1-Pi and porcine Pr3 shown by Western blotting in reducing conditions using anti-human Pr3 antibodies (lanes 3 and 4). Human Pr3 and its complex with α1-Pi were used as a control (lanes 1 and 2). The molecular mass in kDa is indicated on the left-hand side. (C) Inhibition of porcine proteases by reversible inhibitors of human NSPs. The specific inhibition of porcine elastase by EPI-hNE4 (10−6 M final concentration), the preferential inhibition of NE over Pr3 by P0005259 (10−6 M final concentration), the inhibition of both NE and cat G by SLPI (10−6 M final concentration) and the specific inhibition of porcine Pr3 by azapro-3 (5×10−5 M final concentration) reproduced the results obtained with human NSPs, indicating the functional resemblance between pig and human NSPs (median±interquartiles, n=4). * indicate significant (α=5%) inhibition of NSPs.
We confirmed the close functional relationship between the human and pig PMN proteases by testing synthetic or recombinant peptide inhibitors of human NSPs. The HNE-specific inhibitor EPI-hNE4 [32] inhibited cleavage of the NE substrate by the pig neutrophil supernatant (Figure 3C). The PEM cyclic peptide HNE inhibitor P0005259 developed by Polyphor [20] inhibited the cleavage of the NE substrate better than that of the Pr3 substrate (Figure 3C). Incubating the supernatant of pig neutrophils with the specific azapeptide inhibitor of human Pr3 azapro-3 [21] resulted in no cleavage of the Pr3 substrate, whereas SLPI inhibited cleavage of the NE and cat G substrates, but not that of the Pr3 substrate, as expected from the known specificity of this inhibitor for human proteases (Figure 3C) [8]. All of these inhibitors had the same specificities for human and pig NSPs.
Binding of active NSPs to NETs
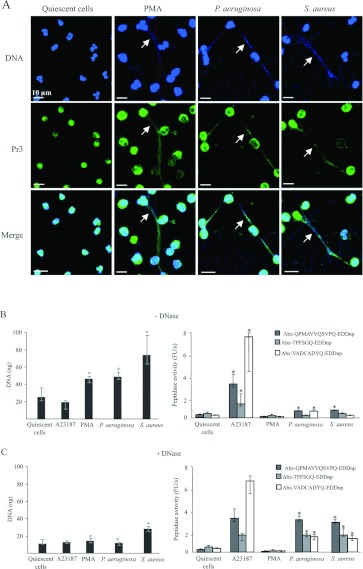
We used PMA or the opportunistic bacteria that infect CF patients, S. aureus and P. aeruginosa [33], to induce purified pig PMNs to secrete NETs, as has been done for human PMNs [9,29]. As expected, our SEM studies showed that PMNs activated with PMA or bacteria secreted NET-like fibrous structures (Figures 4B–4D). These filamentous structures completely disappeared when treated with DNase (Figures 4F–4H), confirming that their backbone was largely made of DNA. SEM also showed that both S. aureus and P. aeruginosa became trapped in the extracellular fibres they had induced (Figures 4C, 4D, 4I and 4J). We then examined the distribution of extracellular proteases in suspensions of NET-producing neutrophils by confocal microscopy using the same antibodies as before. Immunostaining detected proteases at the surface of PMNs activated with PMA or bacteria, and it co-localized with DNA. Figure 5(A) shows a representative immunostain obtained with anti-Pr3 antibodies, but the same result was obtained with all three NSPs antibodies. Figure 5(A) also shows that the nuclei of PMNs activated with PMA and bacteria have lost the polylobular structure that is a feature of both A23187-treated and quiescent PMNs (Figures 2A and 5A).
Figure 4. Secretion of NETs by porcine blood neutrophils in response to PMA and bacteria.
SEM of quiescent porcine neutrophils (A) and of neutrophils activated with PMA (B), S. aureus (C) and P. aeruginosa (D), before (A–D) and after (E–H) DNase treatment. Higher magnification images show the trapping of P. aeruginosa (I) and S. aureus (J) in the fibres of chromatin.
Figure 5. Characterization of NSPs in porcine NET suspensions.
Confocal microscopy showing DNA (blue) and Pr3 (green) in suspensions of PMA- and bacterium-triggered neutrophils (A). Arrows denote the co-localization of Pr3 and chromatin of NETs. (B and C) Quantification of DNA and of proteolytic activities in suspensions of PMA-and bacterium-activated cells before (B) and after (C) DNase treatment (median±interquartiles, n=4). * indicate significant (α=5%) decreases in DNA or increases in protease activity induced by DNase.
Induction of NET secretion by bacteria produced approximately 50 ng of extracellular DNA per 3×105 pig PMNs (Figure 5B), comparable with the amount produced by human neutrophils triggered in the same way (results not shown). This clearly differs from the results obtained with the calcium ionophore A23187, which did not induce the release of any NETs (Figures 2A and 5A). The peptidase activities of all three proteases were only slightly increased in the suspensions of NET-containing pig PMNs (Figure 5B). Treatment of bacterium-activated cells with DNase increased their activities dramatically, indicating that NETs strongly impair access to the active sites of proteases (Figure 5C). However, DNase treatment of PMA-activated cells produced no increase in protease activity (Figure 5C).
DISCUSSION
A major feature of human neutrophil inflammatory lung diseases is the secretion by activated neutrophils of large amounts of proteases whose activities are no longer controlled by endogenous protease inhibitors and thus contribute to the chronicity of the inflammation [5–7]. The NSPs secreted into the extracellular space become distributed between a soluble fraction of free proteases and an insoluble fraction of membrane- and NET-bound proteases [3,9,31,34–37]. This distribution of NSPs in the extracellular environment may compromise their activity and regulation. We have previously shown that natural inhibitors of human NSPs control the activities of membrane-bound proteases secreted by activated PMNs as well as those of soluble proteases [31,36]. However, their inhibition may be strongly impaired when they are bound to anionic macromolecules in lung secretions, and especially to the DNA of NETs [9,38–41]. However, our understanding of how NETs contribute to the development of CF-associated proteolytic lesions and the decline of lung function in CF has been hampered, until recently, by the lack of an animal model that reproduces all of the features of the human disease. CFTR−/− mice do not develop the main features of human CF airways [13,42] and the substrate specificity of their NSPs differs, in part, from that of their human homologues [26]. This means that they cannot be efficiently targeted by therapeutic anti-inflammatory agents raised to control the activities of human NSPs.
CFTR−/− pigs have been recently obtained that develop all of the main features of human CF, especially in the lungs [17]. The anatomy of the pig lung is also closer to that of humans than is the lung of rodents [18]. This could make the pig an appropriate model for testing drugs raised to combat the chronic inflammation that occurs in CF and possibly other lung inflammatory or infectious diseases. However, it is essential to show that the NSPs of the pig model have physicochemical properties and substrate specificities similar to those of their human counterparts. We have shown that pig neutrophils release NETs in response to P. aeruginosa and S. aureus in the same way as human neutrophils, and that pig NSPs are associated with these NETs. Pig NSPs are recognized by anti-human NSP antibodies developed against linear surface peptides, the sequences of which are largely conserved between the two species. More importantly, the selective FRET substrates of human NSPs are also hydrolysed by porcine NSPs, indicating that the proteases of the two species are functionally similar. Thus therapeutic inhibitors developed to target the active sites of human NSPs may be tested in the pig model. Human α1-Pi inhibits both pig elastase and pig Pr3, whereas human ACT inhibits pig cat G. The low-molecular-mass human elastase inhibitors EPI-hNE4 and cyclic PEM P0005259 also inhibit pig elastase. The human Pr3 azapeptide inhibitor azapro-3 [21] selectively targets pig Pr3 in the supernatant of an activated pig neutrophil suspension. Conversely, pig serpin B1 [LNPI (leucocyte neutral proteinase inhibitor)] irreversibly inhibits both porcine and human elastase [43]. Nevertheless, extrapolation of results obtained with the pig model to humans must be done with caution because porcine PMNs are less sensitive to activation with A23187 or PMA than are human PMNs, and the PMNs of different breeds of pig might have different features [44]. However, the overall properties of pig neutrophil proteases are similar enough to those of their human homologues for this species to be a pertinent model for testing drugs targeting NSP active sites. This indicates that the transgenic CFTR−/− pig [15,16] is suitable for evaluating new anti-protease strategies aimed at controlling the lung damage that occurs in CF and related neutrophil-dependent lung inflammatory diseases.
AUTHOR CONTRIBUTION
Sylvie Attucci and François Meurens conceived the whole research project. Sylvie Attucci conceived and designed the present study. The experiments were conducted by Déborah Bréa, Alice Dubois, Julien Gaillard, Claire Chevaleyre and Marie-Lise Jourdan. All authors were involved in the data analysis. Sylvie Attucci, Déborah Bréa, Francis Gauthier and François Meurens wrote the majority of the paper.
ACKNOWLEDGEMENTS
We thank Françoise Mangin and Sandrine Melo for collecting and transporting the pig blood. We thank the UMR Physiologie de la Reproduction et des comportements and the staff of the Unité expérimentale Pluri-espèces Animales de l’Orfrasière (INRA of Tours) for help with animal housing. We also thank the Laboratoire de Bactériologie-virologie, CHU de Tours for providing the bacterial strains. We thank Dr E. Chevalier (Polyphor Ltd, Allschwil, Switzerland) for providing P0005259, Dr B. Korkmaz (INSERM U1100, Tours) for providing azapro-3, Daniel Bourry for help in preparing the Figures, and Owen Parkes and Reuben Ramphal for editing the text before submission.
FUNDING
This work was supported by Vaincre la Mucoviscidose [grant numbers RC0704 and RCB0901] and the Institut National de la Recherche Agronomique (INRA). D.B. was funded by Vaincre La Mucoviscidose.
References
- 1.Pham C. T. Neutrophil serine proteases fine-tune the inflammatory response. Int. J. Biochem. Cell Biol. 2008;40:1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal A. W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Papayannopoulos V., Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. doi: 10.1016/j.it.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Stockley R. A. Neutrophils and protease/antiprotease imbalance. Am. J. Respir. Crit. Care Med. 1999;160:S49–S52. doi: 10.1164/ajrccm.160.supplement_1.13. [DOI] [PubMed] [Google Scholar]
- 6.Griese M., Kappler M., Gaggar A., Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur. Respir. J. 2008;32:783–795. doi: 10.1183/09031936.00146807. [DOI] [PubMed] [Google Scholar]
- 7.Greene C. M., McElvaney N. G. Proteases and antiproteases in chronic neutrophilic lung disease: relevance to drug discovery. Br. J. Pharmacol. 2009;158:1048–1058. doi: 10.1111/j.1476-5381.2009.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korkmaz B., Horwitz M. S., Jenne D. E., Gauthier F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010;62:726–759. doi: 10.1124/pr.110.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois A. V., Gauthier A., Brea D., Varaigne F., Diot P., Gauthier F., Attucci S. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am. J. Respir. Cell Mol. Biol. 2012;47:80–86. doi: 10.1165/rcmb.2011-0380OC. [DOI] [PubMed] [Google Scholar]
- 10.Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., Meller S., Chamilos G., Sebasigari R., Riccieri V., et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanueva E., Yalavarthi S., Berthier C. C., Hodgin J. B., Khandpur R., Lin A. M., Rubin C. J., Zhao W., Olsen S. H., Klinker M., et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholte B. J., Colledge W. H., Wilke M., de Jonge H. Cellular and animal models of cysticfibrosis, tools for drug discovery. Drug Discovery Today. 2006;3:251–259. [Google Scholar]
- 13.Guilbault C., Saeed Z., Downey G. P., Radzioch D. Cystic fibrosis mouse models. Am. J. Respir. Cell Mol. Biol. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 14.Sun X., Sui H., Fisher J. T., Yan Z., Liu X., Cho H. J., Joo N. S., Zhang Y., Zhou W., Yi Y., et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J. Clin. Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers C. S., Stoltz D. A., Meyerholz D. K., Ostedgaard L. S., Rokhlina T., Taft P. J., Rogan M. P., Pezzulo A. A., Karp P. H., Itani O. A., et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klymiuk N., Mundhenk L., Kraehe K., Wuensch A., Plog S., Emrich D., Langenmayer M. C., Stehr M., Holzinger A., Kroner C., et al. Sequential targeting of CFTR by BAC vectors generates a novel pig model of cystic fibrosis. J. Mol. Med. (Berl) 2011;90:597–608. doi: 10.1007/s00109-011-0839-y. [DOI] [PubMed] [Google Scholar]
- 17.Stoltz D. A., Meyerholz D. K., Pezzulo A. A., Ramachandran S., Rogan M. P., Davis G. J., Hanfland R. A., Wohlford-Lenane C., Dohrn C. L., Bartlett J. A., et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci. Transl. Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers C. S., Abraham W. M., Brogden K. A., Engelhardt J. F., Fisher J. T., McCray P. B., Jr, McLennan G., Meyerholz D. K., Namati E., Ostedgaard L. S., et al. The porcine lung as a potential model for cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meurens F., Summerfield A., Nauwynck H., Saif L., Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. 2011;20:50–57. doi: 10.1016/j.tim.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson J. A., Demarco S., Gombert F., Moehle K., Obrecht D. The design, structures and therapeutic potential of protein epitope mimetics. Drug Discovery Today. 2008;13:944–951. doi: 10.1016/j.drudis.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Epinette C., Croix C., Jaquillard L., Marchand-Adam S., Kellenberger C., Lalmanach G., Cadene M., Viaud-Massuard M. C., Gauthier F., Korkmaz B. A selective reversible azapeptide inhibitor of human neutrophil proteinase 3 derived from a high affinity FRET substrate. Biochem. Pharmacol. 2012;83:788–796. doi: 10.1016/j.bcp.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Piriou-Guzylack L., Salmon H. Membrane markers of the immune cells in swine: an update. Vet. Res. 2008;39:54. doi: 10.1051/vetres:2008030. [DOI] [PubMed] [Google Scholar]
- 23.Korkmaz B., Attucci S., Juliano M. A., Kalupov T., Jourdan M. L., Juliano L., Gauthier F. Measuring elastase, proteinase 3 and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrates. Nat. Protoc. 2008;3:991–1000. doi: 10.1038/nprot.2008.63. [DOI] [PubMed] [Google Scholar]
- 24.Attucci S., Korkmaz B., Juliano L., Hazouard E., Girardin C., Brillard-Bourdet M., Rehault S., Anthonioz P., Gauthier F. Measurement of free and membrane-bound cathepsin G in human neutrophils using new sensitive fluorogenic substrates. Biochem. J. 2002;366:965–970. doi: 10.1042/BJ20020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korkmaz B., Attucci S., Moreau T., Godat E., Juliano L., Gauthier F. Design and use of highly specific substrates of neutrophil elastase and proteinase 3. Am. J. Respir. Cell Mol. Biol. 2004;30:801–807. doi: 10.1165/rcmb.2003-0139OC. [DOI] [PubMed] [Google Scholar]
- 26.Kalupov T., Brillard-Bourdet M., Dade S., Serrano H., Wartelle J., Guyot N., Juliano L., Moreau T., Belaaouaj A., Gauthier F. Structural characterization of mouse neutrophil serine proteases and identification of their substrate specificities: relevance to mouse models of human inflammatory diseases. J. Biol. Chem. 2009;284:34084–34091. doi: 10.1074/jbc.M109.042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naudin C., Joulin-Giet A., Couetdic G., Plesiat P., Szymanska A., Gorna E., Gauthier F., Kasprzykowski F., Lecaille F., Lalmanach G. Human cysteine cathepsins are not reliable markers of infection by Pseudomonas aeruginosa in cystic fibrosis. PLoS ONE. 2011;6:e25577. doi: 10.1371/journal.pone.0025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korkmaz B., Moreau T., Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manual M. V. Reference guides. In: Aiello S. E., editor. Merck Veterinary Manual. Whitehouse Station: Merck & Co.; 1998. pp. 2190–2191. [Google Scholar]
- 31.Korkmaz B., Attucci S., Jourdan M. L., Juliano L., Gauthier F. Inhibition of neutrophil elastase by α1-protease inhibitor at the surface of human polymorphonuclear neutrophils. J. Immunol. 2005;175:3329–3338. doi: 10.4049/jimmunol.175.5.3329. [DOI] [PubMed] [Google Scholar]
- 32.Attucci S., Gauthier A., Korkmaz B., Delepine P., Martino M. F., Saudubray F., Diot P., Gauthier F. EPI-hNE4, a proteolysis-resistant inhibitor of human neutrophil elastase and potential anti-inflammatory drug for treating cystic fibrosis. J. Pharmacol. Exp. Ther. 2006;318:803–809. doi: 10.1124/jpet.106.103440. [DOI] [PubMed] [Google Scholar]
- 33.Hauser A. R., Jain M., Bar-Meir M., McColley S. A. Clinical significance of microbial infection and adaptation in cystic fibrosis. Clin. Microbiol. Rev. 2010;24:29–70. doi: 10.1128/CMR.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell E. J., Campbell M. A., Owen C. A. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J. Immunol. 2000;165:3366–3374. doi: 10.4049/jimmunol.165.6.3366. [DOI] [PubMed] [Google Scholar]
- 35.Campbell E. J., Owen C. A. The sulfate groups of chondroitin sulfate- and heparan sulfate-containing proteoglycans in neutrophil plasma membranes are novel binding sites for human leukocyte elastase and cathepsin G. J. Biol. Chem. 2007;282:14645–14654. doi: 10.1074/jbc.M608346200. [DOI] [PubMed] [Google Scholar]
- 36.Korkmaz B., Jaillet J., Jourdan M. L., Gauthier A., Gauthier F., Attucci S. Catalytic activity and inhibition of wegener antigen proteinase 3 on the cell surface of human polymorphonuclear neutrophils. J. Biol. Chem. 2009;284:19896–19902. doi: 10.1074/jbc.M901471200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessenbrock K., Krumbholz M., Schonermarck U., Back W., Gross W. L., Werb Z., Grone H. J., Brinkmann V., Jenne D. E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying Q. L., Kemme M., Simon S. R. Alginate, the slime exopolysaccharide of Pseudomonas aeruginosa, binds human leukocyte elastase, retards inhibition by α1-proteinase inhibitor, and accelerates inhibition by secretory leukoprotease inhibitor. Am. J. Respir. Cell Mol. Biol. 1996;15:283–291. doi: 10.1165/ajrcmb.15.2.8703486. [DOI] [PubMed] [Google Scholar]
- 39.Kainulainen V., Wang H., Schick C., Bernfield M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J. Biol. Chem. 1998;273:11563–11569. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- 40.Belorgey D., Bieth J. G. Effect of polynucleotides on the inhibition of neutrophil elastase by mucus proteinase inhibitor and α1-proteinase inhibitor. Biochemistry. 1998;37:16416–16422. doi: 10.1021/bi981536o. [DOI] [PubMed] [Google Scholar]
- 41.Duranton J., Boudier C., Belorgey D., Mellet P., Bieth J. G. DNA strongly impairs the inhibition of cathepsin G by α1-antichymotrypsin and α1-proteinase inhibitor. J. Biol. Chem. 2000;275:3787–3792. doi: 10.1074/jbc.275.6.3787. [DOI] [PubMed] [Google Scholar]
- 42.Elferink R. O., Beuers U. Are pigs more human than mice? J. Hepatol. 2009;50:838–841. doi: 10.1016/j.jhep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Teschauer W. F., Mentele R., Sommerhoff C. P. Primary structure of a porcine leukocyte serpin. Eur. J. Biochem. 1993;217:519–526. doi: 10.1111/j.1432-1033.1993.tb18272.x. [DOI] [PubMed] [Google Scholar]
- 44.Clapperton M., Bishop S. C., Glass E. J. Innate immune traits differ between Meishan and Large White pigs. Vet. Immunol. Immunopathol. 2005;104:131–144. doi: 10.1016/j.vetimm.2004.10.009. [DOI] [PubMed] [Google Scholar]