Abstract
Apolipoprotein A-V (apoA-V) is postulated to modulate intra-hepatic triglyceride (TG) trafficking. Stably transfected McA-RH7777 hepatocarcinoma cells expressing human apoA-V displayed enhanced neutral lipid staining while conditioned media from these cells had 40 ± 8 % less TG than cells transfected with a control vector. To obtain homogeneous cell lines expressing different amounts of apoA-V, a strategy of clonal selection was pursued. Immunoblot analysis of two distinct apoA-V stable cell lines yielded one that expresses low amounts of apoA-V and another that expresses higher amounts. Confocal fluorescence microscopy of control cells and cells expressing low levels of apoA-V had similar numbers of lipid droplets while cells expressing higher amounts of apoA-V had twice as many lipid droplets, on average. Thus, apoA-V expression promotes lipid droplet accumulation in these cells.
Keywords: triacylglycerol, lipid droplet, apolipoprotein A-V, flow cytometry, confocal fluorescence microscopy
Introduction
The strong positive correlation between hypertriglyceridemia (HTG) and susceptibility to cardiovascular disease has fostered efforts to identify and characterize factors that regulate triglyceride (TG) metabolism. Genome-wide association studies have identified major contributors to HTG, including apolipoprotein (apo) A-V [1,2]. The impact of apoA-V on plasma TG was vividly illustrated in genetically engineered mice [3]. In APOA5 transgenic mice, plasma TG was 3-fold lower than control littermates while apoa5 (−/−) mice displayed a 4-fold increase. Moreover, human population studies reveal a strong association between common APOA5 single nucleotide polymorphisms and plasma TG [4]. Given these associations, defining the molecular basis of apoA-V function is key to translating knowledge of this protein into new approaches for diagnosis, prevention and/or treatment of HTG and related pathological conditions. Practically speaking, plasma TG levels may be influenced by extracellular events (e.g. the rate of lipolysis / clearance of TG-rich lipoproteins) or intracellular events related to production / secretion of TG-rich lipoproteins by liver.
The liver is the only tissue that expresses apoA-V [3,5]. Interestingly, following partial hepatectomy in rats, apoA-V mRNA increases 3.5 fold [5]. Although the function of apoA-V in this physiological setting is unknown, it is likely that, during tissue regeneration, liver cells are programmed to conserve lipid for membrane biogenesis as opposed to its secretion on lipoprotein particles. In another study, it was noted that apoA-V possesses considerable hydrophobic character, and this feature may be related to its poor secretion efficiency [6]. Following adenovirus-mediated overexpression in mice [7,8] or transient overexpression in Hep3B cells [9], apoA-V did not associate with apoB-100 containing lipoprotein particles. On the other hand, Shu et al. [10,11,12] found that ~50 % of newly synthesized apoA-V is retained in the cell in association with cytosolic lipid droplets.
Pamir et al. [13] reported that that APOA5 transgenic mice fed a diet high in fat and sucrose secrete lower amounts of TG. Likewise, apoA-V expression in stably transfected McA-RH7777 cells resulted in decreased TG secretion in a dose dependent manner, generating a VLDL particle population that was ~40 % smaller in volume [14]. In the present study stably transfected McA-RH7777 cells expressing human apoA-V were generated to address whether apoA-V expression drives formation of intracellular lipid droplets. The results provide evidence that apoA-V redirects intracellular TG toward lipid droplet assembly at the expense of TG-rich lipoprotein secretion.
Material and Methods
Cell Culture
McA-RH7777 cells were purchased from American Type Culture Collection (ATCC); growth medium consisted of DMEM containing 10% FBS (Phenix Research), 100 units/ml penicillin and 100 µg/ml streptomycin (Sigma). Stable cell lines were maintained in DMEM growth medium with added 0.25 µg/ml amphotericin B (Sigma) and 100 µg/ml G418 (Invitrogen). Cells were grown in T75 flasks at 37°C in an atmosphere containing 5% CO2. The medium was changed every other day and cells were passaged every 4–5 days.
Stable Transfection
The full-length human apoA-V sequence was subcloned into pcDNA 3.1 and the resulting construct was verified by sequencing. McA-RH7777 cells in 60 mm dishes were transfected at about 30% confluence with 8 µg of apoA-V pcDNA 3.1 or control pcDNA 3.1 plasmid, using Lipofectamine 2000 (Invitrogen). Twenty-four h post-transfection, cells were subjected to selection with DMEM-10% FBS supplemented with 200 µg/ml G418. Selection medium was replaced every other day for 14 days. Individual clones were isolated, expanded, and maintained in 100 µg/ml G418.
Lipid Analysis
Stably transfected McA-RH7777 cells were grown in T75 flasks until 30% confluent. Cells were incubated with serum-free medium 24 h prior to harvesting conditioned medium. Following collection, conditioned media were concentrated, dialyzed against phosphate buffered saline (PBS) and TG measured by L-Type Triglyceride M assay (Wako). Measured TG concentrations were normalized to total cell number. Values reported are percentages of the TG level secreted by control cells transfected with empty vector. Student’s t-test was used to examine statistical differences between cells expressing human apoA-V and control cells; p-value ≤ 0.05 is considered significant.
Flow Cytometry
Stably transfected McA-RH7777 cells were detached from plates with cell dissociation buffer (Invitrogen), washed with PBS, fixed with 4% paraformaldehyde (Sigma) in PBS for 20 min on ice and neutral lipids stained with Nile Red (Sigma) according to Greenspan et al. [15] prior to flow cytometry on a BD LSRFortessa instrument. FlowJo software was used for data processing.
Confocal Fluorescence Microscopy
Stably transfected McA-RH7777 cells were plated on coverslips (Zeiss). Twenty four h post plating, cells were fixed with 4% paraformaldehyde in PBS for 20 min, washed three times with PBS and incubated with Nile Red for 20 min. Cell nuclei were counterstained with Hoechst (Invitrogen) for 20 min and washed three times with PBS. Cells were mounted with vectashield mounting medium (Vector) and viewed with a Zeiss LSM710 Confocal Microscope with a 63× oil objective. Photoshop software was used for lipid droplet analysis. Student’s t-test was performed with a p-value ≤ 0.05 considered significant.
Immunoblot Analysis
Cells were detached from plates with cell dissociation buffer (Invitrogen), washed with PBS, lysed with cold CytoBuster protein extraction reagent (Novagen) lysis buffer plus protease inhibitor cocktail (Sigma) on ice. Total protein amounts in cell lysates were determined by BCA assay (Pierce). Cell lysate contents were separated by electrophoresis on Bio-Rad precast 4–20% acrylamide gradient gels and transferred to 0.2 µm polyvinylidene difluoride membranes using the Trans-Blot Turbo Transfer System (Bio-Rad). The blot was blocked with 20 mM Tris-HCl, pH 7.6, 150 mM NaCl, 0.05% Tween-20 (TBST), containing 5% non-fat milk, and then probed with polyclonal goat anti-human apoA-V solution at 4°C overnight. After washing with TBST, the membrane was incubated with horseradish peroxidase-conjugated bovine anti-goat IgG secondary antibody (Santa Cruz) for one h at room temperature. Signals were detected using Immobilon Western Chemiluminescent horseradish peroxidase substrate (Pierce).
Results
McA-RH7777 cells were stably transfected with either pcDNA 3.1 harboring the full-length coding sequence for human apoA-V or empty vector. To verify that successful integration of the apoA-V coding sequence leads to expression of apoA-V protein, immunoblot analysis was performed. Conditioned media and lysates of cells transfected with apoA-V pcDNA 3.1 were positive for apoA-V while cells transfected with a control plasmid showed no apoA-V reactivity (data not shown). Consistent with previous reports [16,17], McA-RH7777 cells express no detectable apoA-V.
To evaluate the effect of apoA-V expression on cellular neutral lipid content, flow cytometry was performed on cells stained with Nile Red. Compared to cells transfected with empty vector, cells expressing apoA-V displayed increased Nile Red fluorescence intensity, consistent with increased cellular lipid content (Figure 1A). To assess whether the increase in cellular lipid droplet content as a function of apoA-V expression affected TG secretion by these cells, conditioned media were analyzed for TG (Figure 1B). Compared to control cells transfected with empty vector, conditioned media from apoA-V stably transfected cells contained 40 ± 8 % less TG. The data suggest that intracellular accumulation of TG in McA-RH7777 cells as a function of apoA-V expression occurs at the expense of TG secretion.
Figure 1. The effect of apoA-V expression on TG fate in McA-RH7777 cells.
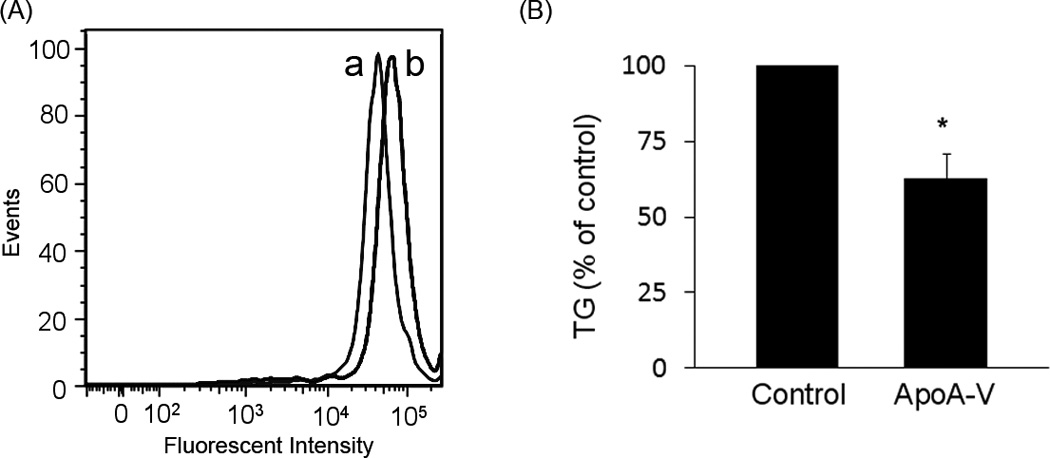
Panel A) Stably transfected McA-RH7777 cells were detached from plates, fixed with 4% paraformaldehyde, washed with PBS and stained with Nile Red prior to flow cytometry. Curve (a) depicts the fluorescence intensity of cells transfected with pcDNA 3.1 control vector while curve (b) shows intensity of cells transfected with human apoA-V pcDNA 3.1. Panel B) Cells were incubated with serum-free medium 24 h prior to harvesting conditioned media. The conditioned media were concentrated, dialyzed against PBS and TG content measured. Values are presented as percentage of TG secreted by control cells expressed as mean ± S.E.M. (n=3). Student t-test was used to examine statistical difference between apoA-V expressing cells and control cells; *, p ≤ 0.05.
In the above studies the population of apoA-V stably transfected cells is heterogeneous, with different cells in the population expressing variable amounts of apoA-V. In an effort to determine whether the level of apoA-V expression correlates with cellular lipid droplet accumulation, a strategy of clonal selection was pursued wherein a heterogeneous population of stably transfected McA-RH7777 cells was serially diluted and cultured in 96 well plates and low and high expressing clones isolated. Immunoblot analysis was performed to evaluate apoA-V expression level in the resulting clonal cell lines (Figure 2). Whereas cells transfected with empty vector do not express apoA-V, two clonal lines from cells transfected with apoA-V pcDNA 3.1 show dramatic differences in apoA-V expression, wherein Clone A (lane 3) has low expression and Clone B (lane 4) has high expression.
Figure 2. Immunoblot analysis of clonal lines of stably transfected McA-RH7777.
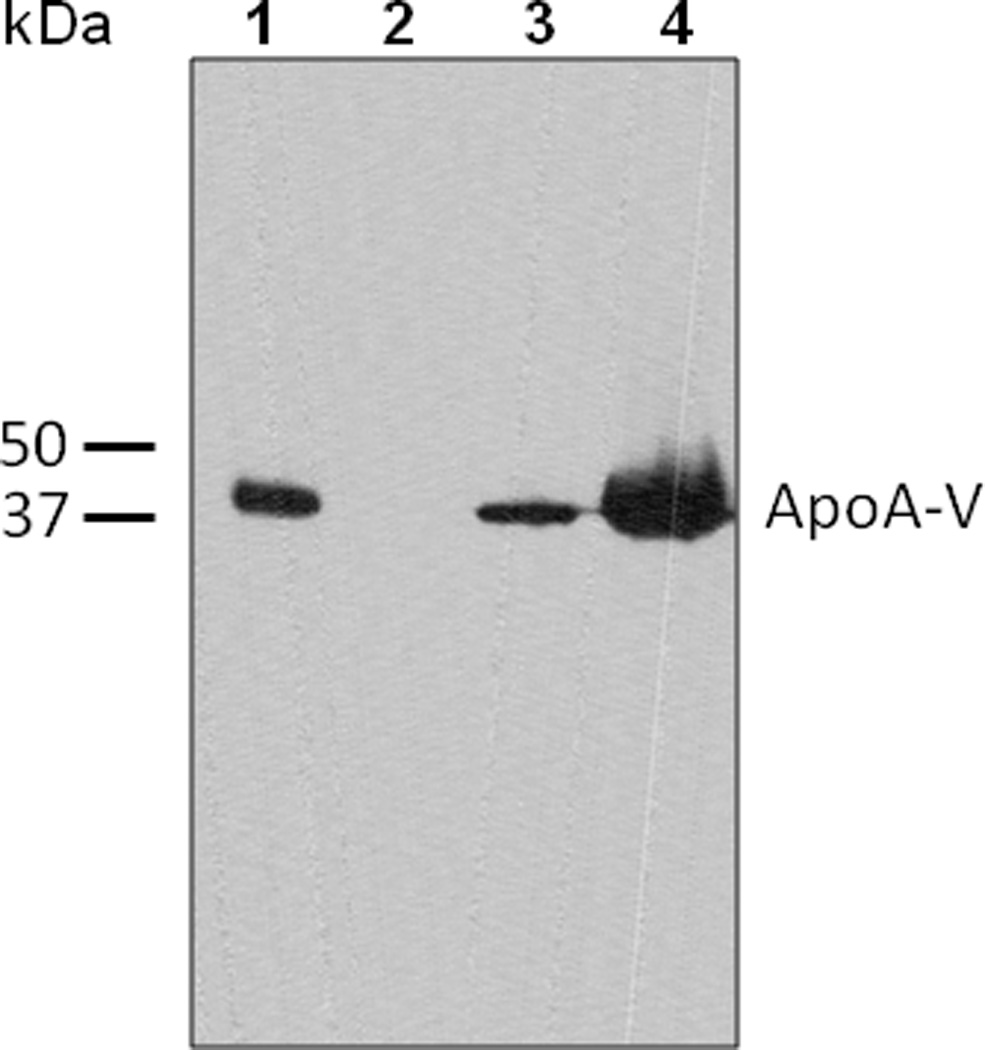
Cell lysates were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane and probed with antibodies against apoA-V. Lane 1) recombinant apoA-V standard (90 ng); Lane 2) cells transfected with empty vector; Lane 3) apoA-V pcDNA 3.1 transfected Clone A and Lane 4) apoA-V pcDNA 3.1 transfected Clone B. Equal amounts of cell protein were loaded in lanes 2 to 4.
Compared to cells transfected with empty vector, Clone A cells express low amounts of apoA-V and display a modest enhancement in Nile Red fluorescence intensity (Figure 3A). By contrast, the clonal cell line expressing higher amounts of apoA-V (i.e. Clone B) displayed increased Nile Red fluorescence. These results support the premise that increased apoA-V expression leads to increased cellular TG accumulation. To extend the flow cytometry results, confocal fluorescence microscopy was performed on control cells and apoA-V stable clones A and B after Nile Red staining (Figure 3B). Whereas the control cells and Clone A cells had similar numbers of lipid droplets per cell (4 ± 2.6 and 4.5 ± 1.8, respectively; n = 6), Clone B cells had significantly increased numbers of lipid droplets per cell (9.5 ± 3.4, n = 6) (p < 0.05 versus control and Clone A). Moreover, the diameter of lipid droplets in Clone B cells was substantially larger (2.6 ± 0.6 µm, n = 57) versus control cells (1.3 ± 0.6 µm, n = 24) and Clone A cells (1.2 ± 0.5 µm, n = 27).
Figure 3. Flow cytometry and confocal fluorescence microscopy analysis of McA-RH7777 clonal cell lines.
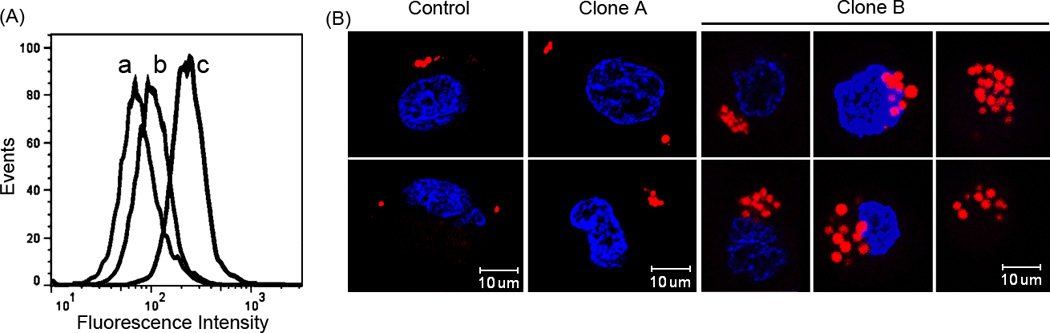
Panel A: Human apoA-V expressing and control pcDNA 3.1 cells lines were detached from plates at 30% confluence, washed with PBS, fixed and stained with Nile Red prior to flow cytometry. Curve a) Control cells transfected with empty vector; curve b) clonal cell line A; and curve c) clonal cell line B. Panel B: Representative confocal fluorescence microscopy images of stable transfected McA-RH7777 cells. Cells were stained with Nile Red and Hoechst nuclear stain prior to microscopy. Left) control cells transfected with empty vector; middle) Clone A cells; right) Clone B cells.
Discussion
The present study reveals an apoA-V concentration dependent accumulation of TG in cytosolic lipid droplets at the expense of TG secretion. The results indicate that both lipid droplet size and number increase as a function of apoA-V expression. A molecular explanation for these results is described schematically in Figure 4. Intrahepatic lipid droplet biogenesis occurs between leaflets of the endoplasmic reticulum (ER) bilayer [12,18,19]. The assembly process begins with formation of a lens of TG within the bilayer. As this TG lens increases in size, it can protrude in the direction of the cytosol, ultimately pinching off to become a cytosolic lipid droplet. Alternatively, the lens can bud toward the ER lumen to be released as a lumenal lipid droplet. Lumenal lipid droplets are involved in TG-rich lipoprotein assembly by fusing with nascent apolipoprotein B containing lipoproteins to generate very low density lipoproteins that migrate to the Golgi apparatus for secretion from the cell [20]. Data presented herein indicates that apoA-V influences the directionality of these competing processes, promoting cytosolic budding of droplets as opposed to lumenal intrusion. The emerging model predicts that apoA-V recognizes and binds to ER membrane defects created by the accumulation of TG between leaflets of the bilayer. By stabilizing the lumenal leaflet in the region of this TG lens, budding preferentially occurs in the direction of the cytosol, leading to increased cytosolic lipid droplet formation. The more apoA-V present in the ER lumen, the less chance a given TG lens will bud in this direction, a step that is necessary for lumenal lipid droplet formation.
Figure 4. Model depicting the effect of apoA-V on the fate of hepatic TG.
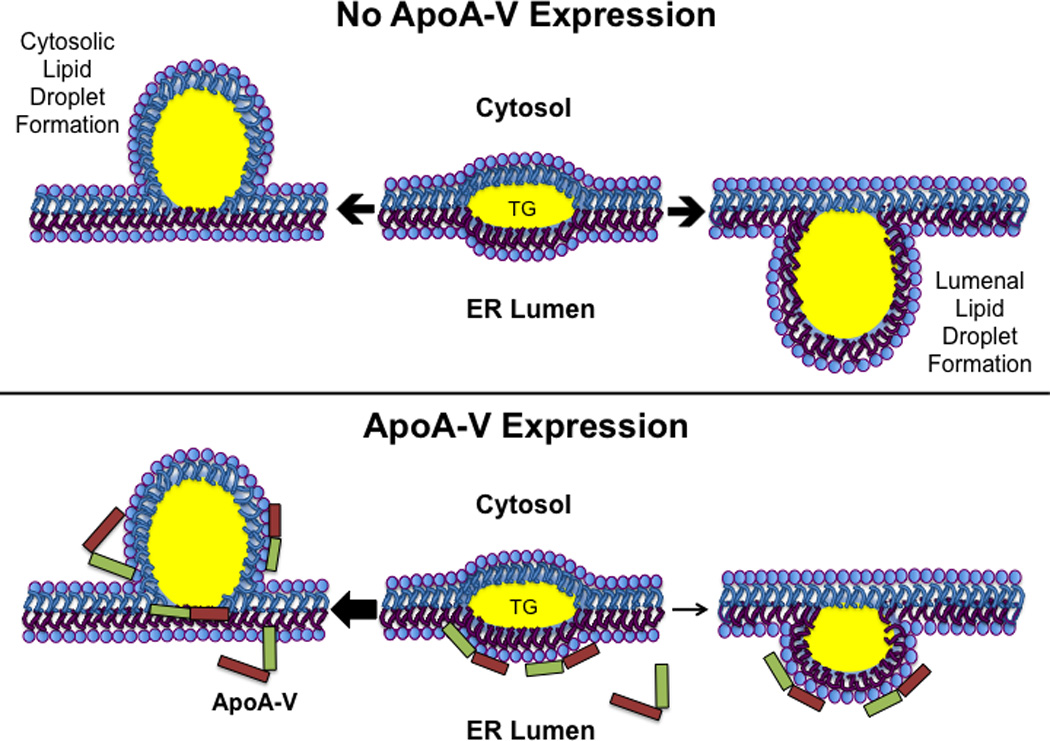
In the absence of apoA-V (Upper Panel) TG accretion forms a lens between leaflets of the ER membrane. Expansion of this lens by continued accrual of TG leads to budding of a nascent lipid droplet from the cytoplasmic leaflet (left) or, alternatively, budding from the lumenal leaflet (right) to create a lumenal lipid droplet for utilization in VLDL maturation. Lower Panel) When present, apoA-V binding to membrane defects created by TG accumulation stabilizes the lumenal leaflet, promoting nascent lipid droplet budding toward the cytosol at the expense of lumenal lipid droplet formation (see arrows).
ApoA-V is synthesized with an N-terminal signal peptide that directs it to the ER – Golgi secretory pathway. This fact, together with its intrinsic lipid surface seeking property, suggests apoA-V is well suited to interact with ER membrane defects caused by TG accumulation. Further, we speculate that interaction with defects on the lumenal leaflet of the ER membrane positions apoA-V such that it is able to translocate to the opposite leaflet during cytosolic lipid droplet maturation, ultimately maintaining contact with the newly formed organelle [8]. The net effect of this process is cellular retention of TG in cytosolic lipid droplets with diversion of some fraction of newly synthesized apoA-V away from the ER – Golgi secretory pathway.
Cytosolic lipid droplets associate with many proteins, including PAT (perilipin, adipophilin and TIP47) family proteins. A major difference between apoA-V and all other hepatic lipid droplet associated proteins relates to the site where it initiates contact with nascent lipid droplets. While apoA-V must associate via the ER lumen, other proteins contact lipid droplets from the cytosolic side. Indeed, when hepatocarcinoma cells were transfected with apoA-V lacking a signal peptide, lipid droplet morphology was significantly affected [10]. The emerging model of apoA-V function in lipid droplet assembly fits with a growing body of evidence including: a) up-regulation of apoA-V mRNA during liver regeneration [5]; b) poor secretion efficiency of apoA-V from cells [6]; c) accrual of apoA-V on cytosolic lipid droplets despite the presence of an N-terminal signal peptide d) inverse relationship between apoA-V production and TG secretion and e) positive correlation between apoA-V expression and cytosolic lipid droplet formation.
In summary, apoA-V appears to function at the crossroads between TG retention in hepatocytes and export on lipoprotein particles. Indeed, apoA-V is the only hepatocyte-derived protein known to exist on lipid droplets as well as plasma lipoproteins, and no other hepatocyte lipid droplet associated protein is synthesized with a signal peptide that targets it to the ER lumen. In the hepatocyte, the ultimate destiny of newly synthesized TG is likely dictated by myriad physiological factors. The balance between TG secretion on lipoproteins or retention as lipid droplets, however, is modulated by apoA-V, which functions as a rheostat that directs TG away from secretion towards cytosolic lipid droplet assembly.
Highlights.
McArdle 7777 hepatocarcinoma cells were transfected with human apolipoprotein (apo) A-V
ApoA-V expression enhances cytosolic lipid droplet (LD) formation
Stable transfection of apoA-V results in decreased triacylglycerol (TG) secretion
A molecular mechanism whereby apoA-V modulates TG trafficking is proposed
ApoA-V binding to ER membrane defects created by TG inhibits lumenal lipid droplet formation
Acknowledgements
The authors thank Dr. Andrzej Witkowski for assistance and Dr. Richard Lehner for valuable suggestions. The authors thank Jennifer Beckstead and Dr. Vineeta Sharma for assistance with Figure 4. This work was supported by a grant from the National Institutes of Health (HL-64159).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johansen CT, Wang J, Lanktree MB, Cao H, McIntyre AD, Ban MR, Martins RA, Kennedy BA, Hassell RG, Visser ME, Schwartz SM, Voight BF, Elosua R, Salomaa V, O'Donnell CJ, Dallinga-Thie GM, Anand SS, Yusuf S, Huff MW, Kathiresan S, Hegele RA. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 2010;42:684–687. doi: 10.1038/ng.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J. Lipid Res. 2011;52:189–206. doi: 10.1194/jlr.R009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 4.Sharma V, Ryan RO, Forte TM. Apolipoprotein A-V dependent modulation of plasma triacylglycerol: A puzzlement. Biochim. Biophys. Acta. 2012;1821:795–799. doi: 10.1016/j.bbalip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J. Biol. Chem. 2001;276:44512–44520. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg RB, Cook VR, Beckstead JA, Martin DD, Gallagher JW, Shelness GS, Ryan RO. Structure and interfacial properties of human apolipoprotein A-V. J. Biol. Chem. 2003;278:34438–34444. doi: 10.1074/jbc.M303784200. [DOI] [PubMed] [Google Scholar]
- 7.Schaap FG, Rensen PCN, Voshol PJ, Vrins C, Van der Vliet HN, Chamuleau RA, Havekes LM, Groen AK, Van Dijk KW. ApoAV reduces plasma triglycerides by Inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 2004;279:27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 8.Van der Vliet HN, Schaap FG, Levels JHM, Ottenhoff R, Looije N, Wesseling JG, Groen AK, Chamuleau RA. Adenoviral overexpression of apolipoprotein A-V reduces serum levels of triglycerides and cholesterol in mice. Biochem. Biophys. Res. Commun. 2002;295:1156–1159. doi: 10.1016/s0006-291x(02)00808-2. [DOI] [PubMed] [Google Scholar]
- 9.Shu X, Chan J, Ryan RO, Forte TM. ApoA-V association with intracellular lipid droplets. J. Lipid Res. 2007;48:1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Shu X, Ryan RO, Forte TM. Intracellular lipid droplet targeting by apolipoprotein A-V requires the carboxyl-terminal segment. J. Lipid Res. 2008;49:1670–1676. doi: 10.1194/jlr.M800111-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shu X, Nelbach L, Ryan RO, Forte TM. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim. Biophys. Acta. 2010;1801:605–608. doi: 10.1016/j.bbalip.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamir N, McMillen TS, Li YI, Lai CM, Wong H, LeBoeuf RC. Overexpression of apolipoprotein A5 in mice is not protective against body weight gain and aberrant glucose homeostasis. Metabolism. 2009;58:560–567. doi: 10.1016/j.metabol.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blade AM, Fabritius MA, Hou L, Weinberg RB, Shelness GS. Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion. J. Lipid Res. 2011;52:237–244. doi: 10.1194/jlr.M010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenspan P, Mayer EP, Fowler SD. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Jimenez CP, mez-Lechon MJG, Castell JV, Jover R. Underexpressed coactivators PGC1 and SRC1 impair hepatocyte nuclear factor 4 function and promote dedifferentiation in human hepatoma cells. J. Biol. Chem. 2006;281:29840–29849. doi: 10.1074/jbc.M604046200. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien PJ, Alborn WE, Sloan JH, Ulmer M, Boodhoo A, Knierman MD, Schultze AE, Konrad RJ. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin. Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 18.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 19.Ploegh HL. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–438. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- 20.Sundaram M, Yao Z. Intrahepatic role of exchangeable apolipoproteins in lipoprotein assembly and secretion. Arterioscler. Thromb. Vasc. Biol. 2012;32:1073–1078. doi: 10.1161/ATVBAHA.111.241455. [DOI] [PubMed] [Google Scholar]


