Abstract
Malignancy is a common cause of disseminated intravascular coagulation and usually presents as a chronic disorder in solid organ tumours. We present a rare case of recurrent acute disseminated intravascular coagulation in neuroendocrine carcinoma after manipulation, firstly, by core biopsy and, later, by cytotoxic therapy causing a release of procoagulants and cytokines from lysed tumour cells. This is reminiscent of tumour lysis syndrome where massive quantities of intracellular electrolytes and nucleic acid are released, causing acute metabolic imbalance and renal failure. This case highlights the potential complication of acute disseminated intravascular coagulation after trauma to malignant cells.
Key words: Disseminated intravascular coagulation, Neuroendocrine carcinoma, Tumour lysis syndrome
Case Report
A 54-year-old man presented with acute right-sided abdominal pain and nausea. A computed tomography scan revealed a diffusely nodular liver (fig. 1a) with multiple lesions of poor contrast enhancement (fig. 1b) and a mass in his pancreas. A fine needle aspirate of one of the liver lesions showed cellular smears with moderate variation in nuclear size and shape with delicate cytoplasm (fig. 2a). Neoplastic cells stained positively with chromogranin (fig. 2b) but synaptophysin, CK7, CK20, TTF-1, Hepar-1, CD10, carcinoembryonic antigen and alpha-fetoprotein were all negative. The morphological and immunohistochemical staining pattern favoured a metastatic neuroendocrine neoplasm. The patient did not have any symptoms suggesting carcinoid syndrome. His chromogranin A was elevated at >4,000 U/l, and urinary 5-hydroxyindoleacetic acid was normal. In addition, a 177lutetium octreatate-positive emission tomography scan showed no accumulation of tracer activity in the multiple hepatic metastases. The diagnosis was consistent with metastatic neuroendocrine tumour with probable pancreatic primary tumour. Three weeks after the biopsy, the patient presented with vomiting and lethargy. He was found to have multi-organ failure with abnormal renal function (creatinine 190 µmol/l), deranged liver enzymes (bilirubin 36 µmol/l; alkaline aminotransferase (ALT) 184 U/l; gammaglutamyl transferase (GGT) 468 U/l; alkaline phosphatase (ALP) 322 U/l), and no radiological evidence of biliary duct obstruction. He developed disseminated intravascular coagulation (DIC) with a platelet count of 10 × 109/l, an international normalised ratio (INR) of 2.8, a fibrinogen level of 0.5 g/l and a D-dimer level of 33.18 mg/l. He showed no signs of bleeding. Over the next 3 days, the patient became anuric and dyspnoeic. Pulmonary embolus was excluded. He was treated with fluid resuscitation, blood products and vitamin K. He gradually improved over a period of 10 days. His renal impairment resolved (creatinine 94 μmol/l), liver function tests improved (bilirubin 24 µmol/l; ALT 59 U/l; GGT 220 U/l; ALP 247 U/l) and the coagulation profile normalised (platelet count 447 × 109/l; INR 1.1; fibrinogen 5.6 g/l). One week later, he received his first cycle of chemotherapy – carboplatin (AUC 5) and etoposide (100 mg/m2) with a 20% dose reduction. He tolerated the treatment without any immediate complications. Three weeks later, he presented with a 5-day history of fatigue, dyspnoea, haematuria and extensive bruising (fig. 3). He had recurrent DIC with active bleeding as evidenced by a drop of haemoglobin to 43 g/l, a platelet count of 11 × 109/l, an INR of 4.3, a fibrinogen level of <0.2 g/l and a D-dimer level of 75 mg/l. His renal and liver functions remained relatively stable initially (creatinine 128 µmol/l; bilirubin 24 µmol/l; ALT 48 U/l; GGT 286 U/l; ALP 254 U/l). He was resuscitated with blood products. He rapidly deteriorated with worsening dyspnoea, ischaemic chest pain and increasing blood product requirement with no improvement in his blood counts. Further chemotherapy was contemplated as it was thought that malignancy was the underlying cause of the DIC. However, due to his rapid decline, this was deemed futile, hence palliative measures were implemented. He died 3 days after his second admission − 2 months after his initial presentation.
Fig. 1.
CT scan. a Diffuse nodular liver. b Lesions in a liver with poor contrast enhancement.
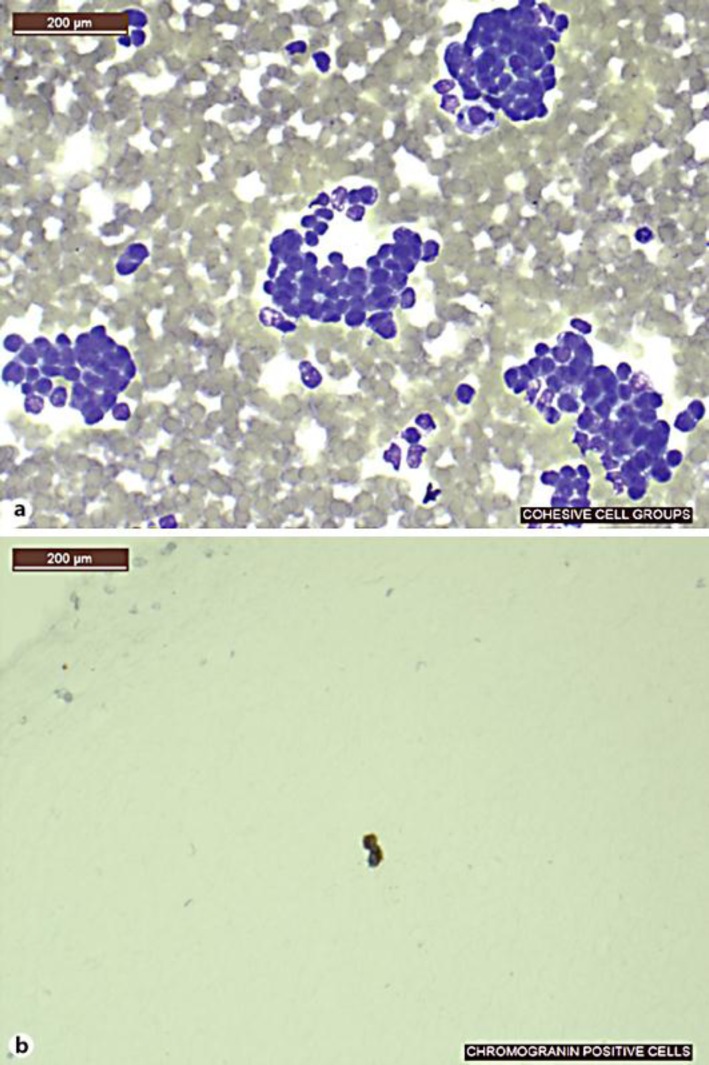
Fig. 2.
Fine needle aspirate of a liver. a Cellular smear with variation in nuclear size and shape with delicate cytoplasm. b Positive chromogranin stain.
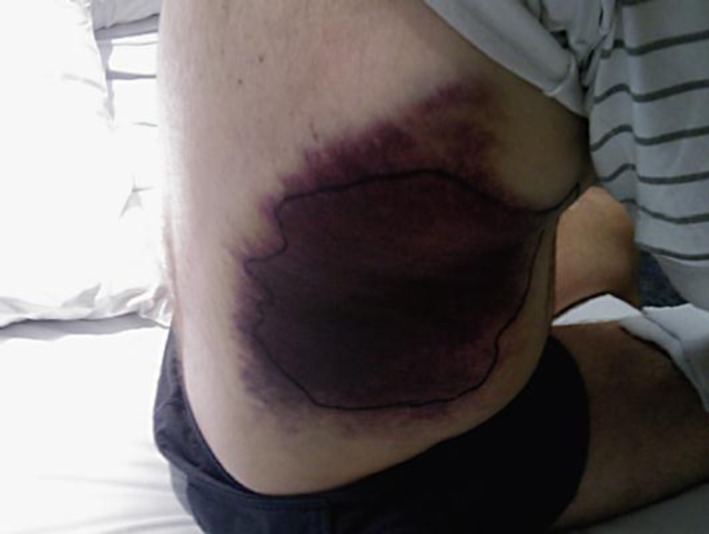
Fig. 3.
Extensive bruising.
Discussion
Neuroendocrine tumours have a wide range of clinical presentations, from the more indolent low-grade carcinoid tumours to aggressive high-grade disease similar to small cell carcinomas [1]. They are often associated with the secretion of hormones and peptides leading to classical syndromes [2, 3, 4, 5]. However, there does not seem to be a relation to the coagulation cascade. The fulminant nature of the DIC in this case is reminiscent of tumour lysis syndrome which is similarly associated with malignancy with a high proliferative rate, large tumour burden and high sensitivity to cytotoxic agents. In tumour lysis syndrome, massive quantities of intracellular contents, including potassium, phosphate and nucleic acid that metabolized to uric acid, are released to the systemic circulation, causing acute metabolic imbalance and renal failure [6]. In our case, it appears that manipulation by core biopsy caused self-limiting and, later, cytotoxic agents caused fulminant DIC by the release of procoagulants and cytokines from lysed tumour cells. DIC is characterised by a widespread, intravascular activation of coagulation leading to intravascular fibrin deposition and simultaneous consumption of coagulation factors and platelets. The consequence of DIC depends on its cause and the rapidity with which the activating event is propagated. If the activation occurs slowly, an excess of procoagulants is produced, leading to thrombosis. The hallmark of chronic, compensated DIC is that the liver can offset the consumption of clotting factors and the bone marrow maintains an adequate platelet count. If the activation is brisk, the clinical picture is dominated by intravascular coagulation leading to profound systemic bleeding diathesis [7].
Our case was an unusual presentation as DIC in cancer usually is a less fulminant presentation compared to DIC associated with sepsis and trauma. Malignancy, particularly solid tumours, is the most common cause of chronic DIC. Tissue factor (TF) and cancer procoagulant-mediated activation of coagulation, platelet aggregation and activation, suppression of the fibrinolytic pathway and cytokine-regulated defective anticoagulant mechanisms form pivotal roles in activating coagulation and the ensuing procoagulant state in cancer. Complications are typically related to excess procoagulants causing venous thrombosis (deep venous thrombosis, superficial migratory thrombophlebitis and, less commonly, arterial thrombosis), digital ischaemia, renal infarction and stroke [8]. The physiological response to the exposure of tumour-specific antigens results in the release of potent inflammatory mediators such as tumour necrosis factor and interleukin-1 from activated macrophages and stimulated T cells. This further propagates the prothrombotic process as besides from being potent mediators of inflammation these cytokines can also induce TF expression, activate platelets and downregulate the protein C pathway. In addition, these cytokines induce endothelial expression of plasminogen activator inhibitor-1 and impair the protein C anticoagulant pathway by downregulating thrombomodulin and endothelial protein C receptor. As a consequence, fibrinolytic and anticoagulant activities are decreased [9]. Laboratory evidence of coagulation activation has been documented with exposure to chemotherapy (e.g. cyclophosphamide, methotrexate and 5-fluorouracil) and hormonal therapy (e.g. tamoxifen), but usually the abnormalities in coagulation markers are modest and do not correlate with the development of DIC. It is postulated that the release of procoagulants and cytokines from lysed tumour cells as well as endothelial cell damage are caused by cytotoxic agents rather than being a direct effect of chemotherapy itself. In addition, by direct surgical trauma of tumour blood vessels, exposure to subendothelial TF causes the release of cytokines with their procoagulant activity [10]. Physicians should be mindful that acute DIC may occur after trauma to malignant cells. Management is limited to supportive care by replacing blood products and treating the underlying malignancy. This poses a dilemma as cytotoxic agents may worsen DIC and may also directly suppress bone marrow production. Unfortunately, the prognosis is often poor despite active intervention.
References
- 1.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 2.Elrington GM, Murray NM, Spiro SG, Newsom-Davis J. Neurological paraneoplastic syndromes in patients with small cell lung cancer. A prospective survey of 150 patients. J Neurol Neurosurg Psychiatry. 1991;54:764–767. doi: 10.1136/jnnp.54.9.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiraki A, Ueoka H, Takata I, Gemba K, Bessho A, Segawa Y, et al. Hypercalcemia-leukocytosis syndrome associated with lung cancer. Lung Cancer. 2004;43:301. doi: 10.1016/j.lungcan.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 4.List A, Hainsworth J, Davis B, Hande K, Greco F, Johnson D. The syndrome of inappropriate secretion of antidiuretic hormona (SIADH) in small-cell lung cancer. J Clin Oncol. 1986;4:1191–1198. doi: 10.1200/JCO.1986.4.8.1191. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd F, Laskey J, Evans W, Goss P, Johansen E, Khamsi F. Cushing's syndrome associated with ectopic corticotropin production and small-cell lung cancer. J Clin Oncol. 1992;10:21–27. doi: 10.1200/JCO.1992.10.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 7.Sallah S, Wan J, Nguyen N, Hanrahan L, Sigounas G. Disseminated intravascular coagulation in solid tumors: clinical and pathologic study. Thromb Haemost. 2001;86:828–833. [PubMed] [Google Scholar]
- 8.Sutherland D, Ilene C, Liebman H. Thromboembolic complications of cancer: epidemiology, pathogenesis, diagnosis, and treatment. Am J Hematol. 2003;72:43–52. doi: 10.1002/ajh.10263. [DOI] [PubMed] [Google Scholar]
- 9.Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol. 2009;22:129–36. doi: 10.1016/j.beha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 10.De Cicco M. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol. 2003;50:187–196. doi: 10.1016/j.critrevonc.2003.10.003. [DOI] [PubMed] [Google Scholar]