Abstract
Pulsatile gonadotropin-releasing hormone (GnRH) release is critical for the central regulation of fertility. There is no method allowing real-time GnRH detection in brain slices. We developed fast-scan cyclic voltammetry (FSCV) using carbon-fiber microelectrodes (CFME) to detect GnRH release and validated it using a biologically relevant system. FSCV parameters (holding potential, switching potential, and scan rate) were determined for stable GnRH detection in vitro, then optimized for GnRH detection in mouse brain slices. Placement of CFMEs in the median eminence (ME) near GnRH terminals allowed detection of both KCl-evoked and spontaneous GnRH release. GnRH release was also detected from GnRH fibers passing near GnRH soma and near fiber–fiber appositions in the preoptic area. No GnRH signal was detected from CFMEs in the ME of hpg mice, which lack GnRH, or in regions not containing GnRH neurons in wild-type mice; application of exogenous GnRH produced a signal similar to that observed for spontaneous/evoked endogenous GnRH release. Using an established mouse model that produces diurnal variations in GnRH neuron activity, we demonstrated corresponding changes in spontaneous GnRH release in the median eminence. These results validate FSCV to detect GnRH in brain slices and provide new information on the sites and amounts of GnRH release, providing insight into its neuromodulatory functions.
Introduction
Pulsatile gonadotropin-releasing hormone (GnRH) release is essential for fertility (Belchetz et al., 1978). GnRH is released from terminals in the median eminence (ME) near pituitary portal vessels. Modulation of GnRH pulse frequency is required for the differential release of luteinizing hormone (LH) and follicle-stimulating hormone from the pituitary that is crucial for driving female reproductive cycles (Wildt et al., 1981). Abnormal GnRH-pulse patterns can cause infertility (McCartney et al., 2002). In addition to release at the ME, GnRH may act as a neuromodulator in local circuits (DePaolo et al., 1987; Xu et al., 2004, 2008; Chen and Moenter, 2009; Han et al., 2010), but release has not been demonstrated from other brain regions in situ.
Recent work has examined the pattern of action-potential firing from GnRH neurons in brain slices and primary cultures (Kuehl-Kovarik et al., 2002; Nunemaker et al., 2003; Abe and Terasawa, 2005; Christian et al., 2005; Pielecka and Moenter, 2006; Pielecka et al., 2006; Lee et al., 2010). There is no empirical link, however, between the firing pattern of GnRH neurons and the pattern of release produced. This is attributable to a lack of a method that allows for GnRH-release detection in preparations used for electrophysiology.
GnRH contains electrochemically active amino acids, in particular tryptophan and tyrosine. Electochemically active species can be detected and identified using fast scan cyclic voltammetry (FSCV) based on their oxidation and reduction properties. This method has been used to detect small molecule neurotransmitters, such as monoamines (Cahill et al., 1996). Our objectives were to adapt FSCV to enable GnRH detection in brain slices, to determine where release occurs, and to test the ability of FSCV to detect biologically relevant changes in GnRH release.
Materials and Methods
Carbon-fiber microelectrodes fabrication.
Carbon-fiber microelectrodes (CFMEs) were fabricated as described previously (Mundroff and Wightman, 2002). Carbon fiber (T-650; a gift from Cytec Engineering Materials) was aspirated into a capillary glass tube (1.65/1.1 mm OD/ID; World Precision Instruments), which was pulled (PE-21; Narishige; or P-97; Sutter Instruments). The fiber was cut to 40–60 μm and sealed to the glass using Epoxy Resin 858 (Miller-Stephenson) and 14% (w/w) m-phenylenediamine (Fluka). Electrodes were incubated overnight at room temperature, baked for 2 h (100°C) and then overnight (150°C). Before experiments, electrodes were washed ≤10 min in isopropanol and then filled with 1 m KCl.
FSCV in vitro.
Chemicals were from Sigma unless otherwise noted. Data were collected using a custom-modified Dagan ChemClamp potentiostat and Tar Heel software (a gift from Mark Wightman, University of North Carolina at Chapel Hill, Chapel Hill, NC) or an extended range (±2 V) EPC10 patch-clamp with PatchMaster (HEKA Elektronic) with Demon Voltammetry (Wake Forest University, Winston-Salem, NC). In vitro experiments were done in pH 7.4 Tris buffer containing the following (in mm): 15 tris(hydroxymethyl)aminomethane, 140 NaCl, 3.25 KCl, 1.2 CaCl2, 1.25 NaH2PO4, 1.2 MgCl2, and 2.0 Na2SO, as described previously (Strand and Venton, 2008). CFMEs were calibrated in vitro using a flow-injection apparatus. Injections (3 s) of compounds of interest (1 μm tryptophan or 5 μm GnRH in Tris buffer) were used to mimic fast concentration changes expected in biological systems. For both tryptophan and GnRH, the waveform parameters (holding and switching potentials, and scan rate) were optimized toward detection of the single, specific oxidation peak.
Animals.
Transgenic mice expressing green fluorescent protein (GFP) under the control of the GnRH promoter (GnRH-GFP) (Suter et al., 2000) were used for spontaneous and evoked release studies. To control for specificity, hypogonadal (hpg) GnRH-GFP-hpg mice (hpg-GnRH-GFP; a gift from Drs. Ursula Kaiser and John Gill, Brigham and Women's Hospital, Harvard Medical School, and Harvard Reproductive Endocrine Sciences Center, Boston, MA) (Gill et al., 2008); these mice lack GnRH peptide (Mason et al., 1986). Tac2-GFP transgenic mice (015495-UCD/ STOCK Tg (Tac2-EGFP)381Gsat; Mouse Mutant Regional Resource Center) were used as a positive control for detecting evoked GnRH release without GFP identification of terminals.
Mice were housed under a 14:10 h light:dark photoperiod with Harlan 2916 chow and water available ad libitum. Adult females (42–60 d) were used. Ovariectomy (OVX) was performed under isoflurane (Burns Veterinary Supply) anesthesia. Bupivicaine (0.25%, 7 μl per surgical site; Abbott Labs) was applied to surgery sites to reduce postoperative pain and distress. During OVX, mice received subcutaneous SILASTIC (Dow-Corning) implants containing 0.625 μg of 17β-estradiol (E) in sesame oil with or without progesterone (P) implants (2.5 mg; Innovative Research of America). OVX+E mice, which generated daily LH surges (Christian et al., 2005), were used 2–4 d postsurgery; OVX+E+P mice were used 5–10 d postsurgery. All procedures were approved by the University of Virginia Animal Care and Use Committee and the University of Michigan University Committee on the Use and Care of Animals.
FSCV in brain slices.
Brain slices were prepared as described previously (Nunemaker et al., 2002; Chu and Moenter, 2005). All buffers were bubbled with 95%O2/5%CO2 ≥15 min before usage. Sagittal (OVX+E+P and OVX+E, morning vs afternoon) or coronal (hpg and Tac2 mice) 300 μm brain slices were cut using a Vibratome 3000 (Ted Pella) in ice-cold sucrose saline containing the following (in mm): 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 glucose, 1.25 NaH2PO4, 1.2 MgSO4, and 3.8 MgCl2. Slices were incubated 30 min at 30–32°C in a 1:1 mixture of sucrose saline and artificial CSF (ACSF) containing the following (in mm): 125 NaCl, 3.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 10 d-glucose, pH 7.4, then transferred to 100% ACSF and incubated 30–300 min at room temperature before study.
For GnRH detection, potential was continuously scanned from 0.5 to 1.45 V at 400 V/s every 100 ms (Fig. 1A). CFMEs were stabilized for 15 min before collecting data. Signals arising from spontaneous release were recorded for 20 min either in the ME or preoptic area (POA), and then secretion was evoked by a 20 mm KCl ACSF. For biological validation of hypothesized changes in GnRH release with time of day in OVX+E mice, GnRH release was monitored continuously for 1 h with CFMEs placed either near GnRH neuron terminals in the ME or in the POA near fiber-soma appositions.
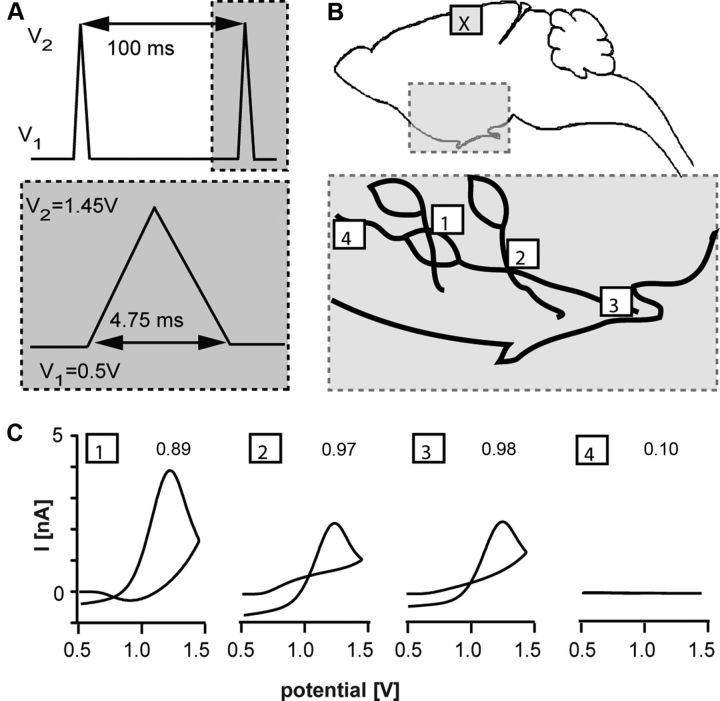
Figure 1.
A, FSCV protocol used to detect GnRH. V1 is holding potential, V2 is switching potential. Gray area is expanded below. B, Illustration of recording sites. Top, Sagittal section; gray area is expanded below. X indicates location of CFMEs for exogenous GnRH application. Bottom, Sites of GnRH release: 1, GnRH soma-fiber contact (POA); 2, GnRH fiber-fiber contact (POA); 3, GnRH terminals in the ME; 4, single GnRH fiber (POA). C, Example CVs taken at peak current of GnRH signal detected in locations indicated in B, R2 is in top right corner.
CFMEs were placed in the cortex or hippocampus, away from GnRH soma/processes, to perform controls. To document the GnRH-generated FSCV signal in the brain slice environment, 5 μm GnRH was applied near CFMEs. To control for specificity, kisspeptin (5 μm), which may be released in the ME, was applied near CFMEs in the cortex/hippocampus. In addition, CFMEs were placed in the ME of hpg-GnRH-GFP mice and Tac2-GFP mice, and KCl-evoked signal examined. Following all recordings, CFMEs were calibrated in 5 μm GnRH in vitro.
Data analysis.
Data were analyzed using Tar Heel or Demon software (Wake Forest University Health Sciences) as described previously (Mundroff and Wightman, 2002). Cyclic voltammograms (CVs) were background-subtracted by averaging 10 background scans. To verify the identity of a spontaneous release peak as GnRH, five control CVs collected after GnRH was injected into a slice were averaged. Each putative GnRH CV was correlated with this average and was considered to be GnRH if R2 ≥ 0.8. This threshold was set to allow for some electrode variability. Ninety-six percent of CVs passed this test; those with R2 < 0.8 were always flanked by events with robust correlations. For the daily surge experiment (OVX+E animals), data were binned at 1 min intervals to facilitate evaluation of the pattern of release. Maximum change in GnRH concentration for each 1 min bin was plotted versus time (Fig. 2A). Event duration was the number of consecutive bins in which a change in GnRH was detected; events separated by a single 1 min bin without detection of GnRH were considered to be single events. Changes in GnRH concentration were estimated based on calibration in 5 μm GnRH. Data are expressed as mean ± SEM and analyzed using ANOVA with Bonferroni post hoc test (GraphPad Prism); p < 0.05 was considered significant.
Figure 2.
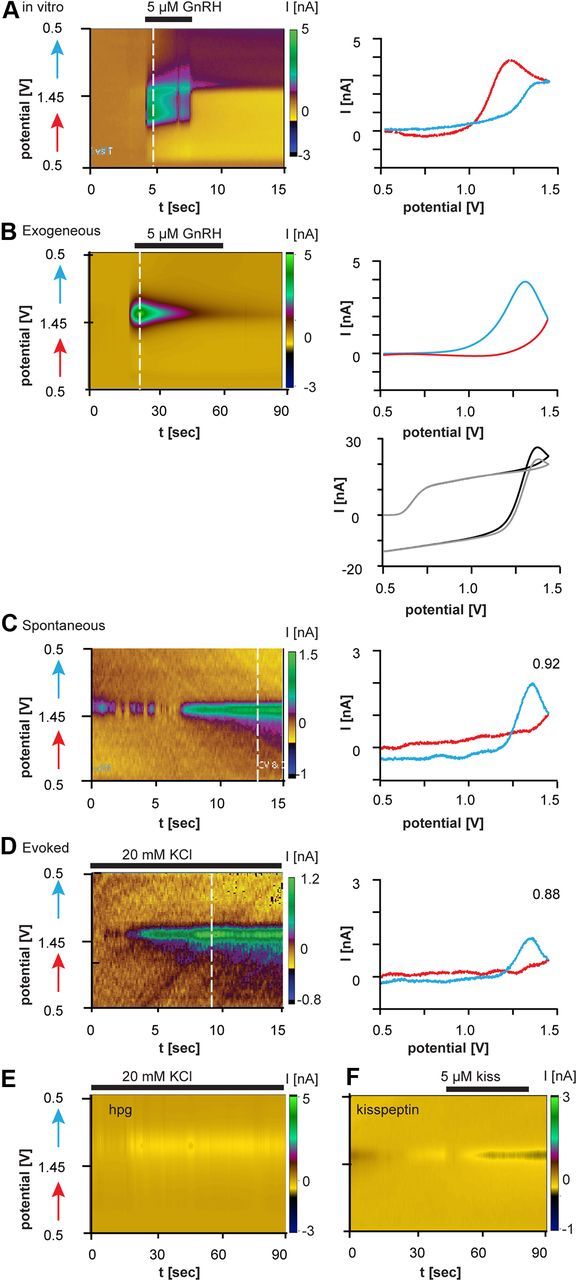
FSCV recordings of evoked and spontaneous GnRH release monitored by CFMEs in brain slices. A, C, D, Recordings from CFMEs in the median eminence. B, F, Recordings from CFMEs in cortex. A–D, Left, Electrochemical current in a color heat plot as a function of voltage and time (left). Red arrows show the forward voltage scan and blue arrows show the reverse scan. Dotted white lines indicate time for which cyclic voltammogram is plotted on the right. A, In vitro GnRH signal. B, Exogenous GnRH applied in cortex generates a similar cyclic voltammogram to endogenous GnRH (C, D). B, Bottom right, Raw background voltammograms in the presence (black trace) and absence (gray) of GnRH. C, Spontaneous GnRH release in GnRH–GFP mouse. D, KCl-evoked GnRH release in GnRH–GFP mouse. For C and D, R2 is in top right corner. E, No GnRH release evoked by KCl in the ME of hpg mice, which lack GnRH. F, Exogenous kisspeptin applied in the cortex does not generate a signal.
Results
Detecting tryptophan and GnRH in vitro
FSCV parameters were optimized for stable in vitro detection of tryptophan (1 μm). Holding potential (potential at the start 0.4–0.6 V), switching potential (potential at the peak 1.1–1.3 V), scan rate (50–500 V/s), and waveforms were varied within the ranges indicated. Tryptophan is known to foul the electrode under some conditions (Paras and Kennedy, 1995), so FSCV parameters were chosen to minimize loss of sensitivity and produce consistent cyclic voltammograms (data not shown). The oxidation peak for 1 μm tryptophan was 0.9 V, whereas the oxidation peak was shifted to higher voltages (∼1.25 V) for 5 μm GnRH (Fig. 2A). This shift in peak may be attributable to slower kinetics of tryptophan oxidation when it is incorporated into the peptide. The peak signal obtained for 5 μm GnRH with the optimized waveform of 0.5 V to 1.45 V at 400 V/s (Fig. 1A, voltage protocol) was 2.5 ± 0.1 nA (n = 5).
FSCV in mouse brain slices
We next recorded from GFP-identified GnRH neurons in brain slices (Suter et al., 2000). Female OVX+E+P mice were used as this treatment produces low endogenous GnRH neuron activity (Pielecka et al., 2006). Because FSCV detects changes in concentration rather than absolute concentration, a low baseline was desired for initial tests. CFMEs were positioned near GFP-identified terminals in the ME. After stabilization, data were collected for 20 min to reveal any spontaneous release, followed by treatment with 20 mm KCl to evoke release. Both spontaneous and evoked GnRH release were detected (Fig. 2C,D; Table 1). The peak oxidation potential was shifted to slightly higher voltages in the brain slice (1.32 ± 0.02 V) than was observed in vitro (1.20 ± 0.05 V). Although the current flow indicates oxidation, the peak occurs during the return of the voltage from switching potential to holding potential. This is likely attributable to slower electron transfer kinetics in electrodes placed in tissue; this was expected as the surrounding milieu for the peptide is different. To test whether changes in milieu between pure buffer and the brain slice would cause a similar shift in cyclic voltammogram, CFMEs were positioned in the cortex or hippocampus and 5 μm GnRH locally applied. The signal obtained (Fig. 2B; Table 1) was similar (R2 > 0.8) to that for spontaneous and evoked release of GnRH detected by CFMEs in the ME (Fig. 2C,D; Table 1). The signal observed for mammalian GnRH differs from that of teleosts (Ishizaki and Oka, 2001), likely due to different materials of the CFME and amino acid sequence.
Table 1.
Summary of control experiments
| Location | Genotype | Treatment | n | nA |
|---|---|---|---|---|
| Cortex/hippocampus | GnRH-GFP | 20 mM KCl | 5 | 0 |
| Cortex/hippocampus | GnRH-GFP | 5 μm GnRH | 10 | 1.6 ± 0.4 |
| Cortex/hippocampus | GnRH-GFP | 5 μm kisspeptin | 3 | 0 |
| Median eminence | hpg | 20 mM KCl | 9 | 0 |
| Median eminence | Tac2-GFP | 20 mM KCl | 7 | 3.3 ± 0.7 |
The presence of tryptophan and tyrosine in many proteins raises an obvious question of specificity. In this regard, tryptophan and tyrosine are hydrophobic, tending to be on the inside of folded proteins (Chothia, 1976; Miller et al., 1987), and thus minimizing interference from large proteins, as these amino acids are inaccessible for oxidation. When tryptophan and tyrosine are accessible as in smaller peptides like GnRH, surrounding amino acids will influence the oxidation profile; thus it should be unique for each substance. We tested specificity in several ways (Table 1; Fig. 2E,F). First, no GnRH signal was observed in brain regions lacking GnRH peptide even in the presence of high potassium to evoke release (data not shown). Second, the small tryptophan-containing peptide kisspeptin-10 (1–5 μm) did not produce a signal similar to that of GnRH (R2 = 0.01; Fig. 2F). Third, no GnRH signal was evoked by high potassium from the ME of hpg mice, which have no detectable GnRH peptide despite a normal distribution of GnRH and other neuroendocrine neurons (Fig. 2E) (Gill et al., 2008). In contrast to the lack of GnRH signal in ME from hpg mice, typical potassium-induced GnRH release was recorded from the ME of control mice, even when CFME placement was guided solely by anatomy (i.e., no GnRH-GFP signal, R2 ≥ 0.8; Table 1). Finally, it should be noted that although voltammetry is used to detect small neurotransmitters such as catecholamines, these neurotransmitters oxidize at much lower voltages, near the holding potential in these experiments (Venton et al., 2002). Thus, no signal would be expected from these substances, as they are already oxidized. Together, these data suggest the signal is specific for GnRH.
Sites of endogenous GnRH release
We next tested sites of GnRH release. CFMEs were positioned: (1) in the POA between GnRH fibers and somata, (2) in the POA where GnRH fibers crossed one another, (3) in the ME among GnRH terminals, and (4) near a single GnRH neuron fiber in the POA (Fig. 1B,C). Release was observed when the electrode was near the intersection of soma and fibers, or between fibers (both ME and POA; Fig. 1B,C), but not near single GnRH fibers even with KCl challenge (Fig. 1B,C). This provides functional evidence of GnRH release at GnRH–GnRH junctions (Witkin and Silverman, 1985; Campbell et al., 2009).
Differential detection of GnRH release in a model exhibiting diurnal changes in GnRH neuron activity
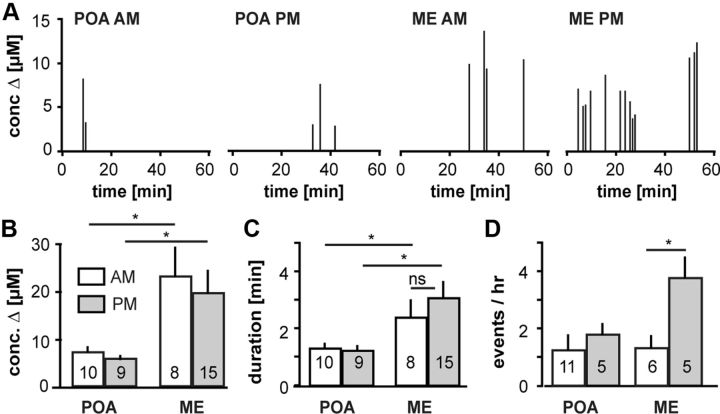
The above correlation analyses revealed that FSCV accurately detects GnRH release in brain slices. We next tested its ability to measure biologically relevant changes using a model in which female mice exhibit estradiol-induced daily changes in GnRH neuron activity that are correlated with circulating LH levels (Christian et al., 2005). This reflects a switch from negative to positive estradiol feedback, and is a critical part of the female reproductive cycle (Döcke and Dörner, 1965; Moenter et al., 1991; Christian and Moenter, 2010). We hypothesized that similar daily changes in GnRH release occur. One-hour FSCV recordings of spontaneous GnRH release were made in the morning (low GnRH neuron activity) and afternoon (high activity) from both soma-fiber appositions in the POA (morning: n = 8, afternoon: n = 5) and ME (morning: n = 6 A.M., afternoon: n = 5 P.M.). No time-of-day differences were observed in the POA for concentration change, release duration, or frequency of release (Fig. 3). Interestingly, three of eight morning POA recordings had no spontaneous GnRH release, but exhibited KCl-evoked release, indicating CFME was placed near a potential release site. The lack of spontaneous release is consistent with reduced activity of GnRH neurons at this time. ME GnRH release was longer in duration (F(41) = 4.292, p < 0.05) and exhibited greater concentration changes (F(41) = 3.943, p < 0.05) than in the POA regardless of time of the day. Further, release events in the ME were more frequent (F(26) = 5.304, p < 0.05) in the afternoon than in the morning. GnRH concentration changes were greater in the ME during both the morning and afteroon, suggesting more release sites than the POA.
Figure 3.
FSCV detects changes in GnRH release between two different biological states. A, Representative examples of the pattern of spontaneous GnRH release detected at CFMEs near GnRH soma/fiber appositions in the POA and fibers in the ME in ovariectomized mice treated with estradiol to induce diurnal changes in GnRH neuron activity (Christian et al., 2005). Data were collected in 1 min bins and each bar shows the maximum concentration in each bin. Multiple spikes in adjacent bin represent one long event. B–D, Mean ± SEM concentration change (B), event duration (C), and number of secretory events/h (D). Numbers in bars are number of cells (D) or number of events examined (B, C). AM, Morning; PM, afternoon; conc., concentration. *p < 0.05.
Discussion
Here we demonstrate that FSCV allows real-time, specific monitoring of GnRH neurosecretion with good spatial resolution in brain sections. FSCV revealed several functional insights into the GnRH network. First, this is the first demonstration in native GnRH neurons that release occurs in regions other than the ME. Second, micromolar changes in GnRH concentration can occur at GnRH–GnRH appositions in the POA and in the ME, demonstrating that GnRH levels that are potentially autoexcitatory are achievable (Xu et al., 2004). Third, estradiol feedback differentially regulates GnRH release in the POA and ME. Together, these data suggest that multiple functions of GnRH and potential site-specific regulation of release help sculpt overall output of this neurosecretory network.
In murine brain slices, GnRH release was detected in the POA only where two GnRH neurons appeared to interact, indicating that GnRH–GnRH junctions may mark release sites. Although the role of GnRH in the neuroendocrine control of the anterior pituitary is well established, it also acts as a neuromodulator within the brain. This was postulated from in vivo studies (DePaolo et al., 1987) and such actions have been demonstrated in brain slices (Xu et al., 2004, 2008; Chen and Moenter, 2009; Han et al., 2010). The detection of GnRH release at GnRH–GnRH appositions in the POA provides intriguing evidence that GnRH neurons may use GnRH for modulation of their own function. Recent work in cultured embryonic GnRH neurons indicated GnRH was released from the soma and proximal processes (Fuenzalida et al., 2011). The present data support and extend these data by demonstrating that there is at least some degree of anatomical specification of release sites to GnRH–GnRH appositions; further, the previous finding of perisomatic release was not attributable to an artifact subsequent to culturing.
FSCV does not allow for detection of absolute concentrations because of the required baseline subtraction; instead, changes in concentration attributable to secretion are monitored. This putative limitation may be an asset in the GnRH neuronal system given the episodic nature of GnRH release. Although absolute concentrations are not determined, the change in concentration measured by FSCV can be used to estimate local concentrations achieved upon release. GnRH release was measured in the micromolar concentration range both at GnRH–GnRH appositions in the POA and within the ME. This high concentration may seem initially surprising given levels measured in pituitary portal blood (Sherwood et al., 1980; Moenter et al., 1992); however, those levels are quantified after diffusion and dilution in the blood. Further, estimates of synaptic cleft concentrations of transmitters are quite high; for example, GABA is in the millimolar range (Mozrzymas et al., 2003). The measurement of micromolar GnRH is of interest with regard to understanding dose-dependent changes in GnRH neuronal activity in response to GnRH (Xu et al., 2004). Nanomolar exogenous GnRH inhibited GnRH neuron activity, whereas micromolar GnRH increased activity (Xu et al., 2004). This may be attributable to different affinities of the GnRH receptor when coupled to different G-proteins (Millar et al., 2008). One hypothesis is that random fusion of GnRH vesicles produces low concentrations that inhibit the network, whereas action potential-driven release of GnRH generates high concentrations that excite the system, perhaps contributing to coordination of pulsatile release or long-term release during the preovulatory GnRH surge (Moenter et al., 1991).
The present work validates FSCV for real-time detection of GnRH in brain slices. The technique is sensitive enough to detect release in the POA between two GnRH neurons, as well as the integrated output of the GnRH population in the ME. Future studies will determine how electrical activity correlates with release from terminals versus dendrites, and examine the function of GnRH release in different brain regions.
Footnotes
This work was supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01 HD34860. We thank Debra Fisher and Laura Burger for expert technical assistance.
References
- Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146:4312–4320. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Cahill PS, Walker QD, Finnegan JM, Mickelson GE, Travis ER, Wightman RM. Microelectrodes for the measurement of catecholamines in biological systems. Anal Chem. 1996;68:3180–3186. doi: 10.1021/ac960347d. [DOI] [PubMed] [Google Scholar]
- Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci U S A. 2009;106:10835–10840. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci. 2009;29:9809–9818. doi: 10.1523/JNEUROSCI.2509-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C. The nature of the accessible and buried surfaces in proteins. J Mol Biol. 1976;105:1–12. doi: 10.1016/0022-2836(76)90191-1. [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102:15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: A possible local feedback circuit. J Neurosci. 2005;25:5740–5749. doi: 10.1523/JNEUROSCI.0913-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo LV, King RA, Carrillo AJ. In vivo and in vitro examination of an autoregulatory mechanism for luteinizing hormone-releasing hormone. Endocrinology. 1987;120:272–279. doi: 10.1210/endo-120-1-272. [DOI] [PubMed] [Google Scholar]
- Döcke F, Dörner G. The mechanism of the induction of ovulation by oestrogens. J Endocrinol. 1965;33:491–499. doi: 10.1677/joe.0.0330491. [DOI] [PubMed] [Google Scholar]
- Fuenzalida LC, Keen KL, Terasawa E. Colocalization of FM1–43, bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses. Endocrinology. 2011;152:4310–4321. doi: 10.1210/en.2011-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JC, Wadas B, Chen P, Portillo W, Reyna A, Jorgensen E, Mani S, Schwarting GA, Moenter SM, Tobet S, Kaiser UB. The gonadotropin-releasing hormone (GnRH) neuronal population is normal in size and distribution in GnRH-deficient and GnRH receptor-mutant hypogonadal mice. Endocrinology. 2008;149:4596–4604. doi: 10.1210/en.2008-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Lee K, Bhattarai JP, Herbison AE. Gonadotrophin-releasing hormone (GnRH) exerts stimulatory effects on GnRH neurons in intact adult male and female mice. J Neuroendocrinol. 2010;22:188–195. doi: 10.1111/j.1365-2826.2009.01950.x. [DOI] [PubMed] [Google Scholar]
- Ishizaki M, Oka Y. Amperometric recording of gonadotropin-releasing hormone release activity in the pituitary of the dwarf gourami (teleosat) brain-pituitary slices. Neurosci Lett. 2001;299:121–124. doi: 10.1016/s0304-3940(01)01492-6. [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci. 2002;22:2313–2322. doi: 10.1523/JNEUROSCI.22-06-02313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:6214–6224. doi: 10.1523/JNEUROSCI.6156-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20:317–326. doi: 10.1055/s-2002-36706. [DOI] [PubMed] [Google Scholar]
- Millar RP, Pawson AJ, Morgan K, Rissman EF, Lu ZL. Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front Neuroendocrinol. 2008;29:17–35. doi: 10.1016/j.yfrne.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Janin J, Lesk AM, Chothia C. Interior and surface of monomeric proteins. J Mol Biol. 1987;196:641–656. doi: 10.1016/0022-2836(87)90038-6. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129:1175–1182. doi: 10.1210/endo-129-3-1175. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology. 1992;130:503–510. doi: 10.1210/endo.130.1.1727719. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Zarmowska ED, Pytel M, Mercik K. Modulation of GABAA receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J Neurosci. 2003;23:7981–7992. doi: 10.1523/JNEUROSCI.23-22-07981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundroff ML, Wightman RM. Amperometry and cyclic voltammetry with carbon fiber microelectrodes at single cells. Curr Protoc Neurosci Chapter. 2002;6 doi: 10.1002/0471142301.ns0614s18. Unit 6.14. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143:2284–2292. doi: 10.1210/endo.143.6.8869. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144:823–831. doi: 10.1210/en.2002-220585. [DOI] [PubMed] [Google Scholar]
- Paras CD, Kennedy RT. Electrochemical detection of exocytosis at single rat melanotrophs. Anal Chem. 1995;67:3633–3637. doi: 10.1021/ac00116a003. [DOI] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74:931–937. doi: 10.1095/biolreprod.105.049619. [DOI] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology. 2006;147:1474–1479. doi: 10.1210/en.2005-1029. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Chiappa SA, Sarkar DK, Fink G. Gonadotropin-releasing hormone (GnRH) in pituitary stalk blood from proestrous rats: effects of anesthetics and relationship between stored and released GnRH and luteinizing hormone. Endocrinology. 1980;107:1410–1417. doi: 10.1210/endo-107-5-1410. [DOI] [PubMed] [Google Scholar]
- Strand AM, Venton BJ. Flame etching enhances the sensitivity of carbon-fiber microelectrodes. Anal Chem. 2008;80:3708–3715. doi: 10.1021/ac8001275. [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Troyer KP, Wightman RM. Response times of carbon fiber microelectrodes to dynamic changes in catecholamine concentration. Anal Chem. 2002;74:539–546. doi: 10.1021/ac010819a. [DOI] [PubMed] [Google Scholar]
- Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- Witkin JW, Silverman AJ. Synaptology of luteinizing hormone-releasing hormone neurons in rat preoptic area. Peptides. 1985;6:263–271. doi: 10.1016/0196-9781(85)90050-6. [DOI] [PubMed] [Google Scholar]
- Xu C, Xu XZ, Nunemaker CS, Moenter SM. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology. 2004;145:728–735. doi: 10.1210/en.2003-0562. [DOI] [PubMed] [Google Scholar]
- Xu C, Roepke TA, Zhang C, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone (GnRH) activates the m-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology. 2008;149:2459–2466. doi: 10.1210/en.2007-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]