Abstract
BACKGROUND AND PURPOSE
The antidepressant efficacy of selective 5-HT reuptake inhibitors (SSRI) and other 5-HT-enhancing drugs is compromised by a negative feedback mechanism involving 5-HT1A autoreceptor activation by the excess 5-HT produced by these drugs in the somatodendritic region of 5-HT neurones. 5-HT1A receptor antagonists augment antidepressant-like effects in rodents by preventing this negative feedback, and the mixed β-adrenoceptor/5-HT1A receptor antagonist pindolol improves clinical antidepressant effects by preferentially interacting with 5-HT1A autoreceptors. However, it is unclear whether 5-HT1A receptor antagonists not discriminating between pre- and post-synaptic 5-HT1A receptors would be clinically effective.
EXPERIMENTAL APPROACH
We characterized the pharmacological properties of the 5-HT1A receptor antagonist DU-125530 using receptor autoradiography, intracerebral microdialysis and electrophysiological recordings. Its capacity to accelerate/enhance the clinical effects of fluoxetine was assessed in a double-blind, randomized, 6 week placebo-controlled trial in 50 patients with major depression (clinicaltrials.gov identifier NCT01119430).
KEY RESULTS
DU-125530 showed equal (low nM) potency to displace agonist and antagonist binding to pre- and post-synaptic 5-HT1A receptors in rat and human brain. It antagonized suppression of 5-hydroxytryptaminergic activity evoked by 8-OH-DPAT and SSRIs in vivo. DU-125530 augmented SSRI-induced increases in extracellular 5-HT as effectively as in mice lacking 5-HT1A receptors, indicating a silent, maximal occupancy of pre-synaptic 5-HT1A receptors at the dose used. However, DU-125530 addition to fluoxetine did not accelerate nor augment its antidepressant effects.
CONCLUSIONS AND IMPLICATIONS
DU-125530 is an excellent pre- and post-synaptic 5-HT1A receptor antagonist. However, blockade of post-synaptic 5- HT1A receptors by DU-125530 cancels benefits obtained by enhancing pre-synaptic 5-hydroxytryptaminergic function.
Keywords: 5-HT1A receptors, antidepressant drugs, serotonin transporter, major depression, prefrontal cortex, raphe nuclei
Introduction
Major depression is a severe psychiatric syndrome with high prevalence and socioeconomic impact (Greenberg et al., 2003; Andlin-Sobocki and Wittchen, 2005; Lopez et al., 2006; World Health Organization, 2008). Most of the prescribed antidepressants, the selective 5-HT reuptake inhibitors (SSRI) and the dual 5-HT and noradrenaline reuptake inhibitors, block physiological reuptake mechanisms in 5-hydroxytryptaminergic axons and thereby they increase extracellular 5-HT concentration in forebrain to activate post-synaptic 5-HT receptors required for clinical effects. However, this process is severely compromised by the simultaneous activation of pre-synaptic 5-HT1A receptors (receptor nomenclature follows Alexander et al., 2011) located somatodendritically on 5-HT neurones (5-HT1A autoreceptors) of the midbrain raphe nuclei (Pazos and Palacios, 1985; Pompeiano et al., 1992). The excess 5-HT produced by reuptake inhibition in midbrain activates 5-HT1A autoreceptors, thereby reducing 5-hydroxytryptaminergic neurone activity and terminal 5-HT release (Bel and Artigas, 1992; Blier and De Montigny, 1994; Romero and Artigas, 1997; Lopez et al., 2006), an effect contrary to that required for therapeutic response.
The limited clinical efficacy of 5-HT-enhancing drugs and their delayed action are partly due to this negative feedback mechanism. Upon chronic treatment, 5-HT1A autoreceptors desensitize, leading to the recovery of 5-hydroxytryptaminergic activity and enhanced 5-HT release (Blier and De Montigny, 1994; Artigas et al., 1996). Hence, pharmacological or genetic suppression of 5-HT1A autoreceptor activity enhances the neurochemical and behavioural effects of SSRI in rodents (Artigas et al., 1996; Romero and Artigas, 1997; Knobelman et al., 2001; Bortolozzi et al., 2004; Richardson-Jones et al., 2010). Moreover, patients with a gene polymorphism leading to high 5-HT1A autoreceptor expression are more susceptible to depression and suicide and respond poorly to antidepressant therapy (Stockmeier et al., 1998; Lemonde et al., 2003; 2004; Neff et al., 2009).
Therefore, 5-HT1A receptor antagonists might be useful to improve antidepressant therapy as they could prevent 5-HT1A-autoreceptor-mediated negative feedback. Hence, the non-selective β-adrenoceptor/5-HT1A receptor antagonist pindolol accelerates and, in some instances, increases the efficacy of SSRIs (Artigas et al., 1994; 2001; Blier and Bergeron, 1995; Perez et al., 1997; Ballesteros and Callado, 2004; Whale et al., 2010). However, its complex pharmacology, including its anti-hypotensive effects, limits it clinical use. Pindolol shows a preferential affinity and occupancy of 5-HT1A autoreceptors compared with post-synaptic 5-HT1A receptors in rodent (Serrats et al., 2004) and human (Martinez et al., 2001) brains. In contrast, the prototypical 5-HT1A receptor antagonist WAY-100635 (not available for human use) interacts equally with pre- and post-synaptic 5-HT1A receptors (Forster et al., 1995; Fletcher et al., 1996). Given the requirement to activate post-synaptic 5-HT1A receptors to achieve antidepressant effects in rodents (Haddjeri et al., 1998; Blier and Ward, 2003), it is unclear whether selective 5-HT1A receptor antagonists with equal potency at pre- and post-synaptic 5-HT1A receptors would be clinically effective. Testing this working hypothesis has not been possible so far due to the lack of 5-HT1A receptor antagonists available for human use.
Preliminary data indicate that the 5-HT1A receptor antagonist DU-125530 shows high affinity for 5-HT1A- receptors and ≥10-fold selectivity versus other monoaminergic receptors (Mos et al., 1997) (see also Table 1) and antagonizes behavioural effects induced by 5-HT1A receptor agonists in rodents (Joordens et al., 1998; Olivier et al., 1998). Likewise, it occupies pre- and post-synaptic receptors in human brain, as demonstrated by PET scan studies (Rabiner et al., 2002). However, a full characterization of its pharmacological properties is lacking. Therefore, we carried out a collaborative translational study in which we examined the ability of DU-125530: (i) to interact with pre- and post-synaptic 5-HT1A receptors; and (ii) to accelerate or enhance the antidepressant action of fluoxetine.
Table 1.
In vitro receptor binding profile of DU-125530 for monoaminergic receptors
| Receptor | Affinity (nM) |
|---|---|
| 5-HT1A | 0.7 |
| 5-HT1B | 890 |
| 5-HT1D | 1200 |
| 5-HT2A | 240 |
| 5-HT2C | 750 |
| 5-HT3 | 1100 |
| α1-adrenoceptor | 6.4 |
| Dopamine D2 | 5.2 |
| Dopamine D3 | 11 |
Data taken from Mos et al. (1997).
Methods
Preclinical studies
Animals
All animal care and experimental procedures followed the European Union regulations (OJ of EC L358/1 18/12/1986) and were approved by the Institutional Animal Care and Use Committee. Male albino Wistar rats (230–300 g; Iffa Credo, Lyon, France; total number used: 69) and C57/Bl6J male mice (10–15 weeks old; Iffa Credo; total number used: 41) were kept in a temperature-controlled environment (12 h light–dark cycle) with food and water provided ad libitum. Stereotaxic coordinates (in mm) were taken from bregma and duramater according to the atlas of Paxinos and Watson (1998).
Methods
To examine the ability of DU-125530 to interact with pre- and post-synaptic 5-HT1AR in rodent brain, we used receptor autoradiography, single unit extracellular recordings of 5-hydroxytryptaminergic neurones in the dorsal raphe nucleus and of pyramidal neurones in medial prefrontal cortex as well as microdialysis studies, following standard methods routinely used in our laboratory and reported elsewhere (Romero and Artigas, 1997; Amargos-Bosch et al., 2004; Serrats et al., 2004; Diaz-Mataix et al., 2005). A detailed description can be found in the ‘Supplementary Methods’ section.
Data treatment
In autoradiographic studies, inhibition curves were statistically analysed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA).
Changes in discharge rate were quantified by averaging the values in the third minute after each drug injection. Drug effects were assessed using Student's t-test or one-way repeated-measures anova, as appropriate. Data are expressed as the mean ± SEM. Statistical significance has been set at the 95% confidence level.
Dialysate 5-HT concentrations were measured as fmol per fraction and are expressed in the Figures as percentages of baseline (set to 100%). Statistical analysis was carried out using repeated-measures anova using treatment and time as variables.
Materials
8-OH-DPAT [8-hydroxy-2-(di-n-propylamino)tetralin] was from Sigma-Aldrich (St. Louis, MO). Fluoxetine [N-methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine] was from Tocris (Bristol, UK). Paroxetine [(3S,4R)-3-[(2H-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine] was generously provided by GSK (London, UK). DU-125530 [2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzodioxin-5-yl)-1-piperazinyl]butyl]-1,2-benzisothiazol-3(2H).-one-1,1-dioxide] was from Solvay Pharma (Brussels, Belgium). (+/−)WAY-100635 [N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide] hydrochloride was from RBI (Natick, MA). Stock solutions were prepared, and aliquots were stored at −20°C. Working solutions were prepared daily by dilution in saline at the appropriate concentrations. Doses are expressed as weight of free bases.
Clinical trial
Patients
Consecutive eligible patients aged 18 to 70, referred by general practitioners of primary care centers or from psychiatric emergency services (Catalonian Public Health Service), were recruited. Inclusion criteria were as follows: diagnosis of unipolar major depression using DSM-IV criteria with moderate to severe symptoms (≥18 on the Hamilton Depression Rating Scale of 17 items, HDRS-17). There was a wash-out of 1 week of any antidepressant drug (except for fluoxetine, 28 days) before entering the study. Written informed consent was obtained from all participants. Exclusion criteria were as follows: concurrent psychiatric disorders (DSM IV axis I, II cluster A or B); failure to respond to drug treatment in current depressive episode; previous resistance to antidepressant drugs, including SSRI; suicide risk score ≥3 on the HDRS; participation in other drug trials within the previous month; presence of delusions or hallucinations; history of substance abuse (including alcohol) in the past three months; pregnancy or lactation; serious organic illnesses in the past 6 months; frequent or severe allergic reactions; concomitant use of other psychotropic drugs (benzodiazepines were allowed) and blockers or catecholamine-depleting agents; current structured psychotherapy.
Study variables
Demographic and clinical data were collected. Likewise, any other relevant clinical information to the study was recorded: number of previous episodes, age at first depressive episode, melancholic features and medical history.
The primary variable of the clinical trial was the HDRS score. Sustained response was defined as a 50% or greater decrease in the admission HDRS score maintained until day 42, allowing a 5% variation during intermediate visits. Sustained remission was defined as an HDRS score of 8 or less maintained until endpoint. Secondary variables were the Montgomery-Asberg Depression Rating Scale (MADRS) and the Clinical Global Impression (CGI). Safety was assessed by means of biochemical variables and vital signs. ECGs were performed at admission, 2 weeks after beginning active treatment and at the end of the study. Plasma concentration of fluoxetine was obtained at 3 weeks of treatment and at the end of the trial.
Study design
The design of the study (Figure 1) was the same as that of a previous study assessing the effect of pindolol addition to fluoxetine treatment (Perez et al., 1997) and had two active treatment arms: fluoxetine + placebo and fluoxetine + DU-125530 after a placebo run-in phase.
Figure 1.
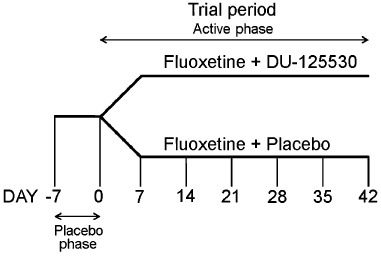
Clinical study design. Patients entered in a single-blind placebo phase of 3–7 days. Patients showing a reduction of 25% or greater of their HDRS score or with a decrease to below 18 at day 0 were excluded. Patients entering the study were randomized on day 0 to one of two treatment arms: fluoxetine + placebo or fluoxetine + DU-125530.
Placebo phase
After obtaining informed consent, patients entered a single-blind placebo run-in period of 3–7 days. Patients showing a ≥25% reduction of their admission HDRS score or an HDRS score lower than 18 during this period were excluded.
Active phase
Patients entering the study were randomized and assigned (day 1) to one of two treatment arms: fluoxetine 20 mg·day−1 plus placebo or fluoxetine 20 mg·day−1 plus DU-125530 (20 mg·day−1). Patients, investigators and all personal participating in the study were unaware of the treatments (double-blind). The active phase lasted for 6 weeks. Clinical assessments were carried out on day 1 and every 7 days (±3 days) until day 42. Compliance was assessed by direct questioning patients and by counting returned pills and capsules at follow-up visits. Side effects were requested at each visit.
The study was approved by the Ethics Committee of the Hospital de Sant Pau and was registered with the US National Institutes of Health Protocol Registration System (NCT01119430). An independent researcher (Ignasi Gich, MD, Department of Clinical Pharmacology, Hospital de Sant Pau), not involved in the clinical trial, carried out the randomization by means of computer-generated random numbers.
Data treatment and statistical analyses
The planned sample size for this study was 100 randomly assigned patients (50 in each treatment group), chosen to provide approximately 75% power to detect a difference in the percentage of responders at endpoint of 60% for fluoxetine plus placebo an 80% for fluoxetine plus DU-125530 using a one-sided 0.05 level test. Given the absence of previous trials using DU-125530, the use of one-sided test was considered to be more appropriate than increasing the sample size. Thus, one-sided P-values were used in safety and efficacy analyses. Data are given as means (SD). All scores were computed using a last observation-carried-forward (LOCF) approach. All analyses were done by intention to treat. Additional analysis of the observed cases (OC) was carried out. An interim analysis was performed at n= 50 (half of the planned sample), which met the criteria to stop the trial.
Main analysis was performed using repeated-measures anova, with time (eight time points) as the within-subjects factor and group (fluoxetine + placebo vs. fluoxetine + DU-125530) as the between-subjects factor. A Huynh–Feldt correction was used where the assumption of sphericity was violated (uncorrected d.f. reported). Further differences were assessed by means of post hoc analyses. All randomized patients who had a baseline and at least one post-baseline score were included in the analyses. One-way anova (treatment group as the between-subjects factor) was used to examine group differences with other clinical variables. Additionally, a survival analysis was done to establish the velocity of each treatment arm. All statistics were performed by means of statistical package for Windows SPSS 17.0.
Results
Characterization of DU-125530 as a 5-HT1A receptor antagonist: preclinical studies
Quantitative receptor autoradiography
The binding of the 5-HT1A receptor agonist [3H]8-OH-DPAT and the corresponding antagonist [3H]WAY-100635 to rat brain structures was inhibited by DU-125530 at low nanomolar concentrations, as illustrated in Figure 2A. Displacement curves of DU-125530 against the two radioligands, as generated from microdensitometric data, fitted to the one site binding competition model (Figure 3). The calculated pIC50 values of DU-125530 for both ligands did not differ among the regions examined (Table 2).
Figure 2.
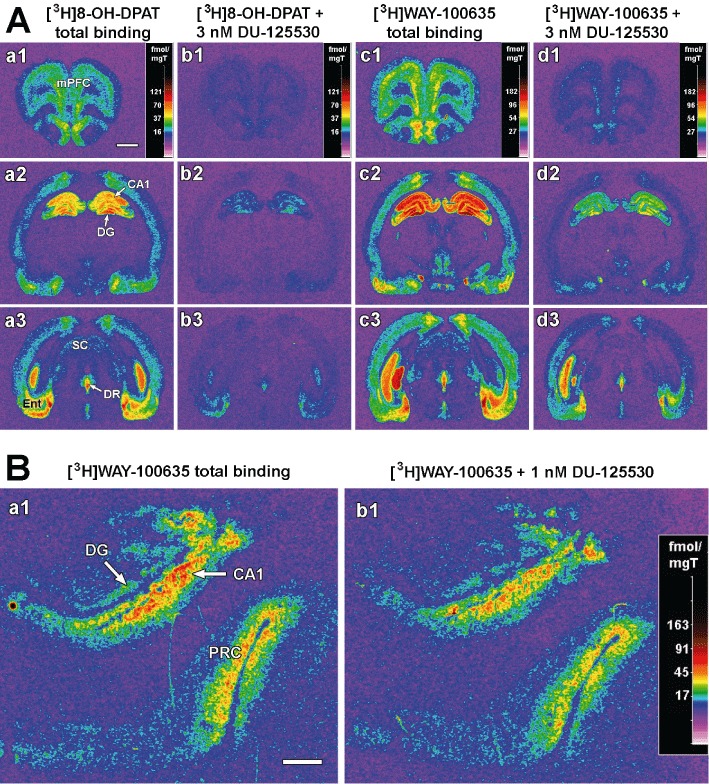
(A) Pseudocolour images from autoradiograms obtained from rat brain sections at different brain levels (prefrontal cortex, hippocampus and midbrain-upper pons) incubated with 0.5 nM [3H]8-OH-DPAT alone (a1–a3) and in the presence of 3 × 10−9 M DU1255530 (b1–b3), or incubated with 0.5 nM [3H]WAY-100635 alone (c1–c3) and in the presence of 3 × 10−9 M DU1255530 (d1–d3). Note that DU-1255530 inhibits [3H]8-OH-DPAT and [3H]WAY-100635 binding in all structures, including CA1, DG (dentate gyrus), DR (dorsal raphe), Ent (entorhinal cortex), mPFC (medial prefrontal cortex) and SC (superior colliculus). Bar = 2 mm. (B) Pseudocolour images from autoradiograms obtained from human hippocampal sections incubated with 0.5 nM [3H]WAY-100635 alone (a1) or in the presence of 10−9 M DU-1255530 (b1). CA1, CA1 hippocampal field; DG, dentate gyrus; PRC, perirhinal cortex. Bar = 2 mm.
Figure 3.
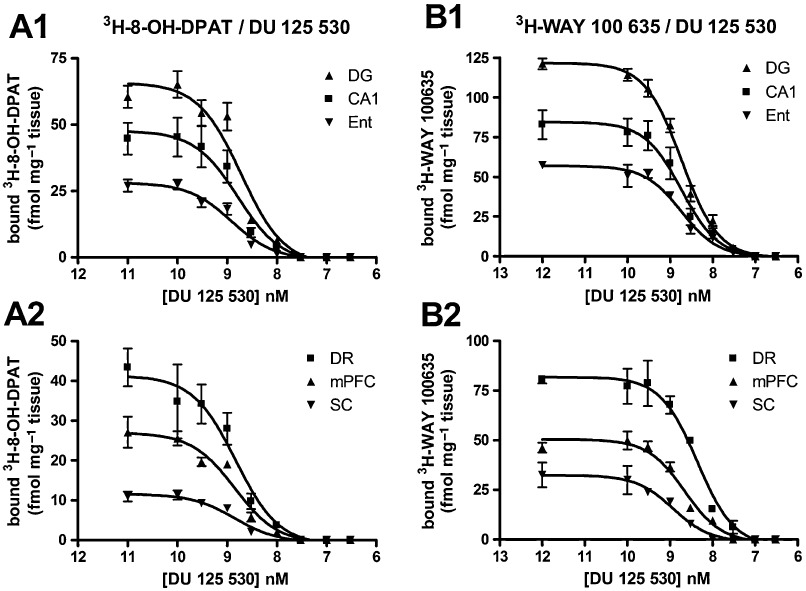
Displacement of [3H]8-OH-DPAT (A1,A2) and [3H]WAY-100635 (B1,B2) binding by DU1255530 in the hippocampus (CA1), DG, DR (Ent), mFPC and SC of the rat. Data points are means ± SEM of three animals and were obtained by microdensitometric analysis of autoradiograms.
Table 2.
Relative binding affinities (pIC50) of DU-125530 for [3H]8-OH-DPAT and [3H]WAY-100635 binding sites in various regions of the rat brain
| Area | [3H]8-OH-DPAT | [3H]WAY-100635 |
|---|---|---|
| pIC50± SD | pIC50± SD | |
| CA1 | 8.8 ± 0.1 | 8.7 ± 0.1 |
| DG | 8.7 ± 0.1 | 8.7 ± 0.1 |
| Ent | 8.9 ± 0.1 | 8.7 ± 0.1 |
| DR | 8.8 ± 0.2 | 8.4 ± 0.1 |
| mPFC | 8.9 ± 0.1 | 8.7 ± 0.1 |
| SC | 8.9 ± 0.1 | 8.9 ± 0.2 |
CA1, Ammon's horn area 1 of hippocampus; DG, dentate gyrus; DR, dorsal raphe nucleus; Ent, entorhinal cortex; mPFC, medial prefrontal cortex; SC, superior colliculus.
In the human hippocampus (Figure 2B), DU-125530 also displaced [3H]WAY-100635 binding with high affinity and produced monophasic displacement curves (not shown). The pIC50 values calculated for the CA1 hippocampal field and the perirhinal cortex are reported in Table 3.
Table 3.
Relative binding affinities (pIC50) of DU-125530 for [3H]WAY-100635 binding sites the CA1 hippocampal field and the perirhinal cortex of two human control cases
| Area | [3H]WAY-100635 |
|---|---|
| pIC50± SD | |
| CA1 case A | 8.6 ± 0.1 |
| CA1 case B | 8.7 ± 0.2 |
| PRC case A | 8.8 ± 0.1 |
| PRC case B | 8.4 ± 0.1 |
CA1, Ammon's horn area 1; PRC, perirhinal cortex.
Electrophysiological studies
We examined the ability of DU-125530 to reverse the inhibition of the discharge rate of DR 5-hydroxytryptaminergic neurones induced by the 5-HT1A receptor agonist 8-OH-DPAT and the SSRI fluoxetine. The administration of DU-125530 (67–134 µg·kg−1 i.v.) did not alter the firing rate of 5-hydroxytryptaminergic neurones by itself (1.9 ± 0.3 spikes per second vs. 1.7 ± 0.3 spikes per second in baseline conditions; n.s.; n= 7). However, DU-125530 fully reversed the decrease in firing rate induced by 8-OH-DPAT (1.2–4.8 µg·kg−1 i.v.) in all neurones examined (F3,12= 3.9; P < 0.04; n= 5; Figure 4A,C). The subsequent administration of increasing doses of WAY-100635 (5–60 µg·kg−1 i.v.) did not increase the 5-hydroxytryptaminergic firing rate further, indicating full antagonism by DU-125530.
Figure 4.
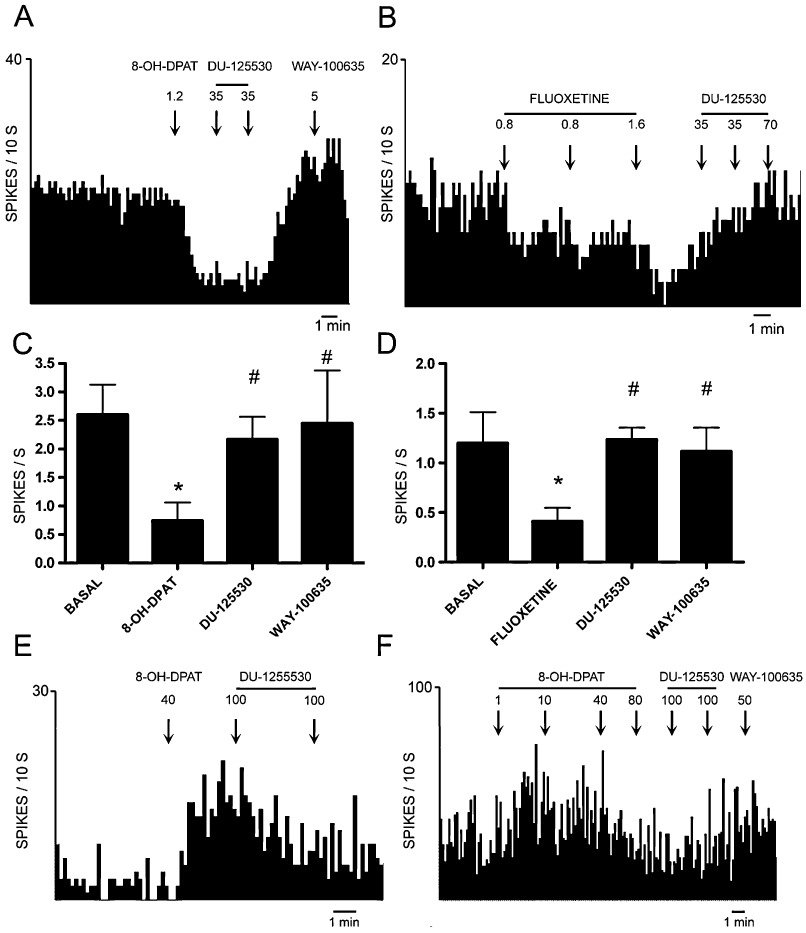
(A,B) Representative integrated firing rate histograms of two 5-hydroxytryptaminergic neurones showing the inhibition of discharge rate induced by the i.v. administration of 8-OH-DPAT (A) and fluoxetine (B) as well as the reversal of the effect by the subsequent administration of DU-125530 in both cases. Note that the administration of the prototypical 5-HT1A receptor antagonist WAY-100635 after DU-125530 did not evoke any further effect on firing rate indicating a complete reversal of the action of 8-OH-DPAT by DU-125530. (C,D) Bar graphs showing the inhibitory effect on 5-HT cell firing produced by 8-OH-DPAT (C) or fluoxetine (D) and the reversal of these effects by DU-125530. (E,F) Integrated firing rate histograms of two pyramidal cells in mPFC, which were identified by antidromic stimulation from the DR. The administration of the 5-HT1A agonist 8-OH-DPAT evokes excitations (E) or excitations at low doses followed by inhibitions at higher doses (F). Both effects are reversed by the subsequent administration of DU-125530, showing its antagonist properties at post-synaptic 5-HT1A heteroreceptors. Arrows mark the time of drug administration.
Similarly, DU-125530 (67–134 µg·kg−1 i.v.) reversed the reduction in DR 5-hydroxytryptaminergic firing rate produced by fluoxetine (0.8–4 mg·kg−1 i.v.) (F3,15= 4.0; P < 0.03; n= 6; Figure 4B,D). Likewise, the subsequent administration of WAY-100635 (5–20 µg·kg−1 i.v.) did not augment the reversal elicited by DU-122530.
DU-12530 also reversed the effect produced by 8-OH-DPAT on medial prefrontal cortex pyramidal neurones in the few cases examined (see two examples in Figure 4E,F).
Microdialysis studies
We assessed the putative antagonist properties of DU-125530 at pre- and post-synaptic 5-HT1A receptors controlling 5-HT release in vivo in rats and mice (WT and 5-HT1A receptor knockout-KO) using four different experimental models: (i) antagonism of systemic 8-OH-DPAT-induced reduction of 5-HT release; (ii) reversal of paroxetine-induced reduction of 5-HT release (with local 5-HT reuptake inhibition in medial prefrontal cortex; mPFC); (iii) reversal of local 8-OH-DPAT application in mPFC; and (iv) augmentation of SSRI effect on extracellular 5-HT in mPFC.
Rat experiments
The systemic administration of DU-125530 (3 mg·kg−1 s.c.) did not significantly modify 5-HT release in mPFC (vehicle + vehicle, n= 6; vehicle + DU-125530, n= 7). However, its administration (3 mg·kg−1 s.c.) prevented the reduction of 5-HT release evoked by 50 µg·kg−1 s.c. 8-OH-DPAT (treatment F1,7= 7.8, P < 0.03; time F15,105= 2.4, P < 0.01 and treatment × time interaction F15,105= 5.3; P < 0.0001; Figure 5A). To test the capacity of DU-125530 to antagonize the actions of 5-HT at somatodendritic 5-HT1A autoreceptors, we used an experimental paradigm in which an SSRI (e.g. paroxetine) is administered systemically while locally blocking the 5-HT transporter (SERT) with citalopram in the sampling forebrain area. In these experimental conditions, the systemically administered SSRI cannot further block SERT in the sampling area (e.g. mPFC), but it does in midbrain, where the increase in extracellular 5-HT activates 5-HT1A autoreceptors, thus reducing terminal 5-HT release (Romero and Artigas, 1997). In these conditions, paroxetine (3 mg·kg−1 s.c.) significantly reduced 5-HT release in mPFC, an effect significantly antagonized by DU-125530 administration (3 mg·kg−1 s.c.) (time F15,120= 38.7; P < 0.0001 and treatment × time interaction F15,120= 3.5; P < 0.0001; Figure 5B).
Figure 5.
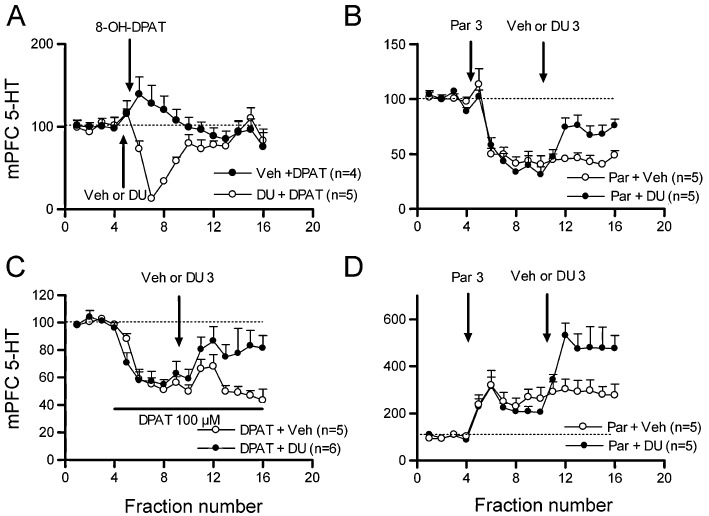
In vivo microdialysis experiments showing the antagonism/reversal exerted by DU-125530 in the different experimental models used in rats. The extracellular 5-HT concentration (shown as percentages of baseline; set to 100, dotted line) in mPFC was used in all instances. (A) Prevention by DU-125530 of the 8-OH-DPAT-induced reduction of 5-HT release. (B) Reversal by systemic administration of DU-125530 of the paroxetine (Par)-induced decrease in 5-HT release in mPFC during the local perfusion of citalopram by reverse dialysis. (C) Reversal by systemic DU-125530 administration of the effects produced by local application of 8-OH-DPAT on mPFC. (D) Augmentation by DU-125530 of the paroxetine-induced increase in mPFC extracellular 5-HT levels. Arrows mark systemic injections. Doses are given in mg·kg−1. Horizontal bars indicate local perfusion by inverse microdialysis. See text for statistical analysis.
To examine the ability of DU-125530 to block post-synaptic 5-HT1A receptors, we locally applied 8-OH-DPAT in the mPFC by inverse microdialysis. The extensive occupancy of 5-HT1A receptors in mPFC by local 8-OH-DPAT inhibits excitatory inputs to the dorsal raphe (DR), thereby reducing 5-HT neuronal activity and terminal 5-HT release (Celada et al., 2001). The local application of 100 µM 8-OH-DPAT in mPFC markedly reduced local extracellular 5-HT concentration. Subsequent systemic administration of DU-125530 (3 mg·kg−1 s.c.) significantly attenuated this reduction (time F15,135= 14.5; P < 0.001 and treatment × time interaction F15,135= 2.7; P < 0.002; Figure 5C) [note that saline rapidly increased extracellular 5-HT due to the injection stress (Adell et al., 1997), yet the effect disappeared rapidly].
Finally, DU-125530 augmented the increase of extracellular 5-HT in mPFC evoked by (i) 3 mg·kg−1 paroxetine (time F15,270= 6.7; P < 0.0001; treatment × time interaction F15,270= 2.3; P < 0.005; Figure 5D); and (ii) 10 mg·kg−1 s.c. fluoxetine (time F15,300= 41.9; P < 0.0001; treatment × time interaction F15,300= 1.8; P < 0.04; fluoxetine + vehicle, n= 9; fluoxetine + DU-125530, n= 13; data not shown).
Mouse experiments
The systemic administration of DU-125530 (3 mg·kg−1 s.c.) alone had no effect on the extracellular 5-HT concentration in mPFC of wild-type mice (WT) (vehicle + vehicle, n= 5; vehicle + DU-125530, n= 5) nor in 5-HT1A receptor knock-out mice (KO) (vehicle + vehicle, n= 5; vehicle + DU-125530, n= 4). However, DU-125530 prevented the reduction of 5-HT release induced by 0.5 mg·kg−1 s.c. 8-OH-DPAT in WT mice (treatment F1,10= 16.4; P < 0.005; time F15,150= 4.1; P < 0.0001; treatment × time interaction F15,150= 2.7; P < 0.005; Figure 6A).
Figure 6.
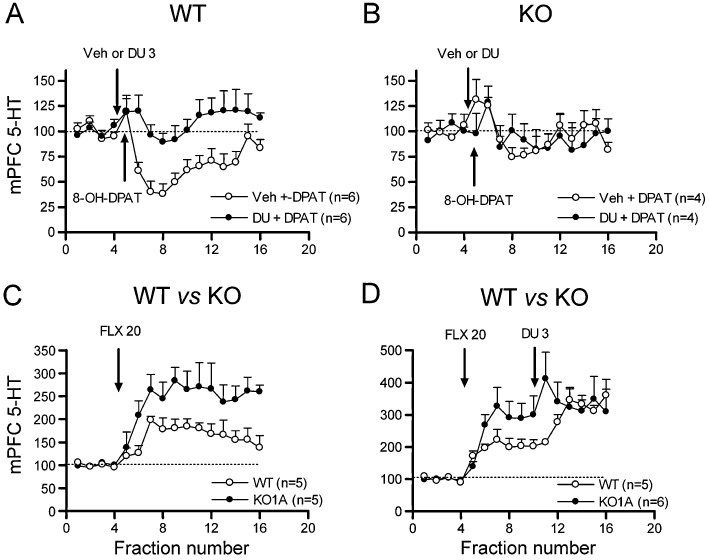
In vivo microdialysis experiments in wild type (WT) and 5-HT1A receptor knock out (KO1A) mice showing the effects of DU-125530. The extracellular 5-HT concentration (shown as percentages of baseline; set to 100, dotted line) in mPFC was used in all instances. (A) Prevention by DU-125530 of the reduction in 5-HT output induced by systemic 8-OH-DPAT administration in WT mice. (B) Lack of effects of systemic administration of 8-OH-DPAT and DU-125530 in KO mice. (C) Comparison of the effects of systemic injections of fluoxetine (FLX) in WT versus KO mice. (D) Augmentation of the effects of fluoxetine by DU-125530 in WT mice but not in KO mice. Note that the treatment of WT with fluoxetine + DU-125530 increases extracellular 5-HT concentration to the same extent than fluoxetine alone in KO mice. Arrows mark systemic injections. Doses are given in mg·kg−1. See text for statistical analysis.
As expected, 0.5 mg·kg−1 s.c. 8-OH-DPAT did not reduce 5-HT release in the mPFC of 5-HT1A receptor KO mice, and the change in 5-HT concentration produced by vehicle + 8-OH-DPAT was identical to that produced by DU-125530 + 8-OH-DPAT (Figure 6B) [a moderate, fast increase in extracellular 5-HT was produced, as a result of handling and injection stress (Adell et al., 1997) ].
The systemic administration of fluoxetine (20 mg·kg−1 s.c.) increased extracellular mPFC 5-HT concentration significantly more in KO mice than in WT mice (genotype effect F1,8= 6.0; P < 0.05; time effect F15,120= 14.7; P < 0.0001; time × genotype interaction F15,120= 2.5; P < 0.005; Figure 6C). The subsequent administration of DU-125530 (3 mg·kg−1 s.c.) significantly enhanced extracellular 5-HT concentration in WT mice, up to the level seen in 5-HT1A receptor KO mice after fluoxetine administration (time F15,135= 20.3; P < 0.0001; genotype × time interaction F15,135= 2.6; P < 0.002; Figure 6D).
Clinical characterization of DU-125530 in accelerating/enhancing fluoxetine antidepressant response
Fifty-seven patients were screened and entered the study between May 2004 and November 2007. Seven patients were excluded before randomization due to placebo response. Therefore, 50 patients with major depression diagnosis (MDD) finally entered the active phase (Figure 7). Twenty-five were randomly assigned to fluoxetine plus DU-125530 arm and 25 to fluoxetine plus placebo arm (Figure 1). No differences were found in demographic or clinical variables between the two groups (Table 4).
Figure 7.
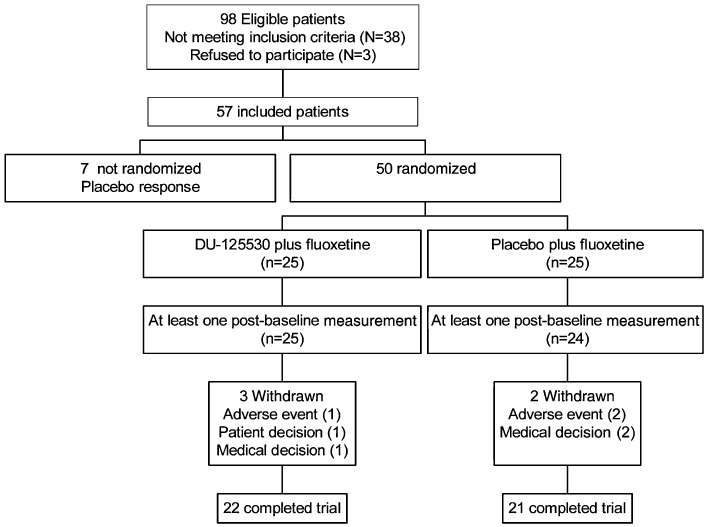
Flow diagram of subject progress through the phases of a randomized trial.
Table 4.
Demographic and clinical variables of the two treatment groups
| Variables | DU-125530 (n= 25) | Placebo (n= 25) | χ2/t | P |
|---|---|---|---|---|
| Gender (% females) | 83.3 | 84 | 0 | n.s. |
| Age | 42.1 (11.5) | 42.5 (13.9) | 0.9 | n.s. |
| Family psychiatric history (% present) | 64 | 36 | 3.7 | n.s. |
| No previous depressive episode (%) | 41.7 | 54.2 | 0.1 | n.s. |
| Age at first depressive episode | 34 (14.3) | 38.2 (15.5) | 0.9 | n.s. |
| Number of depressive episodes (including current episode) | 2.6 (2.8) | 1.7 (1.1) | 1.4 | n.s. |
| Concomitant treatment (% patients taking) | 2.4 | n.s. | ||
| No treatment | 25 | 35.7 | ||
| Benzodiazepines | 56.2 | 28.6 | ||
| Hypnotic | 12.5 | 21.4 | ||
| Benzodiazepines plus hypnotic | 6.2 | 14.3 | ||
| HDRS | ||||
| Pre | 24.7 (3.7) | 25.6 (4.4) | 0.7 | n.s. |
| Post | 13 (9.6) | 11.1 (6.8) | 0.7 | n.s. |
| MADRS | ||||
| Pre | 31.3 (4.5) | 32.6 (5.1) | 0.9 | n.s. |
| Post | 14.8 (12) | 14 (10.3) | 0.2 | n.s. |
| CGI | ||||
| Pre | 4.7 (0.6) | 4.7 (0.6) | 0.3 | n.s. |
| Post | 2.5 (1.3) | 2 (1) | 1 | n.s. |
DU-125530 = patients treated with fluoxetine + DU-125530; Placebo = patients treated with fluoxetine + placebo. Dta are shown as means (with SD). n.s., non-significant; pre, pretreatment; post, post treatment.
Neither the percentage of patients with first depressive episode (51% receiving DU-125530, 48% receiving placebo) nor the percentage of melancholic features (23% and 14%. respectively) differed between groups. Current episode duration ranged from 1 to 6 months for 63.6% of patients receiving fluoxetine + DU-125530 and for 54.5% of those receiving fluoxetine + placebo. Treatments were generally well tolerated with no differences in the incidence of adverse events between the two groups (32% for DU-125530, 16% for placebo, χ2= 1.75; P= 0.16). Regarding sexual dysfunction, one patient treated with DU-125530 reported anorgasmia. Five patients were withdrawn from the clinical trial because of side effects and two due to patient's decision. Repeated-measures anova for blood pressure did not show a significant main effect of time × group (F7,196= 0.5, P= 0.8) nor a group effect (F1,27= 1.3, P= 0.3). Heart rate showed a similar non-significant time × group effect (F7,182= 1.1, P= 0.4) and no group effect (F1,26= 0.1, P= 0.8). These results indicated that vital signs were stable during the study and with no significant differences between groups. Plasma concentration of fluoxetine at days 14 and 42 did not differ between groups. At day 14, fluoxetine mean values were 57.5 ng·mL−1 (SD = 31.7) in the fluoxetine + DU-125530 group and 66.4 ng·mL−1 (SD = 31.9) in the fluoxetine + placebo group (t=−0.9, P= 0.4). At day 42, fluoxetine values were 86.1 ng mL−1 (SD = 46.3) in the fluoxetine + DU-125530 group and 119 ng·mL−1 (SD = 76.3) in the fluoxetine + placebo group (t=−1.7, P= 0.1).
Regarding the main analysis with HDRS scores, repeated-measures anova showed a significant effect of time (F7,280= 96.6; P < 0.001) but not of the group (P= 0.9) nor of time × group interaction (P= 0.6). Figure 8A shows the temporal evolution of cumulative percentages of sustained responses for both groups. A tendency towards higher sustained responses in the fluoxetine + DU-125530 group was seen at 3–4 weeks, but it did not reach statistical significance.
Figure 8.
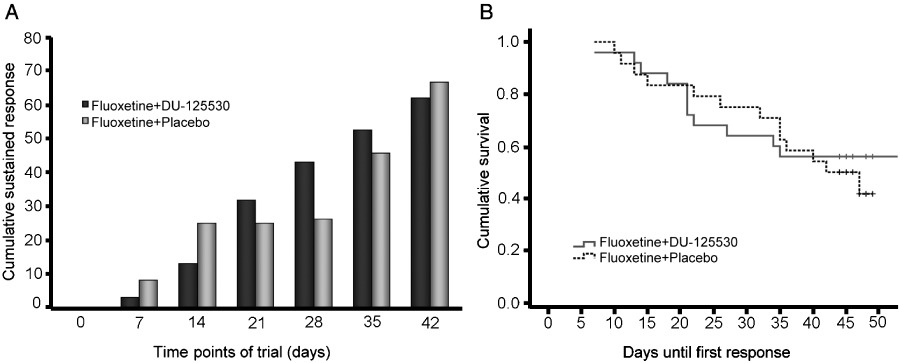
(A) Bar graph showing the cumulative percentages of patients with sustained response throughout the trial period. Repeated-measures anova showed a significant effect of the treatment but not of the group or treatment × group interaction. (B) Kaplan–Meier survival analyses of days until response.
The response rate of patients receiving DU-125530 + fluoxetine was similar to that of patients receiving fluoxetine + placebo. The survival analysis (Figure 8B) confirmed the absence of significant differences between the two treatment arms, being mean survival times until first response 44 days for DU-125530 and 37 days for placebo (log-rank, χ2= 0.3, P= 0.6).
We performed an additional analysis with the OC (n= 21 for DU-125530; and n= 20 for placebo), which gave essentially the same results (group effect: F1,39= 0.1, P= 0.8; time × group effect: F7,273= 0.5, P= 0.8).
Discussion and conclusions
The present study shows that DU-125530 is a high-affinity and silent 5-HT1A receptor antagonist in rodent brain that prevents and reverses the actions of 5-HT and 5-HT1A receptor agonists (8-OH-DPAT) at pre- and post-synaptic 5-HT1A receptors. It binds to rat and human 5-HT1A receptors with low nM affinity, and it antagonizes the actions of the 5-HT1A receptor agonist 8-OH-DPAT and SSRIs (fluoxetine and paroxetine) in electrophysiological and microdialysis experimental paradigms. As a consequence, DU-125530 augments the elevation in forebrain extracellular 5-HT concentration produced by SSRIs by preventing the 5-HT1A autoreceptor-mediated negative feedback evoked by these agents (Artigas et al., 1996). Despite these excellent pharmacological properties, DU-125530 did not accelerate nor enhance the antidepressant action of fluoxetine. To our knowledge, this is the first study testing the 5-HT1A receptor augmentation hypothesis (Artigas, 1993; Artigas et al., 1996) with a selective 5-HT1A receptor antagonist and consequently will affect antidepressant drug design.
Preclinical studies
Overall, the preclinical data support that DU-125530 interacts with 5-HT1A autoreceptors and post-synaptic 5-HT1A receptors in a manner similar to that of the prototypical antagonist WAY-100635 (Forster et al., 1995; Fletcher et al., 1996), which – unlike DU-125530 – is not available for human use. Indeed, DU-125530 displaced the agonist (3H-8-OH-DPAT) and antagonist (3H-WAY-100635) binding to rat and human 5-HT1A receptors with nM affinity and blocked the effects of exogenous (8-OH-DPAT) and endogenous (5-HT) 5-HT1A receptor agonists on (i) 5-HT neurone activity and (ii) 5-HT release, in rats and mice, as previously observed with WAY-100635 using the same experimental paradigms (Romero and Artigas, 1997; Casanovas et al., 1999; Celada et al., 2001; Romero et al., 2003; Lladó-Pelfort et al., 2011). Moreover, DU-125530 augmented the increase in extracellular 5-HT induced by SSRI to an extent comparable with that produced by WAY-100635 (Romero and Artigas, 1997; Hervas et al., 1998). Interestingly, the dose used in the present preclinical experiments appears to fully occupy 5-HT1A receptors, as (i) no further antagonism was produced by WAY-100635 when it was used after DU-125530, and (ii) the 5-HT increase induced by fluoxetine + DU-125530 on extracellular 5-HT in WT mice was identical to that produced by fluoxetine alone in 5-HT1AR KO mice.
The ability of DU-125530 to antagonize post-synaptic 5-HT1A receptors is shown by the following: (i) pilot electrophysiological experiments [reversal of 8-OH-DPAT-induced effects on mPFC pyramidal neurones, an effect depending on post-synaptic 5-HT1A receptor activation (Lladó-Pelfort et al., 2011) ]; and (ii) microdialysis experiments in which the systemic administration of DU-125530 significantly reversed the reduction in 5-HT release evoked by the activation of mPFC 5-HT1A receptors by local 8-OH-DPAT administration, as previously observed with WAY-100635 (Celada et al., 2001). Indeed, the direct activation of pyramidal 5-HT1A receptors in mPFC neurones attenuates the excitatory input onto DR 5-HT neurones (Celada et al., 2001) and evokes a subsequent reduction of forebrain 5-HT release. The data show that the systemic administration of DU-125530 antagonized this effect, showing a clear antagonist action at post-synaptic 5-HT1A receptors.
Despite its in vitro affinity for α1-adrenoceptors (∼10 times lower than for 5-HT1A receptors), DU-125530 did not reduce 5-HT release by itself, as expected from blockade of raphe α1-adrenoceptors (Vandermaelen and Aghajanian, 1983; Bortolozzi and Artigas, 2003). Moreover, no cardiovascular side effects were observed in patients treated with fluoxetine + DU-125530. Both observations allow us to discount a significant occupancy of α1-adrenoceptors at the doses used. Likewise, DU-125530 shows nM affinity for dopamine D2 receptors (Table 1). However, none of the observed preclinical effects of the compound can be attributed to interaction with such D2 receptors. Likewise, no side effects derived from D2 receptor blockade (e.g. extrapyramidal symptoms) were observed in patients treated with fluoxetine + DU-125530.
Thus, the present preclinical results indicate that:
DU-125530 displays equal nM affinity at pre- and post-synaptic 5-HT1A receptors.
DU-125530 is a silent pre- and post-synaptic 5-HT1A receptor antagonist in rodent brain.
DU-125530 cancels the 5-HT1A receptor-mediated negative feedback induced by SSRIs, thereby augmenting their increase of extracellular 5-HT concentration.
Clinical trial
The present clinical trial was conducted to examine whether the augmentation of 5-hydroxytryptaminergic function that resulted from the blockade of 5-HT1A autoreceptors was translated into an increased speed or efficacy of the antidepressant fluoxetine. To this end, the trial design was identical to that used previously to examine the augmenting action of pindolol (Perez et al., 1997). Due to the lack of selective 5-HT1A receptor antagonists available for clinical use, pindolol was used in past studies testing the 5-HT1A receptor augmentation strategy (Artigas, 1993; Artigas et al., 1994; 1996; Perez et al., 1997; Bordet et al., 1998; Zanardi et al., 1998; Ballesteros and Callado, 2004; Portella et al., 2011). However, the addition of DU-125530 to fluoxetine treatment did not enhance nor accelerate its antidepressant action in a population of depressive patients with clinical characteristics similar to those included in previous studies (Perez et al., 1997; 1999). This difference cannot be attributed to pharmacokinetic factors as fluoxetine plasma levels were similar to those previously reported in the fluoxetine + pindolol study (Perez et al., 2001) and did not differ between treatment arms.
However, several remarkable differences exist between pindolol and DU-125530. PET scan studies have revealed a preferential occupancy of pre-synaptic versus post-synaptic 5-HT1A receptors by pindolol, using 11C-WAY-100635 as a ligand (Artigas et al., 2001; Martinez et al., 2001). However, DU-125530 shows a comparable occupancy of pre- and post-synaptic 5-HT1A receptors using the same ligand (Rabiner et al., 2002). Thus, the occupancy of pre- and post-synaptic 5-HT1A receptors by the dose of DU-125530 used herein (20 mg·day−1) is 50–60% in most individuals tested (Rabiner et al., 2002). In contrast, the pindolol dose used in most clinical studies (7.5 mg·day−1) (Martinez et al., 2001) produced an occupancy of 40% pre-synaptic and 18% post-synaptic 5-HT1A receptors. These PET scan studies are paralleled by electrophysiological (Romero et al., 1996) and histological (Castro et al., 2000; Serrats et al., 2004) studies showing a preferential affinity of pindolol for pre- versus post-synaptic 5-HT1A receptors. Hence, pindolol antagonized the 5-HT1A autoreceptor-mediated inhibition of 5-hydroxytryptaminergic cell firing produced by SSRIs (Romero et al., 1996), but not the activation of hippocampal 5-HT1A receptors induced by 5-HT and 5-HT1A receptor agonists (Romero et al., 1996; Tada et al., 1999). In agreement, G-protein activation studies indicated a significantly higher potency of pindolol for 5-HT1A autoreceptors than for post-synaptic 5-HT1A receptors in the hippocampus and entorhinal cortex in rat, guinea pig and human brain (Serrats et al., 2004). Likewise, pindolol showed a greater affinity for pre- than for post-synaptic 5-HT1A receptors in human brain (Castro et al., 2000). A second difference between pindolol and DU-125530 lies in the partial agonist character of pindolol (Newman-Tancredi et al., 1998). Pindolol may increase cortical catecholamine release via activation of mPFC 5-HT1A receptors when administered alone (see Artigas et al., 2001). However, it appears unlikely that this property can be relevant in a pharmacological situation dominated by the excess 5-HT – and therefore high 5-HT1A receptor activation – produced by SSRIs.
The inability of DU-125530 to accelerate or augment the antidepressant action of fluoxetine is likely to be attributable to its simultaneous blockade of pre- and post-synaptic 5-HT1A receptors, given the enhanced post-synaptic 5-HT1A receptor activation produced by several antidepressant drug classes in rodents (Haddjeri et al., 1998; Blier and Ward, 2003). The present data support that this process may also occur in human brain. Thus, while 5-HT1A autoreceptor blockade augments pre-synaptic 5-HT function by preventing the negative feedback at pre-synaptic (raphe) level, the simultaneous blockade of post-synaptic 5-HT1A receptors in corticolimbic areas may cancel this effect. Moreover, the present results indicate that other 5-HT receptors (e.g. 5-HT4) (Lucas, 2009) are involved in the antidepressant action of fluoxetine, because the extensive blockade of post-synaptic 5-HT1A receptors did not cancel the clinical effect of fluoxetine, as it would be expected if post-synaptic 5-HT1A receptors were the only mediators of its antidepressant action.
Limitations of the study
The wide range of techniques and methodologies used in preclinical studies to characterize the action of DU-125530 in rodent brain support a full antagonist action of this agent at pre- and post-synaptic receptors with a low level of uncertainty. In any case, we carried out a reduced number of experiments to examine the action of DU-125530 at post-synaptic 5-HT1A receptors using electrophysiology. However, microdialysis data are fully supportive of such an antagonist action of post-synaptic 5-HT1A receptors. In the clinical trial, the main limitation of the study is that only one dose of DU-125530 was used, based on PET scan data (Rabiner et al., 2002). Given the antidepressant properties of post-synaptic 5-HT1A receptor activation in animal models (see above), it is unknown whether a lower DU-125530 dose, leading to a less post-synaptic 5-HT1A receptor occupancy would have augmented the antidepressant effects of fluoxetine. Trial design does not appear to be a limitation, as we used the same one than in a previous trial (Perez et al., 1997), which was able to detect significant differences between two similar arms (fluoxetine + placebo vs. fluoxetine + pindolol).
In summary, the present study shows that DU-125530 is an excellent antagonist of pre- and post-synaptic 5-HT1A receptors. Despite this, its addition to fluoxetine did not accelerate nor enhance its antidepressant properties in patients with major depression. These results show that simultaneous blockade of pre- and post-synaptic 5-HT1A receptors does not improve the antidepressant actions of SSRI, indicating that post-synaptic 5-HT1A receptor activation is required to achieve an enhancement of the antidepressant effects of SSRIs, a conclusion relevant to antidepressant drug design.
Acknowledgments
This study was supported by grants SAF 2007-62378, FIS PI09/1245 (PN de I+D+I 2008-2011, ISCIII-Subdirección General de Evaluación y Fomento de la Investigación), La Marató TV3, Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM and 2009SGR220 from the Catalan Government. Support and supply of DU-125530 by Advancell is also acknowledged. MCS was the recipient of a post-doctoral fellowship from Fundación Carolina. LL-P was supported by a JAE fellowship from CSIC. SO, DP, RPE, EA and VP were employed by the Hospital de la Santa Creu i Sant Pau. RC and FA are employed by CSIC. MJP was a junior researcher employed by the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM). PC is supported by the Researcher Stabilization Program of the Health Department of the Generalitat de Catalunya.
We thank Judith Ballart, Leticia Campa and Noemí Jurado for skilful technical assistance. Also, Dr Miklos Toth (Cornell Univ.) is gratefully acknowledged for the supply of 5-HT1A receptor knockout mice.
Glossary
- 5-HT
serotonin
- CGI
Clinical Global Impression
- DR
dorsal raphe nucleus
- HDRS-17
Hamilton Depression Rating Scale of 17 items
- KO
knockout
- LOCF
Last observation-carried- forward
- MADRS
Montgomery- Asberg Depression Rating Scale
- MDD
major depression diagnosis
- mPFC
Medial prefrontal cortex
- OC
Observed cases
- SERT
5-HT transporter
- SSRI
selective 5-HT reuptake inhibitors
- WT
wild type
Conflicts of interest
EA has received consulting and educational honoraria from several pharmaceutical companies including Eli Lilly, Sanofi-Aventis, Lundbeck and Pfizer, and he has participated as main local investigator in clinical trials from Eli Lilly, Bristol-Myers and Sanofi-Aventis and also as national coordinator of clinical trials from Servier and Lundbeck. VP has received educational honoraria from the following pharmaceutical companies: Sanofi-Aventis, Lundbeck, Pfizer and Eli Lilly. FA has received consulting or educational honoraria from Boehringer-Ingelheim, Eli Lilly, Lundbeck and Pierre Fabre. The rest of authors declare no conflicts of interest related directly or indirectly to this work.
References
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S3xx. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Andlin-Sobocki P, Wittchen HU. Cost of affective disorders in Europe. Eur J Neurol. 2005;12:34–38. doi: 10.1111/j.1468-1331.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- Artigas F. 5-HT and antidepressants: new views from microdialysis studies. Trends Pharmacol Sci. 1993;14:262. doi: 10.1016/0165-6147(93)90125-4. [DOI] [PubMed] [Google Scholar]
- Artigas F, Perez V, Alvarez E. Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, De Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Artigas F, Celada P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends Pharmacol Sci. 2001;22:224–228. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- Ballesteros J, Callado LF. Effectiveness of pindolol plus serotonin uptake inhibitors in depression: a meta-analysis of early and late outcomes from randomised controlled trials. J Affect Disord. 2004;79:137–147. doi: 10.1016/S0165-0327(02)00404-4. [DOI] [PubMed] [Google Scholar]
- Bel N, Artigas F. Fluvoxamine preferentially increases extracellular 5-hydroxytryptamine in the raphe nuclei: an in vivo microdialysis study. Eur J Pharmacol. 1992;229:101–103. doi: 10.1016/0014-2999(92)90292-c. [DOI] [PubMed] [Google Scholar]
- Blier P, Bergeron R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J Clin Psychopharmacol. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- Blier P, De Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Bordet R, Thomas P, Dupuis B. Effect of pindolol on onset of action of paroxetine in the treatment of major depression: intermediate analysis of a double-blind, placebo-controlled trial. Reseau de Recherche et d'Experimentation Psychopharmacologique. Am J Psychiatry. 1998;155:1346–1351. doi: 10.1176/ajp.155.10.1346. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Artigas F. Control of 5-hydroxytryptamine release in the dorsal raphe nucleus by the noradrenergic system in rat brain. Role of alpha-adrenoceptors. Neuropsychopharmacology. 2003;28:421–434. doi: 10.1038/sj.npp.1300061. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Amargos-Bosch M, Toth M, Artigas F, Adell A. In vivo efflux of serotonin in the dorsal raphe nucleus of 5-HT1A receptor knockout mice. J Neurochem. 2004;88:1373–1379. doi: 10.1046/j.1471-4159.2003.02267.x. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Hervas I, Artigas F. Postsynaptic 5-HT1A receptors control 5-HT release in the rat medial prefrontal cortex. Neuroreport. 1999;10:1441–1445. doi: 10.1097/00001756-199905140-00010. [DOI] [PubMed] [Google Scholar]
- Castro ME, Harrison PJ, Pazos A, Sharp T. Affinity of (+/-)-pindolol, (-)-penbutolol, and (-)-tertatolol for pre- and postsynaptic serotonin 5-HT(1A) receptors in human and rat brain. J Neurochem. 2000;75:755–762. doi: 10.1046/j.1471-4159.2000.0750755.x. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, Celada P, Artigas F. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci. 2005;25:10831–10843. doi: 10.1523/JNEUROSCI.2999-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, et al. A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur J Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, De Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas I, Bel N, Fernandez AG, Palacios JM, Artigas F. In vivo control of 5-hydroxytryptamine release by terminal autoreceptors in rat brain areas differentially innervated by the dorsal and median raphe nuclei. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:315–322. doi: 10.1007/pl00005259. [DOI] [PubMed] [Google Scholar]
- Joordens RJ, Hijzen TH, Olivier B. The effects of 5-HT1A receptor agonists, 5-HT1A receptor antagonists and their interaction on the fear-potentiated startle response. Psychopharmacology (Berl) 1998;139:383–390. doi: 10.1007/s002130050729. [DOI] [PubMed] [Google Scholar]
- Knobelman DA, Hen R, Lucki I. Genetic regulation of extracellular serotonin by 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) autoreceptors in different brain regions of the mouse. J Pharmacol Exp Ther. 2001;298:1083–1091. [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol. 2004;7:501–506. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Santana N, Ghisi V, Artigas F, Celada P. 5-HT1A receptor agonists enhance pyramidal cell firing in prefrontal cortex through a preferential action on GABA interneurons. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr220. doi: 10.1093/cercor/bhr220 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Lucas G. Serotonin receptors, type 4: a new hope? Curr Drug Targets. 2009;10:1085–1095. doi: 10.2174/138945009789735200. [DOI] [PubMed] [Google Scholar]
- Martinez D, Hwang D, Mawlawi O, Slifstein M, Kent J, Simpson N, et al. Differential occupancy of somatodendritic and postsynaptic 5HT(1A) receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology. 2001;24:209–229. doi: 10.1016/S0893-133X(00)00187-1. [DOI] [PubMed] [Google Scholar]
- Mos J, Van Hest A, Van Drimmelen M, Herremans AH, Olivier B. The putative 5-HT1A receptor antagonist DU125530 blocks the discriminative stimulus of the 5-HT1A receptor agonist flesinoxan in pigeons. Eur J Pharmacol. 1997;325:145–153. doi: 10.1016/s0014-2999(97)00131-3. [DOI] [PubMed] [Google Scholar]
- Neff CD, Abkevich V, Packer JC, Chen Y, Potter J, Riley R, et al. Evidence for HTR1A and LHPP as interacting genetic risk factors in major depression. Mol Psychiatry. 2009;14:621–630. doi: 10.1038/mp.2008.8. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Chaput C, Gavaudan S, Verriele L, Millan MJ. Agonist and antagonist actions of (-)pindolol at recombinant, human serotonin1A (5-HT1A) receptors. Neuropsychopharmacology. 1998;18:395–398. doi: 10.1016/S0893-133X(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof TJ, Ronken E, van der Heyden JA. Anxiolytic effects of flesinoxan in the stress-induced hyperthermia paradigm in singly-housed mice are 5-HT1A receptor mediated. Eur J Pharmacol. 1998;342:177–182. doi: 10.1016/s0014-2999(97)01482-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1998. [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Perez V, Gilaberte I, Faries D, Alvarez E, Artigas F. Randomised, double-blind, placebo-controlled trial of pindolol in combination with fluoxetine antidepressant treatment. Lancet. 1997;349:1594–1597. doi: 10.1016/S0140-6736(96)08007-5. [DOI] [PubMed] [Google Scholar]
- Perez V, Soler J, Puigdemont D, Alvarez E, Artigas F. A double-blind, randomized, placebo-controlled trial of pindolol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Grup de Recerca en Trastorns Afectius. Arch Gen Psychiatry. 1999;56:375–379. doi: 10.1001/archpsyc.56.4.375. [DOI] [PubMed] [Google Scholar]
- Perez V, Puigdemont D, Gilaberte I, Alvarez E, Artigas F. Augmentation of fluoxetine's antidepressant action by pindolol: analysis of clinical, pharmacokinetic, and methodologic factors. J Clin Psychopharmacol. 2001;21:36–45. doi: 10.1097/00004714-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portella MJ, Diego-Adelino J, Ballesteros J, Puigdemont D, Oller S, Santos B, et al. Can we really accelerate and enhance the selective serotonin reuptake inhibitor antidepressant effect? A randomized clinical trial and a meta-analysis of pindolol in nonresistant depression. J Clin Psychiatry. 2011;72:962–969. doi: 10.4088/JCP.09m05827blu. [DOI] [PubMed] [Google Scholar]
- Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, Udo de Haes J, de Vries M, et al. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1, 2-benzisothiazol-3-(2H)-one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-py ridinyl)cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301:1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero L, Artigas F. Preferential potentiation of the effects of serotonin uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J Neurochem. 1997;68:2593–2603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- Romero L, Bel N, Artigas F, De Montigny C, Blier P. Effect of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology. 1996;15:349–360. doi: 10.1016/0893-133X(95)00240-E. [DOI] [PubMed] [Google Scholar]
- Romero L, Celada P, Martin-Ruiz R, Diaz-Mataix L, Mourelle M, Delgadillo J, et al. Modulation of serotonergic function in rat brain by VN2222, a serotonin reuptake inhibitor and 5-HT1A receptor agonist. Neuropsychopharmacology. 2003;28:445–456. doi: 10.1038/sj.npp.1300062. [DOI] [PubMed] [Google Scholar]
- Serrats J, Artigas F, Mengod G, Cortes R. An autoradiographic study of the influence of pindolol upon [35S]GTPgammaS binding in rat, guinea pig and human brain. Int J Neuropsychopharmacol. 2004;7:27–34. doi: 10.1017/S1461145703003924. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K, Kasamo K, Ueda N, Suzuki T, Kojima T, Ishikawa K. Anxiolytic 5-hydroxytryptamine1A agonists suppress firing activity of dorsal hippocampus CA1 pyramidal neurons through a postsynaptic mechanism: single-unit study in unanesthetized, unrestrained rats. J Pharmacol Exp Ther. 1999;288:843–848. [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Whale R, Terao T, Cowen P, Freemantle N, Geddes J. Pindolol augmentation of serotonin reuptake inhibitors for the treatment of depressive disorder: a systematic review. J Psychopharmacol. 2010;24:513–520. doi: 10.1177/0269881108097714. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2008. [Google Scholar]
- Zanardi R, Franchini L, Gasperini M, Lucca A, Smeraldi E, Perez J. Faster onset of action of fluvoxamine in combination with pindolol in the treatment of delusional depression: a controlled study. J Clin Psychopharmacol. 1998;18:441–446. doi: 10.1097/00004714-199812000-00004. [DOI] [PubMed] [Google Scholar]


