Abstract
Concerns over cardiac side effects are the largest single cause of compound attrition during pharmaceutical drug development. For a number of years, biophysically detailed mathematical models of cardiac electrical activity have been used to explore how a compound, interfering with specific ion-channel function, may explain effects at the cell-, tissue- and organ-scales. With the advent of high-throughput screening of multiple ion channels in the wet-lab, and improvements in computational modelling of their effects on cardiac cell activity, more reliable prediction of pro-arrhythmic risk is becoming possible at the earliest stages of drug development. In this paper, we review the current use of biophysically detailed mathematical models of cardiac myocyte electrical activity in drug safety testing, and suggest future directions to employ the full potential of this approach.
LINKED ARTICLE
This article is commented on by Gintant, pp. 929–931 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2012.02096.x
Keywords: arrhythmia, Torsade de Pointes, computer model, hERG, QT prolongation
Introduction
The role of safety pharmacology is to evaluate whether any off-target drug interactions may cause unwanted, in particular dangerous, side effects. In this review, we examine the role of computational simulation, using biophysically detailed models of cardiac electrophysiology, in predicting the risk of drug-induced pro-arrhythmic effects.
Torsade de Pointes (TdP) is a ventricular tachycardia, which has been linked to administration of drugs that delay repolarization – the final stage of the electrical cycle underlying the heartbeat. On the ECG, such drugs give rise to a prolongation of the time between onset of ventricular excitation (Q-wave) and the end of repolarization (end of T-wave). This is referred to as QT prolongation. Even in the presence of QT prolongation, however, TdP may occur as rarely as once in every 10 000 patient-years of exposure to a compound. Being a (potentially exceedingly) rare event in human, clinical TdP is notoriously difficult to assess during the pharmaceutical compound development process. Prediction of ‘torsadogenicity’ (the likelihood of a compound initiating TdP) is of high relevance for cardiac safety assessment because, if it occurs, TdP often degenerates into ventricular arrhythmias that may cause sudden cardiac death (unless self-terminated; see Figure 1). TdP as a potentially drug-induced side effect on the heart has become a primary concern in drug development, so that torsadogenicity needs to be assessed and, if possible, excluded as early as possible in the compound development pipeline.
Figure 1.
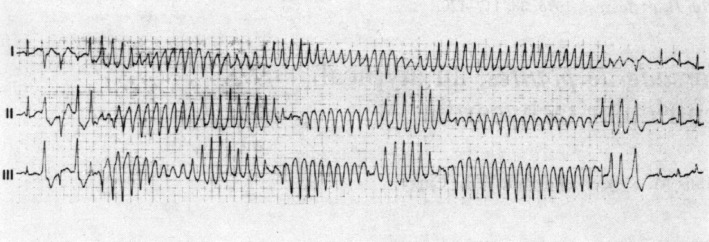
Onset and self-termination of TdP in a patient during ECG observation. From top to bottom: ECG leads I, II and III. Adapted by permission from BMJ Publishing Group Limited. (British Heart Journal, Krikler & Curry, 38:117–120, 1976) (Krikler and Curry 1976).
As a result of the combination of (i) the potential severity of TdP, and (ii) the difficulty in predicting TdP liability at preclinical stages, this has become a significant problem for the industry. TdP risk (both real and perceived) is responsible for failure of countless compounds during development, and it remains a leading cause of drug-withdrawal from the market [e.g. of the antihistamine Terfenadine, and Cisapride (Gottlieb, 1999; Henney, 2000)].
Human ether-a-go-go-related gene (hERG), action potential duration (APD), QT, and Torsade de Pointes
TdP liability has been linked to block of a particular ion channel, expressed in cardiac cells. In humans, the channel's major subunit is encoded by the hERG (Kv11.1), which encodes a protein that forms part of a potassium channel which carries the rapidly activating potassium current (IKr), one of the major repolarizing currents in cardiac tissue. In the following, we use ‘hERG-channel’ to refer to this potassium channel. Unfortunately, the hERG-channel is particularly prone to interaction with a huge range of pharmaceutical compounds (Vandenberg et al., 2001). Block of hERG-channels causes a reduction in repolarizing currents, and, correspondingly, an increase in the length of time that membrane voltage remains at elevated levels. This manifests itself in a prolongation of the cell's APD, as shown in Figure 2. Prolonged APs at the cellular level are one of the mechanisms that give rise to prolongation of the QT interval of the ECG (see Figure 2). Increases in APD, in particular if regionally heterogeneous, are believed to make early after-depolarizations more likely, and these are thought to serve as potential triggers for pro-arrhythmic events (Madhvani et al., 2011).
Figure 2.
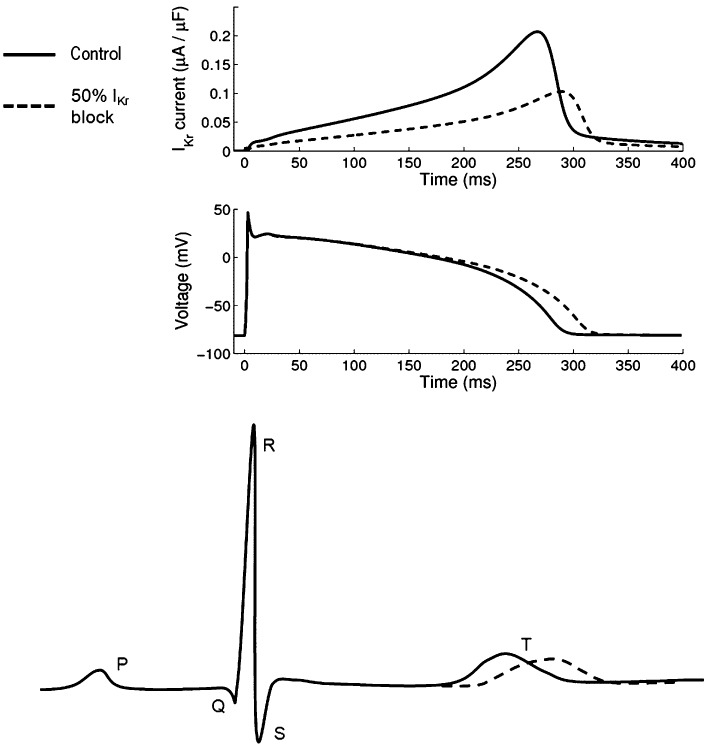
Schematic representation of electrophysiological changes caused by 50% block of the hERG-channel in healthy tissue. Top: hERG current (IKr); middle: action potential (voltage); bottom: ECG annotated with standard labels for characteristic parts of the curve. The QT interval is the period of time from the Q to the T waves. Top and middle panels are from a 1-Hz steady-state simulation of the (Grandi et al., 2010) model, bottom is a schematic. In all panels, control is indicated by a solid line, and the effect of 50% IKr conductance block by a dashed line; simulations illustrating steady-state responses.
As torsadogenicity can be associated with prolongation of the QT interval, it has been concluded that any block of IKr or QT prolongation should be taken as an indication of pro-arrhythmic risk. To address this, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH; http://www.ich.org) has introduced two assays: (ICH, 2005a)
ICH-S7B (ICH, 2005a): suggests in vitro IKr and in vivo QT measurements. These take the form of patch clamp experiments using a hERG expression system, and an in vivo conscious animal QT study;
ICH-E14 (ICH, 2005b): suggests a human phase II ‘thorough QT’ trial. If the 95% confidence interval for prolongation of QT interval, corrected for heart rate, is equal or greater than 10 ms (this generally indicates a mean prolongation of as little as 5 ms), then the compound will be of concern to regulatory bodies (Recanatini et al., 2005; Pollard et al., 2010). Should this occur, extensive further trials are necessary to establish whether a pro-arrhythmic liability exists, and subsequent product warning labels may restrict the market access of a drug. In practice, pharmaceutical companies spend considerable effort on avoiding this, as further trials are expensive and may not result in a positive outcome for the company (unless the clinical benefits of a compound outweighs its TdP risk – i.e. for life-threatening conditions with no existing treatment, or with poor prognosis).
Both guidelines were produced jointly by regulators and pharmaceutical industry, with the effect that the suggested assays are effectively mandatory for regulatory approval to register and market a drug. As a result, pharmaceutical companies have developed a range of earlier and cheaper in-house assays, designed to evaluate whether compounds are likely to run into problems with the regulatory requirements mentioned earlier (Pollard et al., 2008). We outline a ‘typical’ pharmaceutical cardiac side effect screening strategy in Figure 3. This approach has been relatively effective in preventing potentially torsadogenic compounds from reaching the market. However, this process is likely to include false-positive termination of otherwise promising candidate drugs. In addition, a sizeable number of compounds still fail the expensive human ‘thorough QT’ trial, as their QT prolongation risk remains undetected until that late stage (Gintant, 2011).
Figure 3.
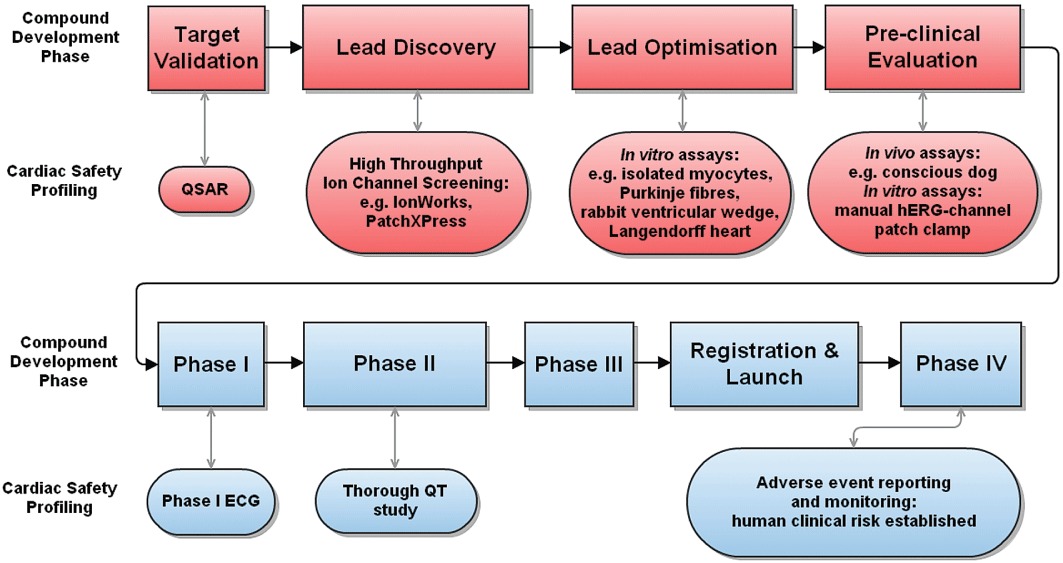
A generic progression strategy for evaluation of QT prolongation risk during pharmaceutical compound development. Top rows (red): preclinical assays, performed on a large number of candidate compounds; bottom rows (blue): clinical assays performed in human. Rectangular boxes: key stages of drug development; oval shapes: profiling activities conducted to assess pro-arrhythmic risk.
However, the sequence that we have outlined so far, hERG-channel block → APD prolongation → QT prolongation → TdP, is far from a definitive description of the problem (Hoffmann and Warner, 2006). Many compounds that would fail the (relatively recently introduced) ICH-S7B and E14 guidelines have reached the market before and are not associated with TdP. Many other possible factors have been suggested (Corrias et al., 2010), including AP triangulation (Hondeghem et al., 2001), reverse-rate dependence (Hondeghem and Hoffmann, 2003), dispersion of repolarization (Valentin et al., 2004), rate adaptation (Green et al., 2011) and beat-to-beat variation in QT (Abrahamsson et al., 2011; Jacobson et al., 2011), among others. Therefore, hERG-channel block and QT prolongation are neither necessary nor sufficient conditions for a torsadogenic risk, despite a strong association (Straus et al., 2005). As the precise mechanisms that lead to initiation of TdP remain undetermined, hERG-channel block and QT prolongation remain the most widely employed biomarkers for detecting TdP risk.
Drug actions on multiple cardiac ion channels may explain some of the discrepancies highlighted earlier (Martin et al., 2004). A drug may block both IKr, which carries a current contributing to repolarization, and other channels, such as fast sodium (INa) or L-type calcium (ICaL), which carry currents that oppose repolarization. A compound therefore may be a hERG-channel blocker, but not markedly prolong AP duration or QT (see Figure 4). This is believed to be the case for Vernakalant [which also blocks INa (Schmitt et al., 2008)]. Similarly, if a drug blocks the hERG-channel only mildly (or not at all), but impedes another AP-shortening channel (e.g. the slowly activating potassium current, IKs), then AP lengthening may be present in spite of an apparently safe hERG assay, potentially causing QT prolongation that exceeds what would have been expected from examining hERG action alone.
Figure 4.
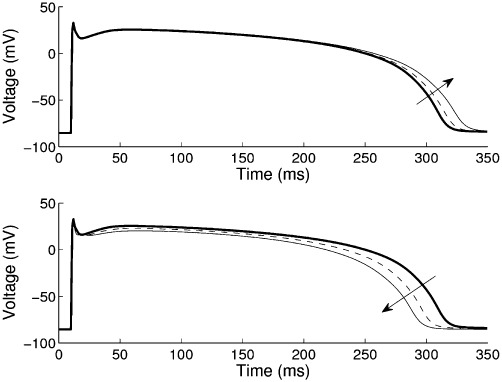
Changes to action potential repolarization during simulated exposure to Verapamil. Using the human epicardial myocyte model of (Ten Tusscher and Panfilov, 2006) at steady 1 Hz pacing, the application of Verapamil is modelled using a conductance-block model with hERG-channel IC50=143 nM and CaL IC50=100 nM [data from (Mirams et al., 2011)]. Top: effect of hERG-channel block only; bottom: combined block of hERG and L-type calcium channels. Arrows indicate changes caused by increasing drug concentrations from 0 nM as control (bold) to 25 nM (dashed) and 81 nM (thin solid). 81 nM is the maximum effective free therapeutic plasma concentration.
Such potential for multiple ion-channel effects is recognized by pharmaceutical companies. Most, if not all, have started to employ high-throughput screens on a range of cardiac ion-channel targets (typically three to six), early in drug development (see Figure 3). The individual ion-channel targets are not yet standardized across the industry, and the results of screens are rarely used quantitatively and in context with one another.
High-throughput screens for multiple targets provide large amounts of data, and novel means are needed for turning this into meaningful information to support flagging-up of potential negative side effects that warrant further investigation in subsequent studies, or indeed to identify drug candidates that should not be progressed further.
Evaluation of compounds in an integrated biological system (e.g. animal-based models) is often a key to understanding the implications of multichannel block. However, these model systems are costly, demand primary tissue and data gathering is low-throughput. For these reasons, they are used later on in the drug development pipeline, after considerable investment has already been made, and when alternative chemical leads have already been deselected. In silico approaches offer the opportunity of improving the decision making at a time when alternative lead compounds are still in scope, and before considerable expense and time has been committed. In Figure 5, we outline the benefits associated with in silico approaches, supporting early and improved decision making.
Figure 5.
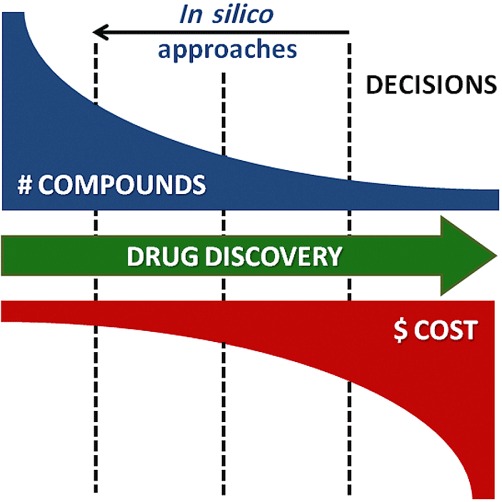
In silico approaches offer the possibility of bringing forward decisions on the viability of compounds for further development. In silico models can identify potentially unsafe compounds earlier in the pipeline, when alternative chemical leads still exist, and before expensive and time-consuming safety testing has occurred. The resulting chemical leads are more likely to succeed in later safety tests. This will reduce costs and improve the chances of developing a successful compound.
In silico tools
A number of approaches are used to quantitatively describe the interactions of a drug compound with cardiac ion channels, including molecular dynamics simulations and quantitative structure activity relationship (QSAR) models.
In molecular dynamics studies, drug and ion channels are represented at an atomic level, and affinity is established in terms of energy minimization [notable recent work includes the study by Silva et al. (2009) linking molecular dynamics to biophysical models]. A drawback is that this approach is computationally very expensive. QSAR modelling is a statistical approach, based on evaluating properties of the molecular structure and estimating target affinity based on a compound's similarity to others contained in a historical dataset (Inanobe et al., 2008).
Because the approach to, and tools for, simulation have matured, both of these models can be used prior to any ‘wet-lab’ experimental work on a compound, relying only on knowledge of the chemical structure. In this review, however, we will focus on another type of in silico simulation: biophysically detailed AP modelling.
Since Hodgkin & Huxley's Nobel Prize winning description of AP formation in nerve cells (Hodgkin and Huxley, 1952), and its subsequent application to cardiomyocytes (Noble, 1960; 1962), cardiac electrophysiology insight has been aided by biophysically based computational models of AP formation […], which we will refer to as ‘AP models’. There is another type of AP model: a ‘phenomenological model’. This represents features of an AP without modelling ion channels (Bueno-Orovio et al. 2008; Walmsley et al., 2010). These simplified models can be helpful in tissue simulations, but they are not generally suited for the mechanistic study of drug actions. So ‘AP models’ in this review refers to biophysically detailed models containing descriptions of individual ionic currents. Today, these AP models represent some of the most detailed and well-tested models in systems biology (Kohl and Noble, 2009). The earliest use of such models to study the action of pharmaceutical compounds in cardiac cells dates back to the 1970s (Katzung et al., 1977). In terms of drug safety testing, these models are used in the context of information on compound actions gathered from experimental data, and to integrate insight across scales of spatial complexity, from the single ion channel to cells, tissue, organ and whole body (Figure 6).
Figure 6.
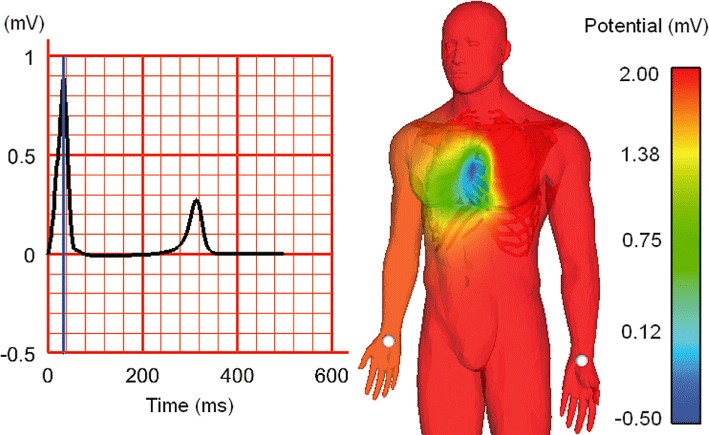
Simulation of human body-surface ECG, using a biophysically detailed cell electrophysiology model, embedded into an anatomically representative human heart mesh inside a whole-body mesh (containing sub-structures with distinct electrical properties). Left: simulated Lead-I ECG, as measured between the white points indicated on the human mesh (right). The time point of body surface voltage snapshot (on the right) is indicated relative to the ECG by a blue line (on the left). Image courtesy of Nejib Zemzemi, University of Oxford, using techniques developed in (Zemzemi et al., 2011).
Several previous reviews have examined the role of cardiac simulation in drug discovery, design and safety assessment (Noble and Colatsky, 2000; Noble 2008; Fink and Noble, 2010; Rodríguez et al., 2010; Amanfu and Saucerman, 2011), and discussed the potential of the ‘systems biology approach’ for modern healthcare challenges (Noble, 2002a; Hunter et al., 2010; Kohl et al., 2010). Here, we focus on the use of AP models in predicting drug-induced pro-arrhythmic risk.
Modelling drug/ion-channel interaction
In this section, we outline the various classes of mathematical models for the drug/ion-channel interaction studies. These were reviewed more thoroughly by Brennan et al., to whom we refer the interested reader for a more complete description of the derivation of these models (Brennan et al., 2009).
In the majority of cases, a drug affects cardiac ion-channel currents by direct binding. Blocking actions are usually attributed to obstruction of the flow of ions through a channel pore, either by forming a physical obstacle, or by changing the conformation of the ion channel. There are exceptions to this, for example pentamidine, which reduces whole-cell IKr by interference with hERG expression and protein trafficking (Cordes et al., 2005). Such ‘unexpected’ mechanisms are one reason why experimental studies cannot be replaced completely by simulation. But as novel assays are developed (e.g. for hERG protein trafficking interference) such data can be included into existing models, and thereby reduce the likelihood of such unexpected results.
To date, modelling efforts have focussed on simulating direct binding of drug compounds to a channel. Ion-channel models fall into two main categories: Hodgkin–Huxley model (HH) formulations (Hodgkin and Huxley, 1952), and their generalizations, which are termed Markov models (MM).
The simplest way to introduce a drug-block into a cardiac electrophysiology model is via ‘conductance-block’, that is by reducing the maximum conductance of an affected ion channel or transporter, using a scaling factor. This factor follows a function for the dose–response curve, describing the effect of a compound on the maximum current flowing through the target. Typically, this scaling factor ‘b’ is related to the drug concentration ‘[D]’ according to:
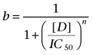 |
(1) |
where IC50 is the drug concentration at which a 50% reduction of the peak current is observed, while n is the Hill coefficient of the dose–response curve (often assumed to be equal to one, i.e. one drug molecule is necessary and sufficient to block one ion-channel). Such models for drug action do not include ‘kinetics’, and drug block is assumed to reach its steady state immediately. Note that it is possible for a simple conductance-block model to exhibit frequency/use dependence at the AP scale, as the roles of a current may change depending on pacing rate. More detailed models of drug/ion-channel interaction take into account the rate at which a drug binds to/unbinds from the ion channel, leading to a differential equation for b (Starmer et al., 1991). Both these types of drug/ion-channel interaction models can be reproduced in either HH or MM ion-channel formulations.
For more complex drug/ion-channel interactions, the MM approach is required (while it may be a relatively simple exercise for a mathematician to convert HH models into MM, the automation of this process is non-trivial). In MM formulations, a drug may be given access only to certain channel states (open, closed, inactivated, etc.) termed state-dependent block. The speed at which overall drug binding (and unbinding) occurs will therefore depend upon the proportion of time that a channel spends in each of the states. This will vary with pacing rates, and allows one to incorporate use/rate-dependence of drug actions (Barber et al., 1991; Tsujimae et al., 2007). The term ‘voltage dependent’ block is often applied to describe this, but this is potentially misleading, as drug binding and block depends primarily on the state of the ion-carrying protein (of course, different states of a channel may become available preferentially at certain voltages). True voltage-dependent block, as seen experimentally, occurs when the charge of a drug compound affects its likelihood of reaching a binding site in a transmembrane voltage-dependent manner [independent of/in addition to channel conformation availability effects (Tikhonov and Magazanik, 1998)]. This may be included in Markov models by making drug binding rates for individual states functions of voltage.
A further intricacy occurs when the bound drug alters the rates of transition between ion-channel states. This is termed allosteric block. Models incorporating this behaviour are complex and require large amounts of experimental data for parameterization (often more than are available).
Many other factors that can influence the binding of a drug to an ion channel are increasingly being modelled, including: temperature, pH (Cardona et al., 2010), or co-administration of other compounds. In practice, however, pharmaceutical ion-channel screening does not usually record sufficient data to evaluate many of the kinetic changes caused by drug/ion-channel interaction, and it will not do so until more advanced high-throughput screening technologies/approaches become available. Simpler dose–response curves, described by the equation above, are often the only available quantitative data. Fortunately, with the exception of drugs that exhibit allosteric effects, the ‘conductance block’ approach is as useful as kinetic models of drug action, when considering steady-state pacing conditions (Brennan et al., 2009). However for conditions with variable pacing rates (such as in arrhythmia) the conductance block approximation may be insufficient for simulation of realistic drug actions. In addition, drug block is rarely at a true ‘steady state’, as compound concentration fluctuates between dosing. The incorporation of, or coupling to, pharmacokinetic/pharmacodynamic models to predict the free concentration of drug compound likely to be found at the cardiac ion channels is a desirable future development (van der Graaf and Benson, 2011).
At present all ‘production simulations’ that we are aware of in pharmaceutical companies take (quite reasonably) the simpler modelling approach of conductance block (Bottino et al., 2006; Davies et al., 2012). The increasing availability of high-throughput ion-channel screens means that required parameters for these models (IC50 and Hill coefficient) can be acquired at low cost for large numbers of candidate compounds, thereby effectively auto-generating the parameters for this class of models. One plea to industry in this context is to consider dose–response curve characterisation as a parameter fitting exercise for equation (1) above. There is information to be gained from the parameters of the dose–response curve, even where a drug effect reaches, for example, only 40% block of an ion channel at the largest concentration tested. Communication of such data is relevant, and much more informative than stating that ‘no IC50 was attained’.
Anything other than simple conductance-block models are typically developed manually, requiring a skilled computational modelling team. The literature on such models is expanding quickly (Noble, 1980; Bean et al., 1983; Gilliam et al., 1989; Weirich and Antoni, 1989; Pásek and Simurda, 2004; Clancy et al., 2007; Comtois et al., 2008; Brennan and Tarassenko, 2010). But manual ‘model building’ is not scalable to the creation of models for every drug candidate and ion channel of interest. The process of ‘model building’, in particular for kinetics and allosteric effects of drug/ion-channel interactions, needs to be automated and established in-house inside pharmaceutical companies. This should be aided by innovative experimental designs, in order to focus on the most relevant data for parameter/model fitting techniques (Ball and Sansom, 1989; Ball et al., 1999; Fink and Noble, 2009; Moreno et al., 2011), and will benefit from academia/pharma consortia, such as the recent PreDiCT initiative (Fletcher et al., 2011).
Modelling the effects of drugs on the AP
There has been some success in the safety pharmacology field using computational models that incorporate drug/ion-channel representations into whole-cell AP models. These models relate the change in membrane potential to the sum of the currents flowing in and out of the cell. They are composed of (typically 10–70) differential equations, with multiple parameters. To facilitate their exchange and re-use, the CellML model description language and associated tools have been vital (Lloyd et al., 2008; Garny et al., 2009; Cooper et al., 2011). The reason for the relevance of whole-cell models is that it is at this spatial scale that the block of single or multiple ion channels may manifest itself in an alteration of AP parameters, a manifestation of key electrophysiologically relevant behaviour of cardiac myocytes (Rudy, 2007). Whole-cell integration of ion currents occurs, of course, also in ‘biological model systems’ of adult heart cells, such as stem-cell derived cardiomyocytes. A recent combined experimental and modelling study has highlighted that the expectation that this may soon lead to patient-specific pharmacological assays could be overly optimistic (Jonsson et al., 2012), as ion current amplitudes and activation properties of stem-cell derived cardiomyocytes are probably closer to neonatal than to adult phenotypes.
In terms of cardiac drug safety assessment, two notable examples of the biophysical simulation approach are related to the development of ivabradine and ranolazine. Ivabradine blocks the so-called ‘funny current’ (If) first observed in 1968, when it was assumed to carry only potassium ions (Noble and Tsien, 1968). The If current was later identified as a mixed cation channel (DiFrancesco, 1981), and simulation work suggested that reduction of If would slow sino-atrial node pacemaker cells by a moderate amount only, due to redundancy with other ion channels (Noble et al., 1992). Ivabradine, marketed by Servier as Procoralan and Corlentor for the symptomatic management of stable angina pectoris, has been reported to be highly effective and safe, causing a small but significant reduction in heart rate. The European Medicines Agency are currently moving to add ‘chronic heart failure’ to the indications for Corlentor, where it has been reported to significantly reduce mortality (Swedberg et al., 2010). Thus, biophysically detailed AP modelling predicted essential aspects of the pharmacological profile of ivabradine, based on the description of underlying ionic current mechanisms.
Ranolazine (Ranexa; CV Therapeutics, now Gilead) is an anti-angina drug. In spite of a large affinity for the hERG-channel, it was observed to cause only moderate QT prolongation. As mentioned earlier, strong hERG-channel block would normally spell the end for a compound, but CV Therapeutics had not observed any early after-depolarizations (as one might otherwise have expected with the degree of hERG-channel block that ranolazine caused). Further studies showed that ranolazine is also a potent blocker of the late/persistent sodium current INa,p (Antzelevitch et al., 2004). At this point, there was still no quantitatively plausible explanation as to why the block of the relatively small sodium current INa,p could curb potentially detrimental effects expected from strong hERG-channel block. The company turned to AP models. These showed that block of INa,p compensated for many of the effects that would otherwise have been associated with hERG-channel block, including AP prolongation. Computer simulations further identified that the reduced sodium loading, caused by INa,p block, led to reduced calcium loading of the cell. The associated smooth repolarization makes QT prolongation, which is still seen in this setting, much less proarrhythmic than usual (Noble and Noble, 2006). Benefiting from this mechanistic insight provided by AP models, Ranolazine is now on the market for angina treatment, and it is being tested as an anti-arrhythmic agent (Belardinelli et al., 2006).
Motivated by the observation that multi-channel effects may alleviate torsadogenicity, a recent modelling investigation (Mirams et al., 2011) examined the effect of including into a human ventricular AP model the IC50 data for three ion channels: hERG/IKr, INa and ICa,L. The study included IC50 data for 31 currently marketed compounds, and assessed the extent to which AP prolongation in the model correlated with the human clinical TdP risk of each drug. By simply including three (instead of IKr only) ion-channel effects, the predictive classification of drugs into the risk categories established by Redfern (Redfern et al., 2003) was substantially improved. This suggests that AP modelling of multiple ion-channel effects may improve early identification of clinical risk. It also implies that torsadogenic effects of hERG block can be eliminated by inhibiting additional channels. Of course, in certain circumstances, this may be detrimental for cardiac function via other mechanisms (e.g. reduced contractility), which represents a separate but important safety issue.
So, how many ion channels should one screen? And how about currents that cannot easily be recorded? This forms part of a conundrum that one might term the ‘inverse problem’: is it possible to infer how a drug affects different ionic currents by measuring an overall change to the AP (which is easier to record than ion currents)? Bottino et al. approached this task with some success, measuring hERG-channel block and associated AP shapes experimentally, and then attempting to fit the IC50 values of five other channels/exchangers present in the cell, using computational AP models (Bottino et al., 2006). As pharmaceutical ion-channel assays expand, this approach may become relevant in the evaluation of drug action on channels/exchangers that cannot yet be screened efficiently in an automated setting, or that may not even be known.
One of the remaining challenges is the variability of data recorded in cardiac preparations of many different types. This can be caused by experimental errors, but more usually, data variability is an expression of genuine differences between individual cells (or hearts), termed extrinsic variability; or to inherent stochasticity in the behaviour of a single cell (or ion channel), termed intrinsic variability. Mathematical consideration of these factors is only just beginning (Dangerfield et al., 2010; Walmsley et al., 2010). First applications to simulating drug action on cardiac cells include the recent study by Davies et al., who re-parameterized a dog ventricular AP model, fitting it to traces from different experiments, thus encapsulating extrinsic variability in the model. They were able to observe different levels of AP prolongation in models representing different animals, potentially explaining some of the observed variation in reaction to drugs as a consequence of natural variation in electrophysiology (Davies et al., 2012). This work points towards the need to develop a spectrum of models, representing such variability as will be present in a patient population (Sarkar & Sobie, 2011). This is a concept that may become important in detecting rare side effects and working towards patient-specific prescription.
Drug effects in tissue models
Like real cells, AP models exhibit different behaviour in isolation, compared to a setting when they are coupled together in tissue, and many of the clinically used biomarkers can be observed only at the tissue level. For this reason, AP models have been coupled together to form tissue simulations (Winslow et al., 1993; Silva and Rudy, 2010), and a number of advanced simulation software packages have been developed to run simulations, from basic cell models (Garny et al., 2009), 1-D (string) or 2-D (sheet) tissue models that can be implemented on a personal computer, to more extensive 3-D (block or anatomical volume) simulations run on supercomputers (Bordas et al., 2009; Pitt-Francis et al., 2009; Niederer et al., 2011a). In recent years, the simulation of drug effects has been an expanding area of application of these tissue models (Soubret et al., 2009).
One reason for this trend is that the most frequently used marker for cardiac side effects in the clinical setting is the QT interval of the ECG (Figure 2), and multicellular simulations are required to reproduce these. Drug actions, incorporated into ion-channel and AP models, can be related to ECG changes at all levels of tissue complexity. Pseudo-ECG behaviour, computed using 1-D tissue strand models, is thought to reproduce certain aspects of ‘real’ ECGs well, such as relative changes in QT interval duration (Viswanathan et al., 1999; Benson et al., 2009). 2-D and 3-D simplified models (Garny et al., 2005), highly structured 3-D whole ventricular representations (Rodríguez et al., 2010), and body surface potential simulations (Zemzemi et al., 2011), as illustrated in Figure 6, can all simulate ECG dynamics. In the remainder of this section, we will illustrate some recent efforts in studying drug actions using these tissue models.
At the tissue strand level, Obiol-Pardo et al. used QSAR models to predict the IC50 values for IKr and IKs, and then simulated conductance-block in 1-D coupled AP models (Obiol-Pardo et al., 2011). This allowed them to compute drug-induced changes in a pseudo-ECG, based purely on projections between compound structure data and historical information on IKr and IKs block by similar compounds. This suggests that it may become possible to simulate ECG effects even before a compound is synthesized, particularly when reliable QSAR models are developed for further ion channels.
Of a particular interest for the prediction of arrhythmogenicity is the identification of drug-induced changes in transmural dispersion of repolarization (Müller and Dhein, 1993), as this may provide the conditions necessary for sustenance of arrhythmic behaviour. For experimental exploration of this concept, coronary-perfused ventricular tissue wedges have become popular, and a matching ‘virtual ventricular wedge’ model of canine tissue has been developed as a corresponding in silico safety screen (Benson et al., 2008; Holden, 2010).
Arrhythmias can be induced in wedges (and other cardiac tissue models) by special pacing protocols, and several studies have examined how drug actions change the vulnerability of cardiac tissue to the induction of arrhythmias, including ventricular fibrillation (Weiss et al., 1999; Qu and Weiss, 2005; Seigneuric et al., 2005). The role of early after-depolarizations in triggering and/or sustaining arrhythmias in this context, has been simulated (Viswanathan and Rudy, 1999; Huffaker et al., 2007), as has been the hypothesis that ischemic tissue may provide a substrate for arrhythmia initiation under drug action (Trénor et al., 2005).
In Moreno et al., allosteric models for block of the fast sodium current, INa, were fitted to experimental data for lidocaine and flecainide (Moreno et al., 2011). Simulations at the human ventricle scale highlighted differences in sustainability of ventricular fibrillation, which matched observed differences in the clinical profiles of the two drugs.
Thus, computer models of drug actions on ion channels, embedded into cell AP models that form part of multi-cellular tissue simulations, may reproduce and partially predict relevant experimental and clinical findings. This is a remarkable achievement, and illustrates the value of representing our understanding of biological structures and functions in quantitative mathematical descriptions that adhere to basic physical laws, such as conversation of mass and charge, or of reaction and diffusion behaviour.
Challenges and opportunities
The field of computational electrophysiology has made large strides towards more accurate simulation of drug-induced changes in cardiac electrical behaviour. Of course, there are many further mechanisms and interactions that need to be considered in order to allow systematic, accurate and high-throughput prediction of drug actions on the heart. Tissue and organ electrophysiological modelling still requires significant computing power that may not be easily available for routine work [although improvements in hardware and model implementation have reduced this overhead very significantly in recent years (Niederer et al., 2011b)].
Present limitations include that fact that the vast majority of ‘whole heart models’ do not include descriptions of the atria, atrio-ventricular activation, mechano-electric coupling and feedback, tissue deformation, coronary flow, and fluid/solid interactions, let alone their combined and interactive effects. So, most of the in silico hearts do not actually pump, even though it is ultimately this mechanical activity of the heart that decides a patient's fate! The heart also contains more non-mycoytes than muscle cells (Camelliti et al., 2005), and contributions of endothelial cells, connective tissue or intra-cardiac neurones (to name but a few) are not yet receiving due attention in modelling (and, arguably, experimental research).
Some of the mechanisms thought to be of relevance for arrhythmogenicity rely on heterogeneous cell behaviour throughout the tissue, for example regional (and temporal) dispersion in excitability, refractoriness and electrical load. There is an ongoing debate about the presence and relevance of cells in the mid-myocardium with prolonged AP duration [see the recent M-cells debate in the Heart Rhythm journal (Janse et al., 2011; Nattel et al., 2011; Wilson et al., 2011)], which highlights that heterogeneity throughout the heart is poorly understood at present. It is, arguably, even more poorly modelled. We are far from a comprehensive appreciation of the physiological relevance of local heterogeneity for homogenous global cardiac function [called ‘homogeneity out of heterogeneity’ (Katz and Katz, 1989)], and are barely touching the surface of patho-physiological changes to this heterogeneity, whose increase – but presumably also reduction – may well underlie disturbances in organ behaviour (Markhasin et al., 2003).
Spontaneous drug-induced arrhythmias at therapeutic concentrations do not tend to occur in silico. But, perhaps, we should not expect them, as: (i) at present our models represent healthy cardiac tissue, with no factors that pre-dispose towards arrhythmias, and (ii) many drug-induced arrhythmias are rare events in the clinic. Disease states, gender differences, electrolyte imbalance, energy availability and use, autonomic control, circadian changes (Jeyaraj et al., 2012), etc. all need to be incorporated into the computational models of the future, and used for cardiac safety testing. This work has begun with models of the AP for patients with hereditary long QT syndromes (Clancy and Rudy, 2001; 2002; Grant et al., 2002), and for models of ischemia (Noble, 2002b). Application of these models to drug safety investigations will open the door to simulating the risk for different patient subgroups. This could serve as an important step towards better stratification of therapeutic approaches, so that patients with no anticipated susceptibility to cardiac adverse events could benefit from a range of drugs that may otherwise not be accessible to them. This concept of ‘rehabilitation of pharmacological compounds’ also benefits from a potentially attractive economical context, as much of the testing and development work has already occurred.
To explore these possibilities, members of preDiCT (http://www.vph-predict.eu), a Europe-wide consortium on cardiac modelling for drug safety, have put forward state-of-the-art models and highlighted their potential use in a recent workshop that was well-attended by representatives from pharmaceutical companies and regulatory agencies, including AstraZeneca, Pfizer, GlaxoSmithKline, Roche, Health Canada, the US Food and Drug Administration, the UK Medicines and Healthcare products Regulatory Agency and the European Medicines Agency [for report see (Fletcher et al., 2011)]. Some of the more important suggestions made at the meeting focussed on the issue of access to, and consistency of, datasets; and we expand upon them here.
Firstly, good-quality experimental data are needed, and we applaud the efforts of teams who are assembling and publishing large ion-channel screens. Notable examples include the University of Kraków who have put together an open-access dataset of hERG-channel IC50 values (Polak et al., 2011), and the AstraZeneca safety pharmacology group for both hERG- and fast sodium channel data (Redfern et al., 2003; Harmer et al., 2011). In this context, the publication of raw datasets, rather than pre-analysed IC50 values and dose–response curves, would be highly desirable, as the original data allow one to glean extra parameters (for example Hill coefficients or, potentially, ion-channel kinetic parameters).
Secondly, because we want to be able to accurately predict when a drug is safe, data from studies that are suggestive of lack of effect are crucial in training and testing the models (a measure of specificity is only possible when such data are included). The current trend of not publishing such results needs to change, as data that is ‘negative for an effect’ is not ‘negative data’.
Thirdly, it is evident that assays are not performed consistently between laboratories. Temperature, species, cell lines, perfusion protocols, etc. are not standardized, and assays may change over time as new technologies become available. To tackle this problem an international group of scientists recently published a draft Minimum Information standard for Cardiac Electrophysiology Experiments (Quinn et al., 2011), in an effort to, at the very least, define the differences between laboratories, including descriptions of the processing, filtering or other conditioning of data that may have occurred. Not only the reporting of (‘positive and negative’) raw data, but more comprehensive metadata are needed to advance this area.
Despite the limitations in models and available data, now is the time to expand the role of cardiac simulation in the pharmaceutical industry. The development costs per marketed compound are becoming prohibitively expensive, and any assays that have the potential to reduce these costs, via more accurate and/or earlier safety predictions (Figure 5), are worth investigating (Mirams and Noble, 2011).
In principle, cardiac AP models now capture a majority of relevant sarcolemmal ion channels, exchangers and pumps that a pharmaceutical compound is likely to interact with. There is no technological reason, or gap in biological knowledge, to suggest that prediction of drug actions will not be possible on this basis. Of course, simulation will not replace the role of animal-based experimental models in cardiac safety testing for the foreseeable future. There will always be novel drug (off-)target effects that are not screened for, which are important in cardiac behaviour, perhaps in genetic toxicology involving up-/down-regulation of channel expression, for example. But models can provide increasingly accurate predictions for a vast majority of compounds with simple affinity for, say, the 10 most promiscuous ion channels and exchangers. Computational models do not need to provide 99% accurate predictions to be useful, as the in vitro models that they complement and/or partially replace tend to be no more than ∼70% specific for the human QT liability. Thus, replacement of experimental assays, including animal-based work (Figure 3) is not an impossible vision, but something that has already begun, while remaining experiments will be conducted more selectively and yield more relevant information.
In the future, we envisage that pharmaceutical companies will not store databases of IC50 values only, but instead create and use repositories of kinetic models that represent compound action on a particular ion channel. These models will be integrated into AP/tissue/organ simulations and analysed to provide a biophysically-based rational risk assessment. The simulation of animal and human tests will be performed in tandem with, and aided by, fewer yet more targeted experimental studies. An agreement of simulation with animal experiments, for example, will provide confidence in the computational projection towards estimation of clinically relevant effects in humans, which may be different due to species differences – yet another domain of mathematical model application. Disagreement will highlight the need for further, more thorough investigation.
Conclusions
Biophysically detailed AP models can offer a ‘short cut’ for cardiac safety testing in the context of QT evaluation. In addition, computational modelling at tissue and organ levels can be helpful in predicting cardiac (and, increasingly, other organ) side effects. Models are already helping to develop an understanding of the mechanisms causing, or predisposing to, cardiac side effects such as TdP. These models do not only allow one to reproduce, and increasingly predict, the result of existing tests, but through an improved understanding of the underlying mechanisms involved in the regulation of cardiac activity, they can be used to propose better safety assessments.
The various challenges outlined earlier can only be addressed via pre-competitive collaboration between industry and academia, bringing together experts with skills in electrophysiology, pharmacology, toxicology, mathematical modelling, numerical methods, computer science, data management and statistics. In the short term, cardiac electrophysiology models offer an opportunity to gain extra information from in vitro and in vivo animal-based model systems. In the medium term, they will allow us to reduce the numbers of experiments that have to be performed, and speed-up the process of safety assessment. In the longer term, simulation offers the hope of replacing certain assays, and suggesting novel ones with greater predictive power.
Acknowledgments
The authors would like to thank Nejib Zemzemi for providing the graphic in Figure 6, and members of the preDiCT consortium and scientific advisory board for fruitful discussions. We acknowledge financial supported by the European Commission's Virtual Physiological Human Initiative, and by the British Heart Foundation. GRM gratefully acknowledges research support from GlaxoSmithKline Grants & Affiliates scheme; PK is a Senior Fellow of the British Heart Foundation.
Glossary
- APD
action potential duration
- hERG
human ether-a-go-go-related gene, encoding the major IKr channel protein
- HH
Hodgkin–Huxley
- ICH
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- If
hyperpolarisation-activated depolarising (pacemaker) current, called ‘funny current’
- IKr
rapidly activating potassium current
- IKs
slowly activating potassium current
- INa
fast sodium current
- INa
p, late/persistent sodium current
- ICaL
L-type calcium current
- MM
Markov model
- QT
the Q to T interval of the ECG
- QSAR
quantitative structure activity relationship
- TdP
Torsade de Pointes
Conflicts of interest
GRM has received research support from GlaxoSmithKline Plc, YC is employed by GlaxoSmithKline Plc, and MRD is employed by AstraZeneca UK Ltd.
References
- Abrahamsson C, Dota C, Skallefell B, Carisson L, Berggren A, Edvardsson N, et al. DeltaT50 – a new method to assess temporal ventricular repolarization variability. J Electrocardiol. 2011;44:477.e1–477.e9. doi: 10.1016/j.jelectrocard.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Amanfu RK, Saucerman JJ. Cardiac models in drug discovery and development: a review. Crit Rev Biomed Eng. 2011;39:379–395. doi: 10.1615/critrevbiomedeng.v39.i5.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball FG, Sansom MSP. Ion-channel gating mechanisms: model identification and parameter estimation from single channel recordings. Proc R Soc Lond B Biol Sci. 1989;236:385–416. doi: 10.1098/rspb.1989.0029. [DOI] [PubMed] [Google Scholar]
- Ball FG, Cai Y, Kadane JB, O'hagan A. Bayesian inference for ion–channel gating mechanisms directly from single–channel recordings, using Markov chain Monte Carlo. Proc R Soc LondSer A Math Phys Eng Sci. 1999;455:2879–2932. [Google Scholar]
- Barber MJ, Starmer CF, Grant AO. Blockade of cardiac sodium channels by amitriptyline and diphenylhydantoin. Evidence for two use-dependent binding sites. Circ Res. 1991;69:677–696. doi: 10.1161/01.res.69.3.677. [DOI] [PubMed] [Google Scholar]
- Bean BP, Cohen CJ, Tsien RW. Lidocaine block of cardiac sodium channels. J Gen Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 2):iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AP, Aslanidi OV, Zhang H, Holden AV. The canine virtual ventricular wall: a platform for dissecting pharmacological effects on propagation and arrhythmogenesis. Prog Biophys Mol Biol. 2008;96:187–208. doi: 10.1016/j.pbiomolbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Benson AP, Al-Owais M, Tong W, Holden A. hERG effects on ventricular action potential duration and tissue vulnerability: a computational study. Funct Imaging Model Hear. 2009;LNCS 5528:172–181. [Google Scholar]
- Bordas R, Carpentieri B, Fotia G, Maggio F, Nobes R, Pitt-Francis J, Southern J. Simulation of cardiac electrophysiology on next-generation high-performance computers. Philos Trans R Soc A Math Phys Eng Sci. 2009;367:1951–1969. doi: 10.1098/rsta.2008.0298. [DOI] [PubMed] [Google Scholar]
- Bottino D, Penland RC, Stamps A, Traebert M, Dumotier B, Georgieva A, et al. Preclinical cardiac safety assessment of pharmaceutical compounds using an integrated systems-based computer model of the heart. Prog Biophys Mol Biol Elsevier Sci. 2006;90:414–443. doi: 10.1016/j.pbiomolbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Brennan T, Fink M, Rodríguez B. Multiscale modelling of drug-induced effects on cardiac electrophysiological activity. Eur J Pharm Sci. 2009;36:62–77. doi: 10.1016/j.ejps.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Brennan TP, Tarassenko L. Effects of Sotalol on T-wave morphology in 24-hour Holter ECG recordings. Comput Cardiol. 2010;2010:689–692. [Google Scholar]
- Bueno-Orovio A, Cherry EM, Fenton FH. Minimal model for human ventricular action potentials in tissue. J Theor Biol. 2008;253:544–560. doi: 10.1016/j.jtbi.2008.03.029. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cardona K, Trénor B, Moltó G, Martinez M, Ferrero JM, Starmer F, Saiz J. Exploring the role of pH in modulating the effects of lidocaine in virtual ischemic tissue. Am J Physiol Circ Physiol. 2010;299:H1615–H1624. doi: 10.1152/ajpheart.00425.2010. [DOI] [PubMed] [Google Scholar]
- Clancy CE, Rudy Y. Cellular consequences of HERG mutations in the long QT syndrome: precursors to sudden cardiac death. Cardiovasc Res. 2001;50:301–313. doi: 10.1016/s0008-6363(00)00293-5. [DOI] [PubMed] [Google Scholar]
- Clancy CE, Rudy Y. Na+ channel mutation that causes both Brugada and long-QT syndrome phenotypes: a simulation study of mechanism. Circulation. 2002;105:1208–1213. doi: 10.1161/hc1002.105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Circ Physiol. 2007;292:H66–H75. doi: 10.1152/ajpheart.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comtois P, Sakabe M, Vigmond EJ, Munoz M, Texier A, Shiroshita-Takeshita A, Nattel S. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Circ Physiol. 2008;295:H1489–H1504. doi: 10.1152/ajpheart.01054.2007. [DOI] [PubMed] [Google Scholar]
- Cooper J, Mirams GR, Niederer S. High throughput functional curation of cellular models. Prog Biophys Mol Biol. 2011;107:11–20. doi: 10.1016/j.pbiomolbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Cordes JS, Sun Z, Lloyd DB, Bradley JA, Opsahl AC, Tengowski MW, et al. Pentamidine reduces hERG expression to prolong the QT interval. Br J Pharmacol. 2005;145:15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrias A, Jie X, Romero L, Bishop MJ, Bernabeu MO, Pueyo E, Rodriguez B. Arrhythmic risk biomarkers for the assessment of drug cardiotoxicity: from experiments to computer simulations. Philos Trans R Soc A Math Phys Eng Sci. 2010;368:3001–3025. doi: 10.1098/rsta.2010.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangerfield C, Kay D, Burrage K. 2010. Stochastic models and simulation of ion channel dynamics. ICCS 2010 – International Conference Computer Science. 1581–1590.
- Davies MR, Mistry HB, Hussein L, Pollard CE, Valentin J, Swinton J, Abi-Gerges N. An in silico canine cardiac midmyocardial action potential duration model as a tool for early drug safety assessment. Am J Physiol Heart Circ Physiol. 2012;302:1466–1480. doi: 10.1152/ajpheart.00808.2011. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Noble D. Markov models for ion channels: versatility versus identifiability and speed. Philos Trans R Soc A Math Phys Eng Sci. 2009;367:2161–2179. doi: 10.1098/rsta.2008.0301. [DOI] [PubMed] [Google Scholar]
- Fink M, Noble D. Pharmacodynamic effects in the cardiovascular system: the modeller's view. Basic & Clin Pharmacol & Toxicol. 2010;106:243–249. doi: 10.1111/j.1742-7843.2009.00534.x. [DOI] [PubMed] [Google Scholar]
- Fletcher K, Shah RR, Thomas A, Tobin F, Rodriguez B, Mirams GR, et al. Novel approaches to assessing cardiac safety-proceedings of a workshop: regulators, industry and academia discuss the future of in silico cardiac modelling to predict the proarrhythmic safety of drugs. Drug Saf. 2011;34:439–443. doi: 10.2165/11591950-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garny A, Noble D, Kohl P. Dimensionality in cardiac modelling. Prog Biophys Mol Biol. 2005;87:47–66. doi: 10.1016/j.pbiomolbio.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Garny A, Noble D, Hunter PJ, Kohl P. Cellular open resource (COR): current status and future directions. Philos Trans R Soc A Math Phys Eng Sci. 2009;367:1885–1905. doi: 10.1098/rsta.2008.0289. [DOI] [PubMed] [Google Scholar]
- Gilliam FR, Starmer CF, Grant AO. Blockade of rabbit atrial sodium channels by lidocaine. Characterization of continuous and frequency-dependent blocking. Circ Res. 1989;65:723–739. doi: 10.1161/01.res.65.3.723. [DOI] [PubMed] [Google Scholar]
- Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther. 2011;129:109–119. doi: 10.1016/j.pharmthera.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Gottlieb S. Antihistamine drug withdrawn by manufacturer. BMJ. 1999;319:7. doi: 10.1136/bmj.319.7201.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaf P, Benson N. Systems pharmacology: bridging systems biology and pharmacokinetics-pharmacodynamics (PKPD) in drug discovery and development. Pharm Res. 2011;28:1460–1464. doi: 10.1007/s11095-011-0467-9. [DOI] [PubMed] [Google Scholar]
- Grandi E, Pasqualini FS, Bers DM. A novel computational model of the human ventricular action potential and Ca transient. J Mol Cell Cardiol. 2010;48:112–121. doi: 10.1016/j.yjmcc.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AO, Carboni MP, Neplioueva V, Starmer CF, Memmi M, Napolitano C, et al. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Investig. 2002;110:1201–1210. doi: 10.1172/JCI15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Diaz GJ, Limberis JT, Houseman KA, Su Z, Martin RL, et al. Ventricular rate adaptation: a novel, rapid, cellular-based in-vitro assay to identify proarrhythmic and torsadogenic compounds. J Pharmacol Toxicol Methods. 2011;64:68–73. doi: 10.1016/j.vascn.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Harmer AR, Valentin J, Pollard CE. On the relationship between block of the cardiac Na+ channel and drug-induced prolongation of the QRS complex. Br J Pharmacol. 2011;164:260–273. doi: 10.1111/j.1476-5381.2011.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney JE. Withdrawal of troglitazone and cisapride. JAMA. 2000;283:228. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis?: A review of emerging trends. J Pharmacol Toxicol Methods. 2006;53:87–105. doi: 10.1016/j.vascn.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Holden AV. Development and application of human virtual excitable tissues and organs: from premature birth to sudden cardiac death. Altern Lab Anim. 2010;38:87–99. doi: 10.1177/026119291003801S12. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Hoffmann P. Blinded test in isolated female rabbit heart reliably identifies action potential duration prolongation and proarrhythmic drugs: importance of triangulation, reverse use dependence, and instability. J Cardiovasc Pharmacol. 2003;41:14–24. doi: 10.1097/00005344-200301000-00003. [DOI] [PubMed] [Google Scholar]
- Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001;103:2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- Huffaker RB, Weiss JN, Kogan B. Effects of early afterdepolarizations on reentry in cardiac tissue: a simulation study. Am J Physiol Circ Physiol. 2007;292:H3089–H3102. doi: 10.1152/ajpheart.01309.2006. [DOI] [PubMed] [Google Scholar]
- Hunter P, Coveney PV, de Bono B, Diaz V, Fenner J, Frangi AF, et al. A vision and strategy for the virtual physiological human in 2010 and beyond. Philos Trans R Soc A Math Phys Eng Sci. 2010;368:2595–2614. doi: 10.1098/rsta.2010.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH. 2005a. The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
- ICH. 2005b. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-anti arrhythmic drugs. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
- Inanobe A, Kamiya N, Murakami S, Fukunishi Y, Nakamura H, Kurachi Y. In silico prediction of the chemical block of human ether-a-go-go-related gene (hERG) K+ current. J Physiol Sci. 2008;58:459–470. doi: 10.2170/physiolsci.RV-0114-08-07-R1. [DOI] [PubMed] [Google Scholar]
- Jacobson I, Carlsson L, Duker G. Beat-by-beat QT interval variability, but not QT prolongation per se, predicts drug-induced torsades de pointes in the anaesthetised methoxamine-sensitized rabbit. J Pharmacol Toxicol Methods. 2011;63:40–46. doi: 10.1016/j.vascn.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Janse MJ, Coronel R, Opthof T. M cells do not have a functional role in the ventricular myocardium of the intact heart. Hear Rhythm. 2011;8:934–937. doi: 10.1016/j.hrthm.2010.10.048. [DOI] [PubMed] [Google Scholar]
- Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson MKB, Vos MA, Mirams GR, Duker D, Sartipy P, de Boer TP, et al. Application of human stem cell-derived cardiomyocytes in safety pharmacology requires caution beyond hERG. J Mol Cell Cardiol. 2012;52:998–1008. doi: 10.1016/j.yjmcc.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Katz AM, Katz PB. Homogeneity out of heterogeneity. Circulation. 1989;79:712–717. doi: 10.1161/01.cir.79.3.712. [DOI] [PubMed] [Google Scholar]
- Katzung B, Hondeghem LM, Morgenstern J. Computer simulation of cardiac arrhythmogenesis and anti-arrhythmic drug action. J Mol Cell Cardiol. 1977;9:33–33. [Google Scholar]
- Kohl P, Noble D. Systems biology and the virtual physiological human. Mol Syst Biol. 2009;5:1–6. doi: 10.1038/msb.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl P, Crampin EJ, Quinn TA, Noble D. Systems biology: an approach. Clin Pharmacol Ther. 2010;88:25–33. doi: 10.1038/clpt.2010.92. [DOI] [PubMed] [Google Scholar]
- Krikler DM, Curry PV. Torsade De Pointes, an atypical ventricular tachycardia. Br Hear J. 1976;38:117–120. doi: 10.1136/hrt.38.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Lawson JR, Hunter PJ, Nielsen PF. The CellML model repository. Bioinformatics. 2008;24:2122–2123. doi: 10.1093/bioinformatics/btn390. [DOI] [PubMed] [Google Scholar]
- Madhvani R, Xie Y, Pantazis A, Garfinkel A, Qu Z, Weiss J, et al. Shaping a new Ca(2+) conductance to suppress early afterdepolarizations in cardiac myocytes. J Physiol. 2011;589:6081–6092. doi: 10.1113/jphysiol.2011.219600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markhasin VS, Solovyova O, Katsnelson LB, Protsenko Y, Kohl P, Noble D. Mechano-electric interactions in heterogeneous myocardium: development of fundamental experimental and theoretical models. Prog Biophys Mol Biol. 2003;82:207–220. doi: 10.1016/s0079-6107(03)00017-8. [DOI] [PubMed] [Google Scholar]
- Martin RL, McDermott JS, Salmen HJ, Palmatier J, Cox BF, Gintant GA. The utility of hERG and repolarization assays in evaluating delayed cardiac repolarization: influence of multi-channel block. J Cardiovas Pharmacol. 2004;43:369–379. doi: 10.1097/00005344-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Mirams GR, Noble D. Is it time for in silico simulation of drug cardiac side effects? Ann N Y Acad Sci. 2011;1245:44–47. doi: 10.1111/j.1749-6632.2011.06324.x. [DOI] [PubMed] [Google Scholar]
- Mirams GR, Cui Y, Sher A, Fink M, Cooper J, Heath BM, et al. Simulation of multiple ion channel block provides improved early prediction of compounds’ clinical torsadogenic risk. Cardiovasc Res. 2011;91:53–61. doi: 10.1093/cvr/cvr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JD, Zhu ZI, Yang PC, Bankston JR, Jeng MT, Kang C, et al. A computational model to predict the effects of class I anti-arrhythmic drugs on ventricular rhythms. Sci Transl Med. 2011;3:98–83. doi: 10.1126/scitranslmed.3002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A, Dhein S. Sodium channel blockade enhances dispersion of the cardiac action potential duration. Basic Res Cardiol. 1993;88:11–22. doi: 10.1007/BF00788526. [DOI] [PubMed] [Google Scholar]
- Nattel S, Antzelevitch C, Noble D. Resolving the M-cell debate: why and how. Heart Rhythm. 2011;8:1293–1295. doi: 10.1016/j.hrthm.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer S, Mitchell L, Smith N, Plank G. Simulating human cardiac electrophysiology on clinical time-scales. Front Physiol. 2011b;2:14. doi: 10.3389/fphys.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer SA, Kerfoot E, Benson AP, Bernabeu MO, Bernus O, Bradley C, et al. Verification of cardiac tissue electrophysiology simulators using an N-version benchmark. Philos Trans R Soc A Math Phys Eng Sci. 2011a;369:4331–4351. doi: 10.1098/rsta.2011.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations. Nature. 1960;188:495–497. doi: 10.1038/188495b0. [DOI] [PubMed] [Google Scholar]
- Noble D. A modification of the Hodgkin–Huxley equations applicable to Purkinje fibre action and pacemaker potentials. J Physiol. 1962;160:317–352. doi: 10.1113/jphysiol.1962.sp006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. Mechanism of action of therapeutic levels of cardiac glycosides. Cardiovasc Res. 1980;14:495–514. doi: 10.1093/cvr/14.9.495. [DOI] [PubMed] [Google Scholar]
- Noble D. The rise of computational biology. Nat Rev Mol Cell Biol. 2002a;3:459–463. doi: 10.1038/nrm810. [DOI] [PubMed] [Google Scholar]
- Noble D. Simulation of Na/Ca exchange activity during ischemia. Ann N Y Acad Sci. 2002b;976:431–437. doi: 10.1111/j.1749-6632.2002.tb04772.x. [DOI] [PubMed] [Google Scholar]
- Noble D. Computational models of the heart and their use in assessing the actions of drugs. J Pharmacol Sci. 2008;107:107–117. doi: 10.1254/jphs.cr0070042. [DOI] [PubMed] [Google Scholar]
- Noble D, Colatsky TJ. A return to rational drug discovery: computer-based models of cells, organs and systems in drug target identification. Emerg Ther Targets. 2000;4:39–49. [Google Scholar]
- Noble D, Noble PJ. Late sodium current in the pathophysiology of cardiovascular disease: consequences of sodium-calcium overload. Heart. 2006;92:1–5. doi: 10.1136/hrt.2005.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Tsien RW. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968;195:185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Denyer J, Brown H, DiFrancesco D. Reciprocal role of the inward currents Ib, Nα and If in controlling and stabilizing pacemarker frequency of rabbit sino-atrial node cells. Proc R Soc Lond Biol Sci. 1992;250:199–207. doi: 10.1098/rspb.1992.0150. [DOI] [PubMed] [Google Scholar]
- Obiol-Pardo C, Gomis-Tena J, Sanz F, Saiz J, Pastor M. A multiscale simulation system for the prediction of drug-induced cardiotoxicity. J Chem Inf Model. 2011;51:483–492. doi: 10.1021/ci100423z. [DOI] [PubMed] [Google Scholar]
- Pásek M, Simurda J. Quantitative modelling of interaction of propafenone with sodium channels in cardiac cells. Med Biol Eng Comput. 2004;42:151–157. doi: 10.1007/BF02344625. [DOI] [PubMed] [Google Scholar]
- Pitt-Francis J, Pathmanathan P, Bernabeu M, Bordas R, Cooper J, Fletcher AG, et al. Chaste: a test-driven approach to software development for biological modelling. Comput Phys Commun. 2009;180:2452–2471. [Google Scholar]
- Polak S, Wisniowska B, Fijorek K, Glinka A, Polak M, Mendyk A. The open-access dataset for insilico cardiotoxicity prediction system. Bioinformation. 2011;6:244–245. doi: 10.6026/97320630006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard CE, Valentin JP, Hammond TG. Strategies to reduce the risk of drug-induced QT interval prolongation: a pharmaceutical company perspective. Br J Pharmacol. 2008;154:1538–1543. doi: 10.1038/bjp.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard CE, Gerges NA, Bridgland-Taylor MH, Easter A, Hammond TG, Valentin JP. An introduction to QT interval prolongation and non-clinical approaches to assessing and reducing risk. Br J Pharmacol. 2010;159:12–21. doi: 10.1111/j.1476-5381.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Weiss JN. Effects of Na+ and K+ channel blockade on vulnerability to and termination of fibrillation in simulated normal cardiac tissue. Am J Physiol Circ Physiol. 2005;289:H1692–H1701. doi: 10.1152/ajpheart.00241.2005. [DOI] [PubMed] [Google Scholar]
- Quinn TA, Granite S, Allessie MA, Antzelevitch C, Bollensdorff C, Bub G, et al. Minimum information about a cardiac electrophysiology experiment (MICEE): standardised reporting for model reproducibility, interoperability, and data sharing. Prog Biophys Mol Biol. 2011;107:4–10. doi: 10.1016/j.pbiomolbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanatini M, Poluzzi E, Masetti M, Cavalli A, De Ponti F. QT prolongation through hERG K+ channel blockade: current knowledge and strategies for the early prediction during drug development. Med Res Rev. 2005;25:133–166. doi: 10.1002/med.20019. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- Rodríguez B, Burrage K, Gavaghan DJ, Grau V, Kohl P, Noble D. The systems biology approach to drug development: application to toxicity assessment of cardiac drugs. Clin Pharmacol Ther. 2010;88:130–134. doi: 10.1038/clpt.2010.95. [DOI] [PubMed] [Google Scholar]
- Rudy Y. Modelling the molecular basis of cardiac repolarization. Europace. 2007;9:vi17–vi19. doi: 10.1093/europace/eum202. [DOI] [PubMed] [Google Scholar]
- Sarkar AX, Sobie EA. Quantification of repolarization reserve to understand interpatient variability in the response to proarrhythmic drugs: a computational analysis. Heart Rhythm. 2011;8:1749–1755. doi: 10.1016/j.hrthm.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Ehrlich JR, Hohnloser SH. New antiarrhythmic drugs for the treatment of atrial fibrillation. Herz. 2008;33:562–567. doi: 10.1007/s00059-008-3151-z. [DOI] [PubMed] [Google Scholar]
- Seigneuric RG, Chassé JL, Auger P, Bardou A. Simulated interactions between a class III antiarrhythmic drug and a figure 8 reentry. Acta Biotheor. 2005;53:265–275. doi: 10.1007/s10441-005-4879-y. [DOI] [PubMed] [Google Scholar]
- Silva JR, Rudy Y. Multi-scale electrophysiology modeling: from atom to organ. J Gen Physiol. 2010;135:575–581. doi: 10.1085/jgp.200910358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JR, Pan H, Wu D, Nekouzadeh A, Decker KF, Cui J, et al. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc Natl Acad Sci U S A. 2009;106:11102–11106. doi: 10.1073/pnas.0904505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubret A, Helminger G, Dumotier B, Bibas R, Georgieva A. Modeling and simulation of preclinical cardiac safety: towards an integrative framework. Drug Metab Pharmacokinet. 2009;24:76–90. doi: 10.2133/dmpk.24.76. [DOI] [PubMed] [Google Scholar]
- Starmer CF, Lastra AA, Nesterenko VV, Grant AO. Proarrhythmic response to sodium channel blockade. Theoretical model and numerical experiments. Circulation. 1991;84:1364–1377. doi: 10.1161/01.cir.84.3.1364. [DOI] [PubMed] [Google Scholar]
- Straus SMJM, Sturkenboom MCJM, Bleumink GS, Dieleman JP, Lei J, Graeff PA, et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur Hear J. 2005;26:2007–2012. doi: 10.1093/eurheartj/ehi312. [DOI] [PubMed] [Google Scholar]
- Swedberg K, Komajda M, Böm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- Ten Tusscher K, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol. 2006;291:1088–1100. doi: 10.1152/ajpheart.00109.2006. [DOI] [PubMed] [Google Scholar]
- Tikhonov DB, Magazanik LG. Voltage dependence of open channel blockade: onset and offset rates. J Membr Biol. 1998;161:1–8. doi: 10.1007/s002329900309. [DOI] [PubMed] [Google Scholar]
- Trénor B, Ferrero JM, Rodríguez B, Montilla F. Effects of pinacidil on reentrant arrhythmias generated during acute regional ischemia: a simulation study. Ann Biomed Eng. 2005;33:897–906. doi: 10.1007/s10439-005-3554-4. [DOI] [PubMed] [Google Scholar]
- Tsujimae K, Suzuki S, Murakami S, Kurachi Y. Frequency-dependent effects of various IKr blockers on cardiac action potential duration in a human atrial model. Am J Physiol Circ Physiol. 2007;293:H660–H669. doi: 10.1152/ajpheart.01083.2006. [DOI] [PubMed] [Google Scholar]
- Valentin JP, Hoffmann P, De Clerck F, Hammond TG, Hondeghem LM. Review of the predictive value of the Langendorff heart model (Screenit system) in assessing the proarrhythmic potential of drugs. J Pharmacol Toxicol Methods. 2004;49:171–181. doi: 10.1016/j.vascn.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Walker BD, Campbell TJ. HERG K+ channels: friend and foe. Trends Pharmacol Sci. 2001;22:240–246. doi: 10.1016/s0165-6147(00)01662-x. [DOI] [PubMed] [Google Scholar]
- Viswanathan PC, Rudy Y. Pause induced early afterdepolarizations in the long QT syndrome: a simulation study. Cardiovasc Res. 1999;42:530–542. doi: 10.1016/s0008-6363(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Viswanathan PC, Shaw RM, Rudy Y. Effects of IKr and IKs heterogeneity on action potential duration and its rate dependence: a simulation study. Circulation. 1999;99:2466–2474. doi: 10.1161/01.cir.99.18.2466. [DOI] [PubMed] [Google Scholar]
- Walmsley J, Mirams GR, Bahoshy M, Bollensdorff C, Rodriguez B, Burrage K. Phenomenological modeling of cell-to-cell and beat-to-beat variability in isolated Guinea Pig ventricular myocytes. Eng Med Biol Soc. 2010;2010:1457–1460. doi: 10.1109/IEMBS.2010.5626858. [DOI] [PubMed] [Google Scholar]
- Weirich J, Antoni H. Modelling frequency-and voltage-dependent effects of a class I antiarrhythmic drug (nicainoprol) on V max of the cardiac action potential from guinea-pig papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1989;340:456–464. doi: 10.1007/BF00167049. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Garfinkel A, Karagueuzian HS, Qu Z, Chen PS. Chaos and the transition to ventricular fibrillation: a new approach to antiarrhythmic drug evaluation. Circulation. 1999;99:2819–2826. doi: 10.1161/01.cir.99.21.2819. [DOI] [PubMed] [Google Scholar]
- Wilson LD, Jennings MM, Rosenbaum DS. M-cells are Present in the Ventricular Myocardium. Hear Rhythm Off J Hear Rhythm Soc. 2011;8:930–933. doi: 10.1016/j.hrthm.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Varghese A, Noble D, Adlakha C, Hoythya A. Generation and propagation of ectopic beats induced by spatially localized Na–K pump inhibition in atrial network models. Proc Biol Sci. 1993;254:55–61. doi: 10.1098/rspb.1993.0126. [DOI] [PubMed] [Google Scholar]
- Zemzemi N, Bernabeu M, Saiz J, Rodriguez B. Simulating drug-induced effects on the heart: from ion channel to body surface electrocardiogram. Funct Imaging Model Hear. 2011;LNCS 6666:259–266. [Google Scholar]


