Abstract
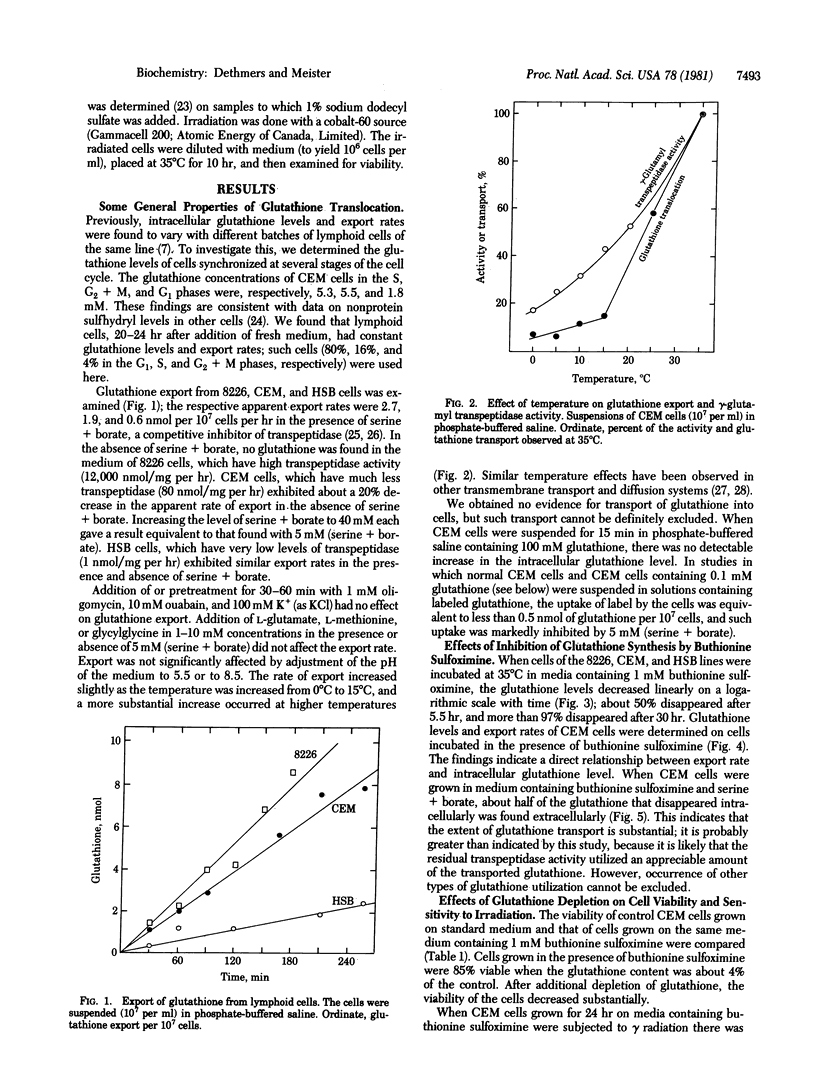
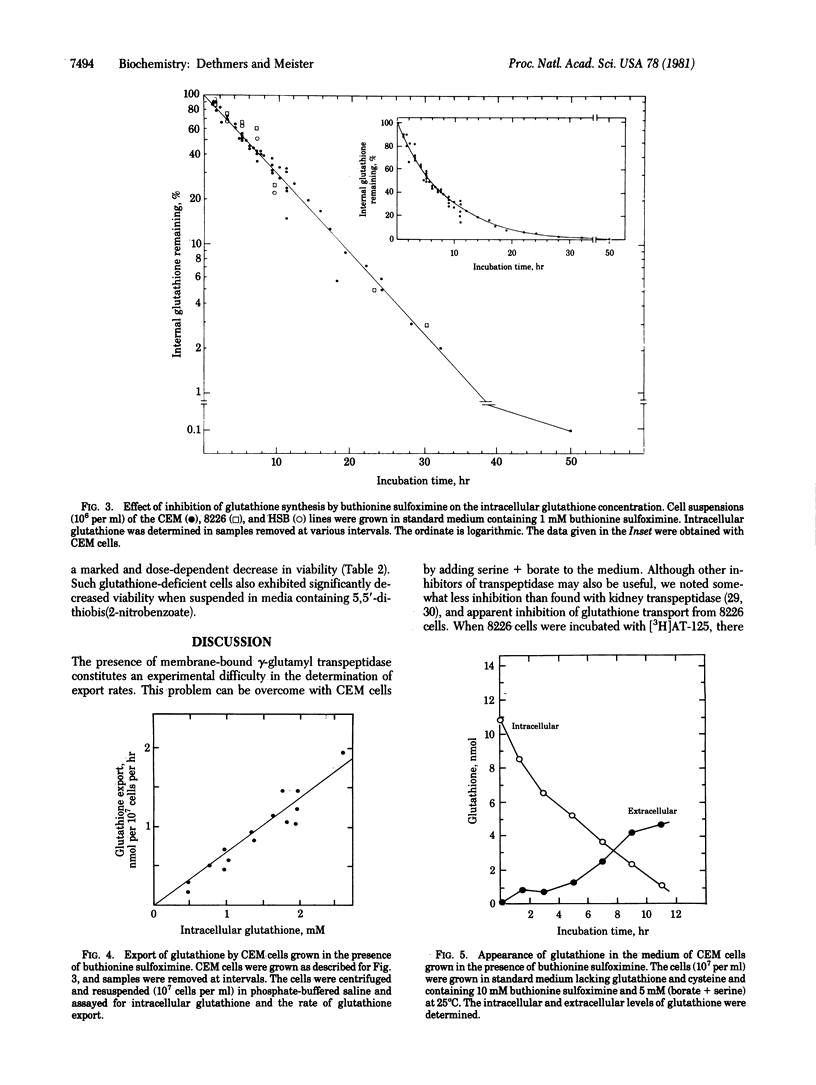
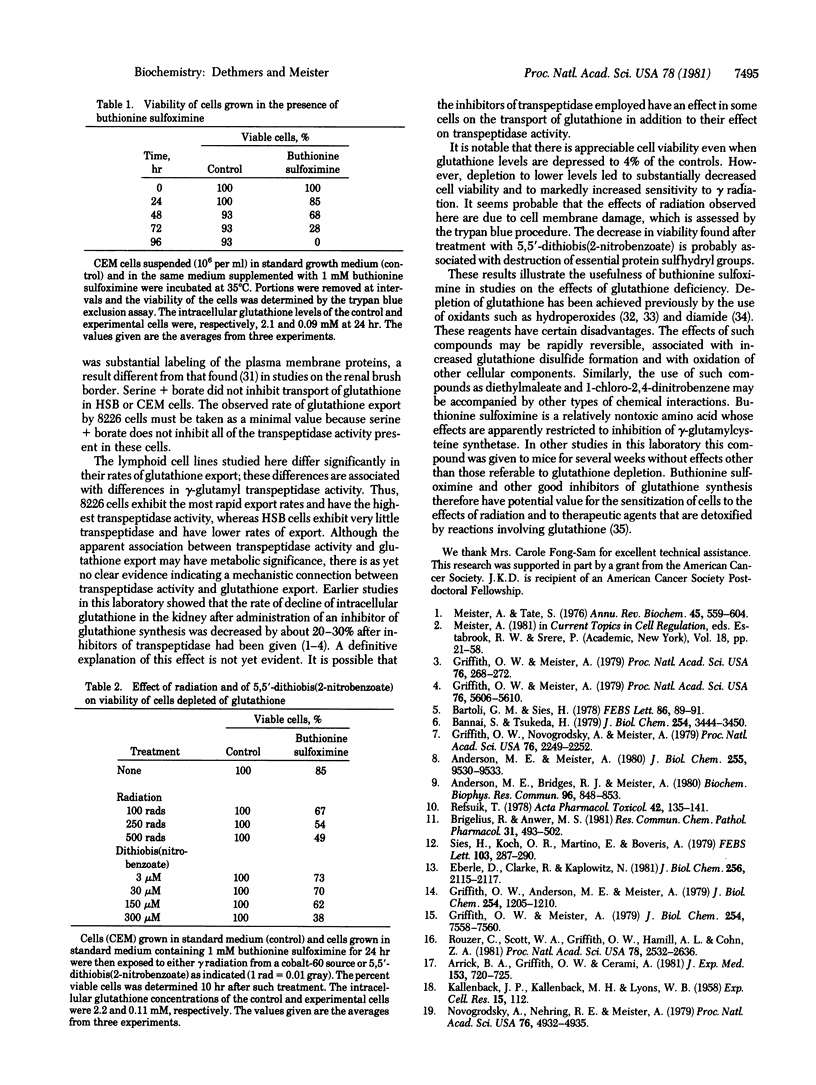
Glutathione (in the form of GSH) is transported out of cultured human lymphoid cells at rates proportional to the intracellular glutathione levels. Inhibition of glutathione synthesis by buthionine sulfoximine, a potent selective inhibitor of gamma-glutamylcysteine synthetase, leads to exponential decrease in intracellular glutathione, a large fraction of which appears extracellularly, indicating that glutathione turnover is associated with its export. Although cells with 0.09 mM glutathione (4% of controls) were 85% viable, further decrease was associated with marked loss of viability. Cells with 4-5% of control glutathione levels were much more sensitive than control cells to the effects of gamma radiation and of 5,5'-dithiobis(2-nitrobenzoate). Depletion of glutathione by use of buthionine sulfoximine has advantages over other reagents (such as diamide, other oxidizing agents, and diethylmaleate, which affect other cellular components and may increase glutathione disulfide levels) and therefore has potential usefulness in sensitizing cells to the effects of radiation and to therapeutic agents that are detoxified by reactions involving glutathione.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Bridges R. J., Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980 Sep 30;96(2):848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Dynamic state of glutathione in blood plasma. J Biol Chem. 1980 Oct 25;255(20):9530–9533. [PubMed] [Google Scholar]
- Arrick B. A., Griffith O. W., Cerami A. Inhibition of glutathione synthesis as a chemotherapeutic strategy for trypanosomiasis. J Exp Med. 1981 Mar 1;153(3):720–725. doi: 10.1084/jem.153.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai S., Tsukeda H. The export of glutathione from human diploid cells in culture. J Biol Chem. 1979 May 10;254(9):3444–3450. [PubMed] [Google Scholar]
- Bartoli G. M., Sies H. Reduced and oxidized glutathione efflux from liver. FEBS Lett. 1978 Feb 1;86(1):89–91. doi: 10.1016/0014-5793(78)80105-7. [DOI] [PubMed] [Google Scholar]
- Brigelius R., Anwer M. S. Increased biliary GSSG-secretion and loss of hepatic glutathione in isolated perfused rat liver after paraquat treatment. Res Commun Chem Pathol Pharmacol. 1981 Mar;31(3):493–502. [PubMed] [Google Scholar]
- Eberle D., Clarke R., Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem. 1981 Mar 10;256(5):2115–2117. [PubMed] [Google Scholar]
- Gardell S. J., Tate S. S. Affinity labeling of gamma-glutamyl transpeptidase by glutamine antagonists. Effects of the gamma-glutamyl transferase and proteinase activities. FEBS Lett. 1980 Dec 29;122(2):171–174. doi: 10.1016/0014-5793(80)80430-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Novogrodsky A., Meister A. Translocation of glutathione from lymphoid cells that have markedly different gamma-glutamyl transpeptidase activities. Proc Natl Acad Sci U S A. 1979 May;76(5):2249–2252. doi: 10.1073/pnas.76.5.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. W., Patt H. M. Non-protein sulfhydryl content and cell-cycle dynamics of Ehrlich ascites tumor. Exp Cell Res. 1969 Jul;56(1):134–141. doi: 10.1016/0014-4827(69)90406-6. [DOI] [PubMed] [Google Scholar]
- KALTENBACH J. P., KALTENBACH M. H., LYONS W. B. Nigrosin as a dye for differentiating live and dead ascites cells. Exp Cell Res. 1958 Aug;15(1):112–117. doi: 10.1016/0014-4827(58)90067-3. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- Kozak E. M., Tate S. S. Interaction of the antitumor drug, L-(alpha S, 5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid (AT-125) with renal brush border membranes. Specific labeling of gamma-glutamyl transpeptidase. FEBS Lett. 1980 Dec 29;122(2):175–178. doi: 10.1016/0014-5793(80)80431-5. [DOI] [PubMed] [Google Scholar]
- Kwok S. Y., Litwin S. D. Immunoglobulin expression of cells from human lymphoblastoid lines. III. Cell cycle phase and surface immunoglobulin expression. Cell Immunol. 1976 Aug;25(2):256–265. doi: 10.1016/0008-8749(76)90116-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meister A., Griffith O. W. Effects of methionine sulfoximine analogs on the synthesis of glutamine and glutathione: possible chemotherapeutic implications. Cancer Treat Rep. 1979 Jun;63(6):1115–1121. [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Nehring R. E., Jr, Meister A. Inhibition of amino acid transport into lymphoid cells by the glutamine analog L-2-amino-4-oxo-5-chloropentanoate. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4932–4935. doi: 10.1073/pnas.76.10.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL J. P., BALL E. G. The reaction of glutathione with amino acids and related compounds as catalyzed by gamma-glutamyl transpeptidase. J Biol Chem. 1959 Mar;234(3):577–582. [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Griffith O. W., Hamill A. L., Cohn Z. A. Depletion of glutathione selectively inhibits synthesis of leukotriene C by macrophages. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2532–2536. doi: 10.1073/pnas.78.4.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H., Koch O. R., Martino E., Boveris A. Increased biliary glutathione disulfide release in chronically ethanol-treated rats. FEBS Lett. 1979 Jul 15;103(2):287–290. doi: 10.1016/0014-5793(79)81346-0. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Serine-borate complex as a transition-state inhibitor of gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4806–4809. doi: 10.1073/pnas.75.10.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]



