Abstract
BACKGROUND & PURPOSE
Loperamide is a selective µ opioid receptor agonist acting locally in the gastrointestinal (GI) tract as an effective anti-diarrhoeal but can cause constipation. We tested whether modulating µ opioid receptor agonism with δ opioid receptor antagonism, by combining reference compounds or using a novel compound (‘MuDelta’), could normalize GI motility without constipation.
EXPERIMENTAL APPROACH
MuDelta was characterized in vitro as a potent µ opioid receptor agonist and high-affinity δ opioid receptor antagonist. Reference compounds, MuDelta and loperamide were assessed in the following ex vivo and in vivo experiments: guinea pig intestinal smooth muscle contractility, mouse intestinal epithelial ion transport and upper GI tract transit, entire GI transit or faecal output in novel environment stressed mice, or four weeks after intracolonic mustard oil (post-inflammatory). Colonic δ opioid receptor immunoreactivity was quantified.
KEY RESULTS
δ Opioid receptor antagonism opposed µ opioid receptor agonist inhibition of intestinal contractility and motility. MuDelta reduced intestinal contractility and inhibited neurogenically-mediated secretion. Very low plasma levels of MuDelta were detected after oral administration. Stress up-regulated δ opioid receptor expression in colonic epithelial cells. In stressed mice, MuDelta normalized GI transit and faecal output to control levels over a wide dose range, whereas loperamide had a narrow dose range. MuDelta and loperamide reduced upper GI transit in the post-inflammatory model.
CONCLUSIONS AND IMPLICATIONS
MuDelta normalizes, but does not prevent, perturbed GI transit over a wide dose-range in mice. These data support the subsequent assessment of MuDelta in a clinical phase II trial in patients with diarrhoea-predominant irritable bowel syndrome.
Keywords: opioid, irritable bowel syndrome, visceral pain, motility, gastrointestinal secretion, δ opioid receptor
Introduction
Morphine and synthetic opioids, such as loperamide, are potent inhibitors of gastrointestinal (GI) transit. Loperamide, at the recommended doses, has GI effects with low liability for CNS-related adverse effects (Awouters et al., 1993). It is a widely used anti-diarrhoeal and, although not specifically indicated for diarrhoea-predominant irritable bowel syndrome (d-IBS), is often used to control the symptoms of d-IBS (Callahan, 2002). IBS is characterized by unexplained abdominal pain, discomfort and bloating associated with altered bowel habits. Loperamide reduces diarrhoea and urgency in d-IBS patients (Cann et al., 1984) but is also associated with constipation, which can be a serious problem in some of these patients (Talley, 2003). Therefore, an effective treatment option that reduces the likelihood of constipation would be attractive.
The G-protein-coupled opioid receptors are of three major types, δ, κ, and µ, with amino acid sequence differences primarily in the extracellular tails (Quock et al., 1999). In the gut, δ, κ and µ opioid receptors are present in the enteric nervous system (Wood and Galligan, 2004), pacemaker cells (Bagnol et al., 1997) and smooth muscle cells (Kuemmerle and Makhlouf, 1992). Perhaps not surprisingly, drugs being investigated or already marketed with actions at opioid receptors may be beneficial for IBS patients. Loperamide is a selective µ opioid receptor agonist, without δ opioid receptor activity or κ opioid receptor affinity (DeHaven-Hudkins et al., 1999); trimebutine is a weak agonist at µ, δ and κ opioid receptors (Delvaux and Wingate, 1997); asimadoline is a κ opioid receptor selective agonist (Camilleri, 2008).
Enteric neurons expressing µ opioid receptors contribute to opioid-induced constipation (Sternini et al., 1996) where local µ opioid receptor activation would be expected to reduce secretion and motility, based on the known literature and marketed µ opioid receptor agonists. From the literature, a role for the δ opioid receptor in propulsive motility is more controversial due partly to species and GI regional differences (Broccardo et al., 1998; Shahbazian et al., 2002; Holt et al., 2005; Feng et al., 2006). Some reports show little effect of δ opioid receptor activation (Shahbazian et al., 2002; Feng et al., 2006), whereas δ opioid receptor agonists were shown to decrease colonic propulsion in guinea pig colon (Foxx-Orenstein et al., 1998). In the same model, the δ opioid receptor antagonist naltrindole acted synergistically with 5-HT4 agonists to increase propulsion (Foxx-Orenstein et al., 1998), suggesting that δ opioid receptor antagonism could oppose the inhibition exerted by µ opioid receptor agonism. When µ and δ opioid receptor agonists are combined, the inhibition of GI transit is greater than that induced by a µ opioid receptor agonist alone in a rodent model of GI inflammation (Pol et al., 1994). However, we are not aware of pharmacological experiments on the effects of combining δ opioid receptor antagonists with µ opioid receptor agonists on GI transit specifically, although recent evidence suggests that this combination has potential analgesic advantages over morphine (Dietis et al., 2009; Mosberg et al., 2011). Therefore, we tested whether blockade of δ opioid receptors modulates the inhibitory effects of µ opioid receptor agonists on the GI tract. We used combinations of reference compounds and selected a molecule from a series of potent phenylimidazoles (Breslin et al., 2006) that is a mixed µ opioid receptor agonist and δ opioid receptor antagonist (MuDelta). The synthesis and structure of MuDelta are reported separately (Breslin et al., 2012). A comparison of the effects of MuDelta with loperamide in several rodent models of perturbed GI motor function in mice revealed differences that could support the use of MuDelta in treating patients with d-IBS.
Methods
The nomenclature used throughout conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Animal experiments
All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010;McGrath et al., 2010). All protocols utilizing animals were carried out in accordance with the Federal Animal Welfare Act and with methods approved by the Institutional Animal Care and Use Committee of Johnson and Johnson Pharmaceutical Research and Development, LLC.
The animals used in these experimental protocols were as follows: 3 female cynomolgus monkeys (average 3.7 kg, maintained in house); 12 male or female Hartley guinea pigs (400-500 g, Charles River, Kingston, NY); 992 male CD-1 mice (Charles River Laboratories, Kingston, NC); 4 male Wistar rats (150-250 g, Charles River, Kingston, NY) and 83 male Sprague-Dawley rats (115-135 g, Charles River Laboratories Raleigh, NC). Rodents were group housed (4-6 per plastic cage) at an ambient temperature of 21-23 C with an automated 12/12-h light/dark cycle and access to water and a commercial rodent food ad libitum. All animals were deprived of food but not water overnight before any treatments.
In vitro opioid receptor binding
MuDelta was assessed in radioligand binding assays, as follows. Human µ opioid receptor over-expressing membranes (Perkin-Elmer, Waltham, MA, USA; Receptor Biology) were homogenized (50 mM Tris pH 7.5 and 5 mM MgCl2) and incubated with 3.6 nM [3H]-Tyr-DAla-Gly-[NMePhe]-NH(CH2)2 (DAMGO; Perkin-Elmer NEN) for 2 h (RT). Non-specific binding was determined by the addition of 1 µM unlabelled DAMGO. Male Wistar rats (150–250 g; Charles River, Kingston, NY) were killed by CO2, and forebrains were homogenized (ice cold Tris–HCl buffer 50 mM, pH 7.4) and centrifuged. Rat δ opioid receptor expressing forebrain cell membranes were incubated with the δ opioid receptor selective peptide ligand (2 nM [3H]-cyc[DPen2, DPen5]enkephalin; DPDPE) or 1 nM [3H]-DAMGO (25°C for 2.5 h). Human κO opioid receptors recombinantly expressing HEK293 cells and guinea pig cerebellum membranes were homogenized (50 mM Tris–Cl, pH 7.8, 5 mM MgCl2 and 1 mM EGTA) and incubated (80 min at 22°C) with 0.7 nM [3H]-U-69593 (Perkin-Elmer NEN) in the absence or presence of MuDelta. Non-specific binding was determined in the presence of 10 µM naloxone.
All assay mixtures were filtered through GF/C Filterplates (Perkin-Elmer NEN) and radioactivity determined by membrane-bound [3H]-DAMGO and [3H]-U-69593 (Packard Instrument Co., Meriden, CT USA: Topcount-NXT Microplate Scintillation Counter) and [3H]-DPDPE (Wallac 1205 BetaPlate liquid Scintillation Counter; Perkin-Elmer).
Opioid receptor activity in cell-based and isolated tissue functional experiments
Rat δ opioid receptor activity was determined in NG108-15 cell membranes (5 mg·mL−1; Applied Cell Sciences, Rockville, MD) suspended in 10 mM Tris–HCl (pH 7.2) with 2 mM EDTA and 10% sucrose. P2 membranes (75 µg·mL−1) were incubated with 0.1 nM [35S]-GTPγS in buffer containing 100 µM GDP. Non-specific binding was determined in the presence of 10 µM unlabelled GTPγS. Compounds were assessed for stimulation of [35S]-GTPγS binding alone and in the presence and absence of δ opioid receptor antagonists.
MuDelta activity at the rodent δ opioid receptor was assessed by investigating its effects on electrical field stimulation (EFS)-evoked contractions of hamster vas deferens. Segments of hamster vas deferens were suspended in 20 mL organ baths containing an oxygenated (95% O2 and 5% CO2) and pre-warmed (37°C) physiological saline (pH 7.4) of the following composition (in mM): NaCl (118.0), KCl (4.7), CaCl2 (2.5), KH2PO4 (1.2), NaHCO3 (25.0) and glucose (11.0), with yohimbine (1 µM) and atropine (1 µM) present to block α2-adrenoceptors and muscarinic receptors, respectively. Following equilibration for 30 min, EFS was applied to the tissues using a constant current stimulator (rectangular constant current pulses, 0.5 ms; 10 V, voltage; 0.05 Hz). Tissues were exposed to MuDelta or reference δ opioid receptor antagonist naltrindole in the presence or absence of δ opioid receptor agonist DPDPE (0.1 µM), with consistent values compared to historical data, and the results are expressed as % of control twitch contraction amplitude (n = 2).
MuDelta activity at rodent µ opioid receptors was determined in segments of guinea pig distal ileum induced to contract by transmural EFS, where DAMGO is a selective µ opioid receptor agonist. Activity at rodent κ opioid receptors was assessed by EFS-evoked contractions of guinea pig proximal colon muscularis externa, and selectivity was determined in the presence of a κ opioid receptor antagonist, nor-binaltorphimine (norBNI). Segments (15 mm) of guinea pig, intact distal ileum or proximal colon muscularis externa (mucosa-free), oriented along the circular muscle axis were mounted in water-jacketed organ baths, maintained at 36°C, in an oxygenated (95% O2 and 5% CO2) and 37°C buffer (mM): NaCl (121.0), KCl (5.95), NaHCO3 (14.3), NaH2PO4 •H2O (1.34), MgCl2 (1.2), CaCl2 (2.5), dextrose (12.7). Segments were attached to solid-state isometric force transducers (FORT-10, WPI, Sarasota, FL) coupled to a bridge amplifier (OCTAL Bridge, AD Instruments, Colorado Springs, CO) with the output via an analogue-to-digital converter and continuously monitored with Chart™ software (PowerLab 8sp, AD Instruments). Tissues at a resting tension of 0.5 g were equilibrated (1 h) before EFS stimulation (rectangular constant current pulses, 0.5 ms; 1.5× voltage required for maximal contraction; 0.05 Hz). Drugs were added cumulatively, and results are expressed as % variation of the control twitch contraction amplitude, where mean values were unaffected by the conditions.
In vivo motility in mice
The effects of MuDelta and loperamide were assessed in untreated mice and two models of enhanced GI transit, as follows.
Mild stress-induced increases in GI transit were induced in male, CD-1 mice (30–35 g) with 10 mice per dose group. Acute ‘novel environment stressed’ mice were placed individually in 20 × 20 × 15 cm cages, equipped with a wire mesh bottom without prior acclimatization. Non-stressed controls had a 16–18 h period of acclimatization to their novel environment.
Post-inflammatory altered GI transit was induced in male CD-1 mice (9–10 weeks old). Freshly opened oil of mustard (95% or 98% pure allyl isothiocyanate; Sigma-Aldrich, St. Louis, MO) was administered intracolonically (50 µL of a solution of 0.5% in 30% ethanol) as reported previously (Kimball et al., 2005). GI motility was evaluated in these mice 4 weeks later, when GI transit increases are noted without overt GI inflammation, and compared with age-matched controls (Kimball et al., 2005). Upper GI transit was measured 1 h after oral gavage of carmine red. Mice were killed by cervical dislocation, a laparotomy was performed and the intestines were removed and leading edge of red dye measured. The % of the upper GI tract over which transit had occurred was calculated and reported at % control group that received intracolonic 30% ethanol 4 weeks earlier, with no effect at testing time.
Faecal pellet output h-1 was calculated after vehicle or test compounds (in 0.5% w v-1 methylcellulose at 0.1 mL·10 g−1 body wt) were administered by intragastric gavage. Where the number of pellets was reported as % control, the control group had faecal output that was not affected by the vehicle. Entire GI transit was measured after carmine red (0.25 mL, 6% carmine in 0.5% methylcellulose) was administered by oral gavage 30 min after vehicle or test compounds. The number of mice that excreted a carmine-containing faecal pellet at the end of each hour post-carmine administration was recorded, until the end of 6 h.
Upper GI transit was also determined by geometric centre (GC) 45 min after oral gavage of FITC conjugated to dextran (70 000 MW; FITC–dextran 5 mg·mL−1 in 0.5% methylcellulose). Animals were killed by administration of isoflurane with exsanguinations, and the entire GI tract was harvested into 15 segments: the stomach, 10 equal segments of small bowel, the caecum and 3 equal segments of colon. The fluorescent signal for the contents of each segment was determined by a fluorescence plate reader (Cytofluor™; excitation wavelength, 530 nm and emission, 590 nm). GC was calculated as: Σ(S1 × 1 + S2 × 2 +… S11 × 11), where S is the fraction of the total signal detected in each of the 15 segments (Miller et al., 1981).
δ Opioid receptor immunohistochemistry
Segments of GI tract of mice subjected to novel environment stress (n = 6) and control (n = 3) as well as banked human colonic tissue (obtained with informed consent) were paraffin-imbedded and sectioned for immunostaining with rabbit anti-δ opioid receptor polyclonal antisera (Affinity BioReagents, Golden, CO, USA; 1:4000). Digital microscope images were obtained using 20× magnification (5 fields per tissue sample), and δ opioid receptor immunostaining in mucosa (epithelium and lamina propria) was quantified using Quantimet digital image acquisition and analysis software. The imaging software was first calibrated against a series of gradated grey level images for optical density (OD) reading. The number of pixels at each OD intensity was then plotted against the OD measurements over the full range from 0.04 to 2.55 for each image. Means of five plots were then calculated to produce a mean OD plot for each sample. Background and ‘specific’ staining could be separated by discrimination of a threshold between the two. The total area above and below this threshold was quantified. Each data point was taken from an average of five images per section, with one or two sections obtained from each animal.
GI secretion
Epithelial ion transport was evaluated in full-thickness distal colon and jejunoileum segments from CD-1 male mice (30–40 g) mounted in Ussing-type flux chambers. Tissues were mounted as flat sheets exposing each surface (0.3 cm2) to a 6 mL chamber oxygenated Krebs buffer solution (pH 7.4, 37°C). Spontaneous transmural potential difference was clamped at 0 mV electronically with a voltage–current clamp amplifier. Baseline and stimulated changes in short-circuit current (Isc), an index of net unidirectional active ion transport, were continuously monitored using an IBM computer with Chart software (AD Instruments). Tissue conductance (Gt) was calculated in mS from changes evoked in baseline Isc (µA) in response to rectangular 1 mV bipolar pulses. Neurally mediated peak Isc changes (ΔIsc) were evoked by EFS (60–100 V, 0.5 ms, 10 Hz, 5 s). Compounds were added to the serosal reservoir, and effects were determined after a 20 min incubation period. Forskolin (10 µM) was added at the end of the experiment to determine tissue viability. Data were normalized to tissue surface area and expressed cm-2.
Colorectal distention and visceral hypersensitivity
Male Sprague–Dawley rats (311 ± 25 g) were anaesthetized by induction with 5% isoflurane in 100% O2 and then administered 80 mg·kg−1 ketamine, 0.2 mg·kg−1 medetomidine and 0.01 mg·kg−1 atropine by i.m. injection. The presence of full surgical anaesthesia was monitored in these rats during EMG electrode instrumentation by the absence of a corneal reflex or withdrawal to paw pinch.
An incision was made to expose the right oblique external abdominal muscle, into which two EMG electrodes (40 gauge stainless steel wire; Cooner Wire, Chatsworth, CA) were inserted. Teflon-insulated lead wires from the EMG electrodes together with a bare ground wire were passed s.c. to the nape of the neck where they were attached to a transcutaneous connector (Plastics One, Inc., Roanoke, VA) and sutured in place.
During a 1 week post-surgery recovery period, the rats were acclimatized to a restraint tube and colorectal balloon distention. Rats were briefly anaesthetized (70% CO2:30% O2), and a 6 cm long polyethylene colorectal balloon was inserted 2 cm proximal to the anus with the catheter (4.5 mm; Tygon® tubing obtained from Fisher Scientific, Pittsburgh, PA, USA) secured to the tail and connected to a miniature barostat (Distender Series IIR; G & J Electronics, Inc.; Toronto, ON) while the rat was placed in a cylindrical plastic restrainer. Pressure and volume outputs from the barostat, and EMG electrodes were connected to an analogue-to-digital converter (PowerLab 16sp, AD Instruments) and data acquisition, display and off-line analyses were performed using Chart™ software (AD Instruments).
Control baseline recordings were the average of six 40 mmHg barostat-controlled inflations, and the effects of subsequent treatments are reported as % control response for each rat. Visceral hyperalgesia was induced by intracolonic administration of 1.5 mL of zymosan A (2.5% in 30% ethanol) as previously reported (Coutinho et al., 1996). Four hours later, EMG responses to 30 repeated 40 mmHg distensions (duration 20 s at 4 min intervals) were recorded for 126 min total. Compounds and vehicle were given by oral gavage 18 and 4 h before a colorectal distention period, which was 4 h after intracolonic zymosan A.
Where EMG analysis was suboptimal post surgery, manometric data analysis was used to evaluate VMR responses (Tammpere et al., 2005) as follows. The signal was conditioned by a 1 Hz high-pass filter, and rectified. The baseline-subtracted manometric response to each distension stimulus was defined as the integral of the rectified signal for the first 15 s of the CRD, minus the integral of the rectified signal for 15 s immediately preceding the CRD.
Statistics
Data are presented as mean ± SEM. For generation of IC/EC50 values from concentration–effect curves obtained in the in vitro and ex vivo experiments, a non-linear regression curve fitting function for a sigmoidal dose–response curve with variable slope was applied (GraphPad Prism®, La Jolla, CA, USA). For in vivo assays, anova was performed with appropriate post hoc multiple comparison test, as indicated. Student's paired t-test (δ opioid receptor immunohistochemistry) was used to compare the means (GraphPad Prism®). Differences were considered significant at P < 0.05.
Results
δ Opioid receptor antagonism opposes µ opioid receptor inhibition of GI contractility and transit
In guinea pig isolated ileum longitudinal smooth muscle, a δ opioid receptor antagonist (naltrindole) and a µ opioid receptor agonist (DAMGO) were applied separately or in combination in the presence of EFS. Naltrindole (Figure 1A) enhanced (EC50 = 18.4 nM), and DAMGO (Figure 1B) inhibited (EC50 = 1.8 nM) the EFS-evoked contractions. Naltrindole and DAMGO combined at their maximally effective concentrations (1 µM) or at their EC50s, attenuated the inhibition of contractility in response to DAMGO alone (Figure 1B).
Figure 1.
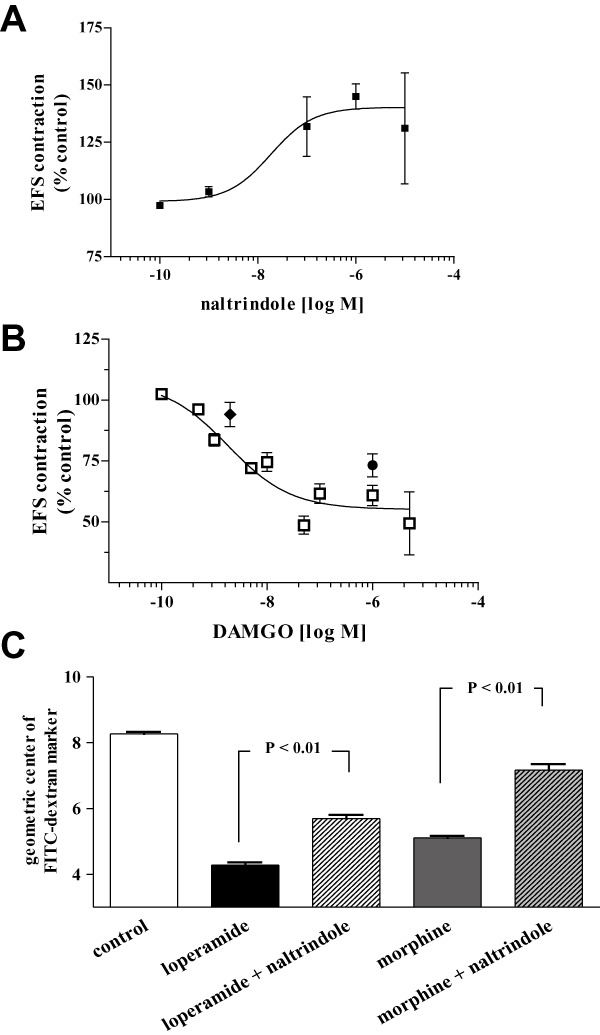
Combining reference compounds in guinea pig isolated ileum and mouse in vivo experiments supported the idea that δ opioid receptor antagonist opposes µ opioid receptor agonist effects. (A) The δ opioid receptor antagonist naltrindole, enhanced the EFS-evoked ileal contractions (EC50 = 18.4 nM). (B) The µ opioid receptor agonist DAMGO inhibited the EFS-evoked response (EC50 = 1.8 nM) in guinea pig. The effect of DAMGO was attenuated when combined with naltrindole each at 1 µM or at their EC50 concentrations. (C) Upper GI transit in mice measured by GC of FITC dextran was inhibited by µ opioid receptor agonists loperamide (3 mg·kg−1, p.o.) and morphine (3 mg·kg−1, p.o.). The inhibition of propulsive motility by either µ opioid receptor agonist was reversed by co-administration of naltrindole (30 mg·kg−1, p.o.).
Mice were administered FITC-dextran by oral gavage 1 h before either loperamide or morphine (3 mg·kg−1 p.o.) alone or with a δ opioid receptor antagonist (naltrindole 30 mg·kg−1 p.o.), and upper GI transit was measured 45 min later. Oral administration of morphine or loperamide inhibited upper GI transit, as measured by Geometric Center of FITC-dextran. Co-administration of the δ opioid receptor antagonist naltrindole with the µ opioid receptor agonists reduced the extent to which GI transit was inhibited by each µ opioid receptor agonist alone (Figure 1C). These in vitro and in vivo data support the hypothesis that δ opioid receptor antagonism functionally opposes µ opioid receptor agonism.
Receptor characterization of MuDelta
MuDelta had high affinity for rat δ opioid receptors (Ki = 1.3 nM) and human µ opioid receptors (Ki = 1.7 nM) but modest affinity for human κ opioid receptors (Ki = 55 nM). To determine the affinity of MuDelta for the human δ opioid receptor, a human SK-N-BE(2) neuroblastoma cell line endogenously expressing δ opioid receptors was used since we were unable to use δ opioid receptor recombinantly over-expressing cells. In SK-N-BE(2) cells, MuDelta binding (Ki = 366.6) was comparable with that of the δ opioid receptor peptide agonist DPDPE (Ki = 621.0 nM; Figure 2A). A different δ opioid receptor agonist, naltriben, had 100-fold higher affinity (Ki = 1.3 nM) in these cells (Figure 2A). It should be noted that SK-N-BE(2) cells endogenously express multiple opioid receptor subtypes.
Figure 2.
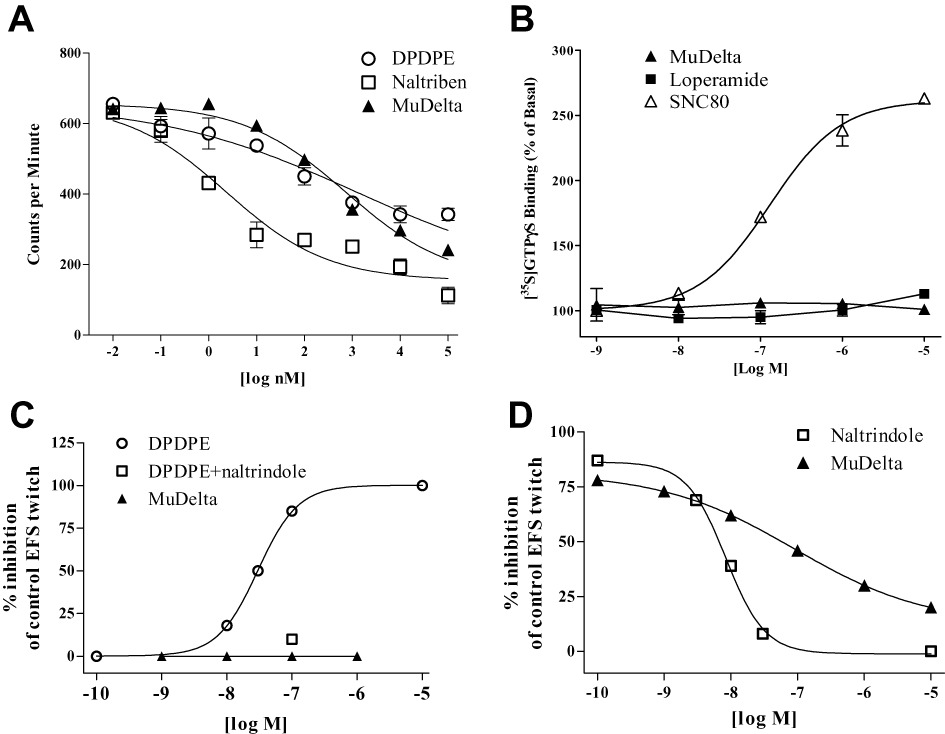
(A) In human SK-N-BE(2) cell membranes endogenously expressing δ opioid receptors MuDelta binding affinity was similar to that of the δ opioid receptor peptide agonist DPDPE but with ∼100-fold lower affinity than naltriben. (B) Neither MuDelta nor loperamide had activity at the δ opioid receptor, whereas SNC80 was an agonist, as measured by [35S]-GTPγS binding. (C) Electrically-evoked twitches in the hamster vas deferens were dose-dependently inhibited by DPDPE but not MuDelta at concentrations up to 1 µM. The inhibitory effect of 100 nM DPDPE was reversed by naltrindole. (E) The maximal effect of DPDPE to inhibit the hamster vas deferens' electrically-evoked twitch was dose-dependently reversed by the δ opioid receptor antagonist naltrindole and MuDelta with IC50 s of 8.4 and 89 nM, respectively. Data in (D) and (E) are the averages of duplicate experiments.
In rat δ opioid receptor expressing NG108-15 cell membranes, 1 µM SNC80 (a δ opioid receptor agonist) stimulated [35S]-GTPγS binding (basal cpm = 1310 ± 117) by over 200%, (3146 ± 14 cpm), whereas neither MuDelta (1376 ± 29 cpm) nor loperamide (1539 ± 18 cpm) had any agonist effect on the δ opioid receptors (Figure 2B). The stimulant effect of SNC80 was completely abolished by 10 µM MuDelta (1364 ± 44 cpm), but not loperamide (2819 ± 39 cpm), suggesting that the MuDelta is a δ opioid receptor antagonist. This was confirmed in hamster vas deferens where another δ opioid receptor agonist (DPDPE) concentration-dependently inhibited electrically evoked contractions, as reported previously (McKnight et al., 1985), but MuDelta was inactive in this preparation (≤1 µM; Figure 2C). The effect of DPDPE was reversed by a selective δ opioid receptor antagonist (naltrindole, IC50 8.4 nM) and by MuDelta (IC50 89 nM; Figure 2D).
Similar to DAMGO, MuDelta behaved as a potent full µ opioid receptor agonist (EC50 ≍ 1 nM), inhibiting EFS-stimulated guinea pig distal ileum contractions (Figure 3A). Naloxone reversed the effect of MuDelta (1 µM) on EFS-evoked contractions (Figure 3A).
Figure 3.
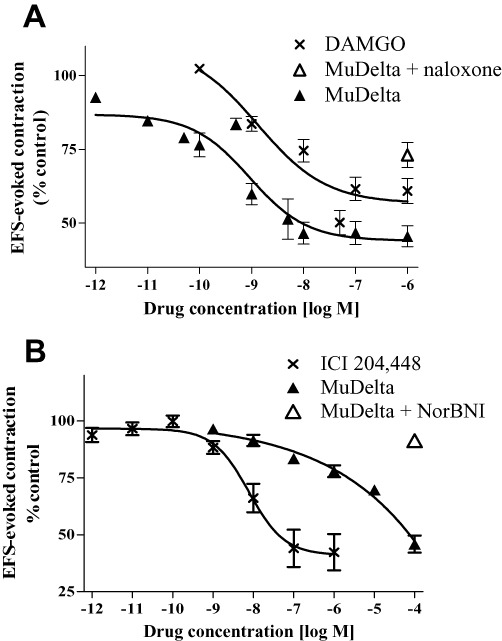
(A) In the guinea pig isolated ileum, MuDelta reduced EFS-evoked contractions similar to DAMGO, with Kis of 1.0 and 1.3 nM, respectively (n = 8 per group); 10 µM naloxone reversed the effects of 1 µM MuDelta. (B) In guinea pig isolated proximal colon, MuDelta had weak κ opioid receptor agonist activity compared with the κ opioid receptor agonist ICI 204 448, with IC50 = 1.6 µM and 1.7 nM, respectively (n = 4–8). A selective κ opioid receptor antagonist, norBNI (10 µM), reversed the effects of 100 µM MuDelta (n = 4).
MuDelta had weak κ opioid receptor agonist activity (EC50 = 1.6 µM) in the guinea pig isolated proximal colon circular smooth muscle, in the presence of µ and δ opioid receptor receptor blockade (naloxonazine and naltrindole; Figure 3B). The activity of MuDelta (100 µM in the presence of naloxonazine and naltrindole) was reversed by the selective κ opioid receptor antagonist, nor-BNI (Figure 3B).
A summary of the receptor binding and functional activity of MuDelta compared with loperamide, is shown in Table 1. The selectivity of MuDelta was also evaluated by determining its ability to inhibit the binding of selective ligands in a panel of 50 receptors at a test concentration of 10 µM. At this concentration, MuDelta inhibited binding of only five ligands by over 30%.
Table 1.
Summary of the binding affinities and functional activity of MuDelta at specific opioid receptors
| Opioid receptor subtype | Loperamide1 | MuDelta | |||||
|---|---|---|---|---|---|---|---|
| Ki | Ki | Binding assay | IC50 | Functional assay | |||
| µ | 3.3 nM | 1.7 nM | Recombinantly expressed human µOR | 1.0 nM | guinea pig ileum | ||
| δ | 48 nM | 1.3 nM | Rat membrane preparation (also equivalent to DPDPE in human SK-NBE cells) | 89 nM | hamster vas deferens | ||
| κ | 1.2 µM | 55 nM | Recombinantly expressed human κOR | 1.6 µM | guinea pig colon | ||
Loperamide data for comparison (DeHaven-Hudkins et al., 1999).
MuDelta has low oral bioavailability
Single doses of MuDelta dissolved in 0.5% OH-propyl methylcellulose were administered by oral gavage to adult male CD-1 mice (average wt. 45 g; four per each time point), and values averaged. A Tmax at 30 min of 15.4 ng·mL−1 MuDelta (∼0.03 nM) was obtained, which is consistent with low systemic oral bioavailability of less than 1%.
Single doses of MuDelta were administered to rats with 0.5% hydroxypropyl methylcellulose, adjusted to pH 3.3. Blood samples were taken at 0.5, 1, 2, 4, 6 and 8 h following oral administration. MuDelta was below the limit of detection in each of the samples from all four rats. In hepatic portal vein-cannulated rats, low concentrations of MuDelta were detected within 5 min of oral gavage (10 mg·kg−1 MuDelta) and remained constant over the 4 h sampling period (data not shown). In the same animals, jugular vein concentrations were mostly below the limits of detection.
Three female cynomolgus monkeys (average wt. 3.7 kg) were fed their normal morning meal and 1 h later were given a single oral dose of MuDelta (5 mg·kg−1), and were fed again 4 h after dosing. None of the animals vomited during the study. The compound was below the limit of detection in all blood samples (obtained at 0.25, 0.5, 1, 2, 4, 7 and 24 post administration) in all three monkeys.
Expression of δ opioid receptors in mouse and human GI tract
In the human colon, δ opioid receptor immunoreactivity was observed in mucosal epithelial cells (Figure 4A) and in ganglia of the enteric nervous system (Figure 4B). In the proximal and distal colon of mice exposed to novel environment stress, the expression of δ opioid receptors in the GI mucosal epithelium appeared more intense, as illustrated in the example from the distal colon (Figure 4C and D). The majority of δ opioid receptor staining appeared to be in epithelial cells within the crypt region (Figure 4D) but was also noted in enteric neurons. When the increase in δ opioid receptor immunostaining within the mucosa and lamina propria was quantified for both proximal and distal colon (Figure 4E and F), the proximal colon δ opioid receptor immunostaining attained a level of statistical significance in stressed mice. In the distal colon (Figure 4E), jejunum and ileum, δ opioid receptor background-subtracted staining tended to be increased in stressed mice (jejunum control = 0.03 ± 0.01 vs. stressed = 0.23 ± 0.12; ileum control = 0.06 ± 0.02 in vs. stressed = 0.08 ± 0.03, P > 0.05). There was no difference in background staining of all GI regions between the control and stressed groups.
Figure 4.
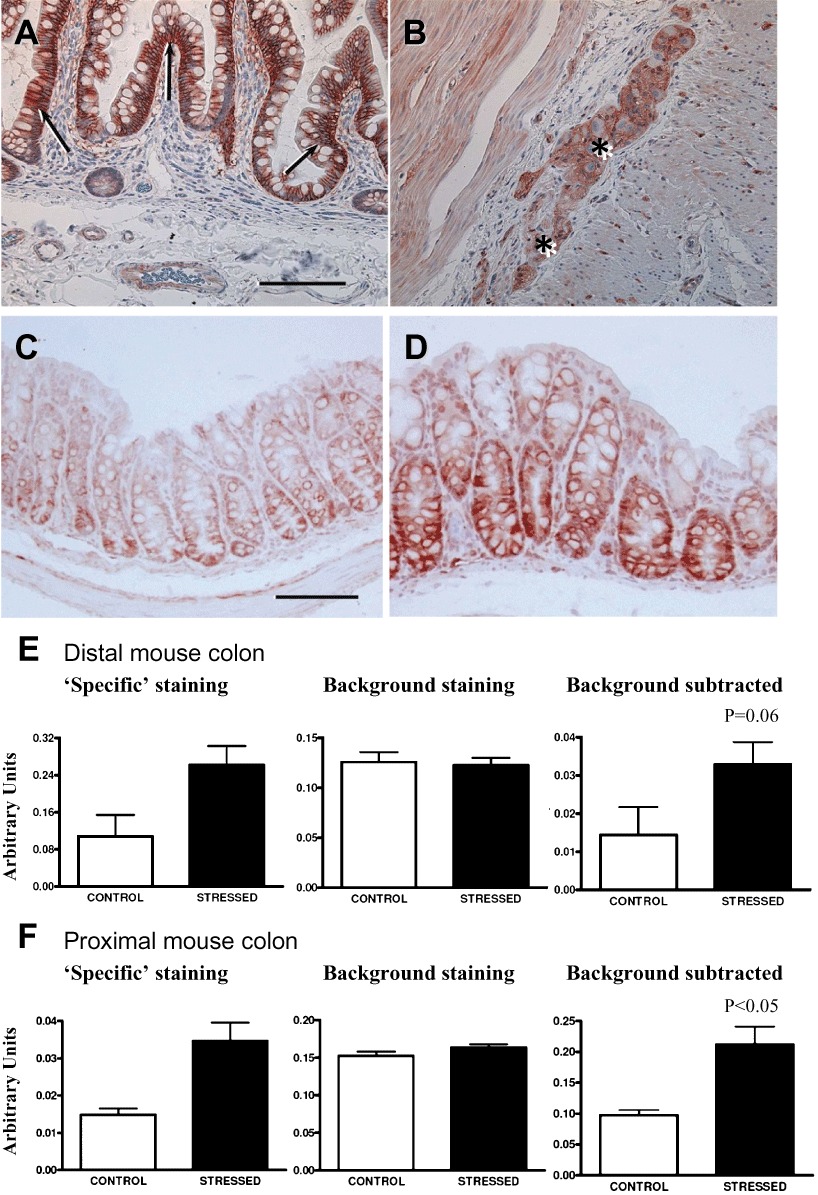
Digital images of immunohistochemical localization of δ opioid receptors in human colon (A, B) and mouse distal colon (C, D). In human colon, δ opioid receptor immunoreactivity was noted in (A) mucosal epithelium, which was densely immunostained, and (B) in a myenteric ganglion (asterisks). In mouse distal colon from control mice (C), δ opioid receptors can be seen in mucosal epithelium and was markedly increased in the same region in stressed mice (D). Bar = 100 µm. Quantification of δ opioid receptor mucosal immunostaining in distal colon (E) and proximal colon (F) of three control and six stressed mice illustrates that ‘specific’ background-subtracted δ opioid receptor immunostaining was significantly increased in the proximal colon.
Opioid receptor-mediated effects on GI functions
In non-stressed control mice, the faecal output h-1 was generally low (2–3 faecal pellets per hour), and MuDelta inhibited this at 50 mg·kg−1, but not at 5–25 mg·kg−1, whereas loperamide inhibited faecal output at 3–10 mg·kg−1 (Figure 5A and B), as would be expected from µ opioid receptor agonists. In mice exposed to novel environment-stress, 1 h faecal output was increased two- to threefold, and MuDelta normalized the stimulated faecal output over a wide dose range (5–100 mg·kg−1, p.o.; Figure 5C). This is shown by the lack of difference between stressed mice dosed with MuDelta compared to the control acclimatized mice. Notably, MuDelta reduced faecal output to within control levels at a range of doses administered (5–50 mg·kg−1, p.o.) and even at the highest dose (100 mg·kg−1) the inhibition of faecal output was not significantly different from control faecal output (100%). In comparison, loperamide significantly inhibited faecal output in stressed mice below control levels at 10 mg·kg−1 (Figure 5D).
Figure 5.
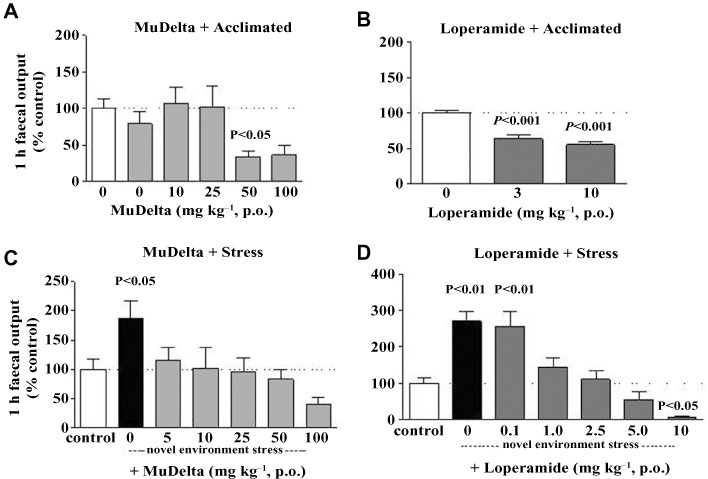
Comparison of the effects of MuDelta and loperamide on mouse 1 h faecal output in control acclimatized (A and B) and stressed (B and D) mice. Both MuDelta (50 mg·kg−1; A) and loperamide (3 and 10 mg; B) reduced faecal output in control mice. (C) Stress enhanced 1 h faecal output and MuDelta (5–100 mg·kg−1) dose-dependently reversesd this but did not reduce it to below that in the acclimatized controls. (D) Loperamide reversed stress-induced faecal output (1–5 mg·kg−1), but at 10 mg·kg−1, it reduced it to below that in the acclimatized controls. n = 24 mice/control groups, n = 10–12 in all other groups. P-value is shown with respect to control acclimatized group.
MuDelta and loperamide were also assessed, over a 6 h period, for effects on faecal output (Figure 6A and B) and entire GI tract transit (Figure 6C and D). In non-stressed mice, MuDelta (5, 10 and 25 mg·kg−1 p.o.) had little effect on 6 h faecal output, and at the higher doses (50 and 100 mg·kg−1), faecal output was reduced but not completely prevented (cumulative number of faecal pellets was ∼4 at 6 h; Figure 6A). In comparison, loperamide (5 and 10 mg·kg−1, p.o.) completely suppressed faecal output (cumulative number of faecal pellets was 0 at 6 h; Figure 6B) in non-stressed mice. Entire GI tract transit was enhanced in stressed compared with acclimatized mice (Figure 6C and D) and this enhancement was reduced dose-dependently by MuDelta (Figure 6C); however, even at the highest dose (100 mg·kg−1), entire GI transit was not abolished since the carmine red marker was noted in the faeces in 25% of the stressed mice. By contrast, loperamide (10 mg·kg−1) completely prevented the entire GI tract transit (Figure 6D).
Figure 6.
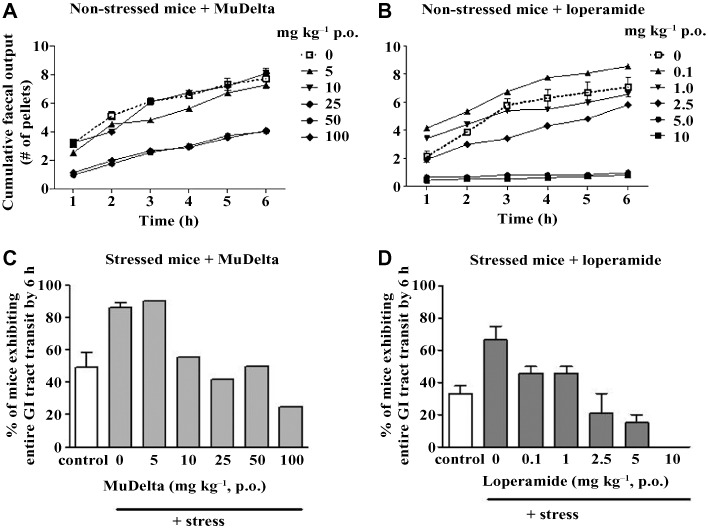
Comparison of dose-ranging effects of MuDelta (A and C) and loperamide (B and D) on cumulative faecal pellet output (A and B) and entire GI tract transit over 6 h (C and D), with a minimum of 10 mice per group. (A) Cumulative pellet output in non-stressed mice was dose-dependently decreased by MuDelta up to 100 mg·kg−1. (B) Loperamide reduced cumulative faecal output at 0.1–2.5 mg·kg−1 but at 5 and 10 mg·kg−1 completely abolished it. (C and D) The % mice exhibiting total GI tract transit after 6 h was approximately doubled by novel environment stress compared with acclimatized mice. (C) This effect was dose-dependently decreased by MuDelta, but the entire GI tract transit was not abolished at doses up to 100 mg·kg−1. (D) Loperamide abolished the entire GI tract transit at the highest dose (10 mg·kg−1). Bars illustrate the range where groups were repeated.
Upper GI tract transit was enhanced in mice 4 weeks after intracolonic oil of mustard, a model that shares similarities with post-inflammatory IBS (Kimball et al., 2005). In this model, MuDelta (10 mg·kg−1) inhibited upper GI transit significantly more than the same dose in age-matched controls (Figure 7A). Both MuDelta (25, 50 and 100 mg·kg−1) and loperamide (1 and 5 mg·kg−1) induced dose-related inhibition of upper GI tract transit in this model (Figure 7B).
Figure 7.
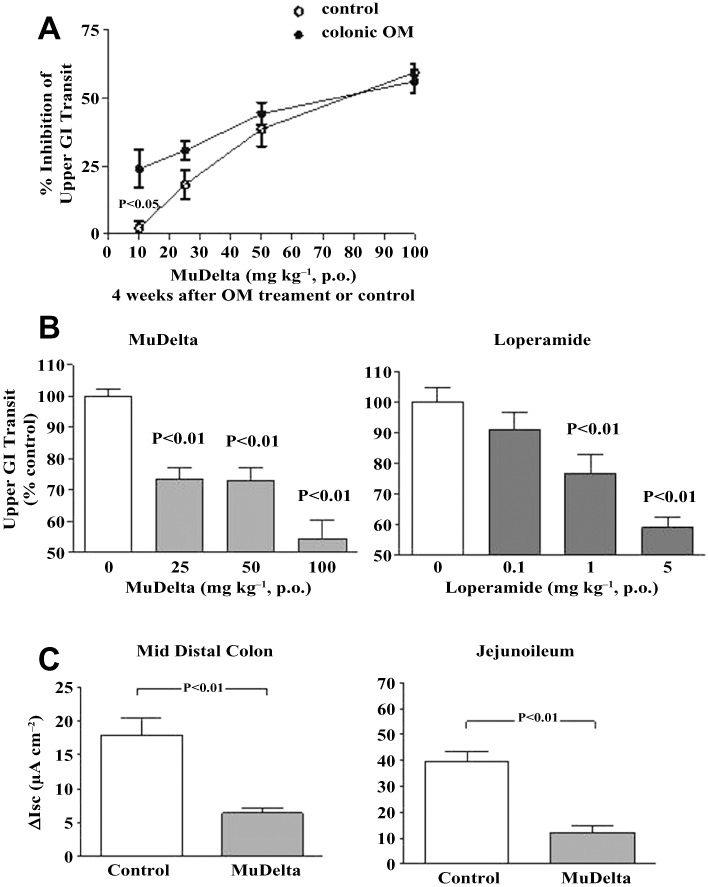
(A) MuDelta (10 −100 mg·kg−1) inhibited upper GI transit in a mouse model of post-inflammatory altered bowel function more potently at 10 mg·kg−1 compared with age-matched mice that did not receive intracolonic oil of mustard (n = 12–14 per group). (B) Dose-dependent inhibition of upper GI transit by MuDelta (25–100 mg·kg−1) and loperamide (1–5 mg·kg−1) in the same mice 4 weeks after intracolonic mustard oil (n = 11–14 per group). (C) MuDelta (1.0 µM) decreased neurogenic secretion in the colon and small bowel of mice in both the colon (control n = 29; MuDelta n = 10) and jejunoileum (control n = 35; MuDelta n = 11).
In isolated segments of CD-1 mouse intestine, increases in fluid/ion transport were evoked by EFS in Ussing chambers. The neurogenically stimulated secretory response was inhibited by MuDelta (1 µM) in both the colon and small intestine (Figure 7B) but not by loperamide (data not shown).
Mudelta and loperamide were assessed in a rat model of intracolonic zymosan A-induced visceral hyperalgesia. Each rat served as its own control, with EMG baseline control recordings compared with those obtained 4 h after intracolonic zymosan A. Zymosan A increased the pseudoaffective response to colorectal distention by 230 ± 50% and 333 ± 80% before loperamide and MuDelta, respectively (Figure 8A and B). However, neither orally administered MuDelta (50 mg·kg−1; Figure 8A) nor loperamide (5 mg·kg−1; P = 0.053; Figure 8B) were able to reverse the increased pseudoaffective response over the 2 h testing period. Manometric balloon pressure was analysed in three rats as another measure of the pseudoaffective response (Tammpere et al., 2005). Based on this analysis, MuDelta (50 mg·kg−1, p.o.) had an anti-hyperalgesic effect (P < 0.05 vs. pre-zymosan control, n = 3) in the first 30 min of repetitive distentions.
Figure 8.
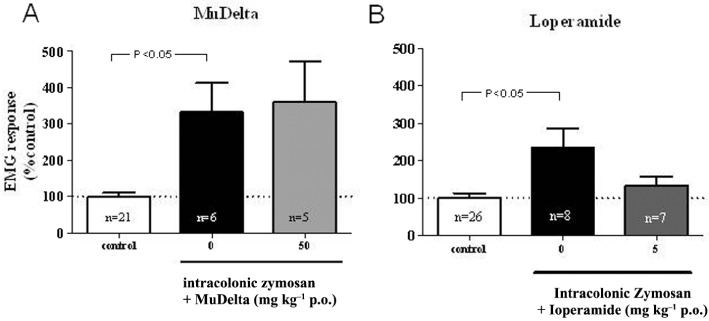
Acute colonic inflammation induced by intracolonic zymosan increased the pseudoeffective response (electromyograph) to repetitive colorectal balloon distension (40 mmHg) in rats, although variations in the magnitude of the hypersensitivity response were observed for each group. Neither MuDelta (at left, 50 mg·kg−1 p.o.) nor loperamide (at right, 5 mg·kg−1) reduced the pseudoeffective response after zymosan.
Discussion
The key findings of this study are that a combination of a δ opioid receptor antagonist with a µ opioid receptor agonist ameliorates the anti-transit effects of µ opioid receptor agonism alone. This effect was observed on both guinea pig isolated ileum contractility and on mice in vivo motility. Peripherally restricted compounds MuDelta (combined µ opioid receptor agonist/δ opioid receptor antagonist) and loperamide (µ opioid receptor agonist) both decreased transit in mice after oral administration. The difference between these compounds is based on dose range, and not on single dose, comparisons in models of perturbed transit. MuDelta normalized motility over a wide dose range, whereas loperamide normalized motility over a narrow dose range. For example, in stressed mice, MuDelta reduced GI transit to control levels but did not completely prevent it, at 10- to 20-fold the minimum effective dose. In contrast, loperamide prevented GI transit completely at three- to fourfold the minimum effective dose. These data indicate that MuDelta can ‘normalize’ transit without constipation over a wide dose range and provide preclinical evidence for the assessment of MuDelta in diarrhoea-predominant IBS patients.
MuDelta showed high affinity for human µ opioid receptors, based on binding in recombinantly expressing human µ opioid receptor membranes and its potent inhibition of the twitch response in guinea pig ileum. Therefore, there is agreement across human/guinea pig species for its binding and efficacy at µ opioid receptors in the low nM range. For δ opioid receptors, consistent data were obtained for MuDelta at rodent δ opioid receptors, with high-affinity binding in rat forebrain and a slightly lower IC50 in a hamster vas deferens assay. In endogenous human δ opioid receptor-expressing cells, SK-N-BE(2), MuDelta had a lower binding affinity (Ki = 337) but this was comparable to that of the δ opioid receptor agonist DPDPE (Ki = 621). Previously a much higher affinity (Ki = 2 nM) was reported for DPDPE in human δ opioid receptor-recombinantly expressing CHO cells (Parkhill and Bidlack, 2002). The low values for DPDPE and MuDelta may reflect the low δ opioid receptor receptor density in endogenously expressing cells. In addition, SK-N-BE(2) cells express several δ opioid receptor subtypes, and naltriben, a δ opioid receptor subtype 2 selective ligand (Stewart et al., 1994), had 100-fold higher affinity in these cells, suggesting an alternative explanation that MuDelta may have selectivity for δ opioid receptor subtype 1. Unfortunately, we were unable to use cell lines that over-express human δ opioid receptor subtype 2 (Law et al., 1994) to test this idea. MuDelta had a modest binding affinity to human κ opioid receptors in recombinantly expressing cells, and at the rodent κ opioid receptor, the EC50 was in the µM range. Therefore, in the subsequent discussion, we will focus on the actions of µ and δ opioid receptors in the gut.
Pharmacokinetic studies of MuDelta in rats, mice and primates demonstrated low systemic exposure to MuDelta after oral administration, which is consistent with a local site of action of the compound. Since levels were detected in hepatic portal vein cannulated rats, there is likely to be local penetration into the lamina propria followed by extensive first pass metabolism in the liver. Therefore, the site of action of MuDelta could include the myenteric plexus and smooth muscle where it is well accepted that opioid receptors mediate their transit effects (Greenwood-Van Meerveld et al., 2004; Wood and Galligan, 2004). However, because MuDelta is a mixed opioid receptor modulator, it is difficult to determine its site (s) of action, partly because of differences in the presence and activity of opioid receptor subtypes in different mammalian species (Greenwood-Van Meerveld et al., 2004). For example, although δ and µ opioid receptors extensively colocalize in enteric neurons of rats (Gray et al., 2006), only δ opioid receptors are present in pigs (Townsend et al., 2004). Even within rodents, the activity of δ opioid receptor agonists can vary depending on the region of the GI tract and the preparation. For example, δ opioid receptor agonists had no effect on motility when given systemically in rodents (Burks et al., 1988) or in µ opioid receptor-knockout mice (Roy et al., 1998), but decreased propulsion in the guinea pig isolated colon (Foxx-Orenstein et al., 1998). If a δ opioid receptor agonist increases motility, then a δ opioid receptor antagonist would be expected to block the response to endogenous enkephalins (µ > d opioid receptor agonists) released by enteric neurons (Greenwood-Van Meerveld et al., 2004; Wood and Galligan, 2004) and β-endorphins (µ = d opioid receptor agonist) released by mucosal enteroendocrine cells (Nihei and Iwanaga, 1985). Indeed, endogenous opioid release activates δ opioid receptors in organotypic cultures (Poole et al., 2011). Consistent with this, a selective δ opioid receptor antagonist had a synergistic effect with a 5-HT4 agonist to increase colonic propulsion (Foxx-Orenstein et al., 1998). Therefore, part of the effect of MuDelta on intestinal motility could be due to δ opioid receptor antagonism that enhances propulsion.
In the present study, δ opioid receptor immunostaining was noted in enteric neurons in both human and mouse colon. Intense δ opioid receptor immunostaining was also visualized in colonic mucosa of both species and was apparently increased in small and large intestine of stressed compared with acclimatized mice. However, quantification of mucosal δ opioid receptor staining revealed that this increase was significant only in the proximal colon of stressed mice. Quantification of δ opioid receptor staining specifically within enteric neurons was not done, since this would be unlikely to show significant changes. Consequently, we are unable to correlate the increase in δ opioid receptor expression after animals experienced stress with pharmacological sensitivity measured by motility, which would be mediated via enteric neurons. Electrophysiological whole-mount studies would be more appropriate to investigate δ opioid receptor myenteric sensitivity.
A stress-induced increase in δ opioid receptor expression is consistent with other models of GI pathophysiology. Specifically, up-regulation of δ opioid receptor gene expression is noted in croton oil-induced inflammation (Pol et al., 2001; Pol and Puig, 2004) and after intracolonic oil of mustard (Kimball et al., 2007). The inhibitory effect of morphine is enhanced by inflammation (Pol et al., 1994), presumably due to up-regulation of µ opioid receptors. Similarly, the potency of MuDelta to decrease upper intestinal transit in mice after intracolonic mustard oil was increased, which could reflect an up-regulation of µ opioid receptors in the small intestine in this model. In models of enhanced GI transit (novel environment stress and intra-colonic mustard oil), loperamide was a potent inhibitor of GI transit, similar to previous reports in castor oil-induced diarrhoea in mice (Puig and Pol, 1998; Greenwood-Van Meerveld et al., 2004).
In intestinal smooth muscle cells, µ and δ opioid receptor expression and functional activity are noted in rabbit, guinea pig and human (Grider and Makhlouf, 1991; Kuemmerle and Makhlouf, 1992), although the µ opioid receptor was not visualized in rat intestinal smooth muscle cells (Fickel et al., 1997). Therefore, although the present studies demonstrate that MuDelta has effects in rodents, translation of these effects to humans should be viewed with caution. Characterization of MuDelta in human isolated intestinal smooth muscle cells and myenteric neurons would determine their relative contributions to its overall effects in this species.
MuDelta decreased the short-circuit current in ex vivo preparations of neurogenically-evoked secretion in the mouse small intestine and colon consistent with the expression of δ opioid receptors in mouse submucosal neurons (Poole et al., 2011). Whether δ opioid receptor agonists or antagonists act directly on submucosal neurons was not tested, and there may be species differences since a δ opioid receptor agonist was found to inhibit submucosal neurons in guinea pig caecum (Mihara and North, 1986). Loperamide had no effect in this preparation, consistent with previous findings that µ opioid receptor agonists are ineffective at altering the short-circuit current (Kachur et al., 1980). However, loperamide has been shown to reduce fluid secretion evoked by PGE2 in rodents (Beubler et al., 1993). Hence, differences in the way in which secretion is promoted in these studies may account for discrepancies in the responses to loperamide.
Based on peripheral analgesic properties (DeHaven-Hudkins et al., 1999), one might expect loperamide and MuDelta to reduce visceral hyperalgesia. In the rat colonic zymosan A-induce inflammatory hyperalgesia model, loperamide tended to reduce the pseudoaffective response to distention. MuDelta was ineffective over the 2 h period overall, although some improvements were noted within the first 30 min. However, the ability of colorectal distention methods (clinically or pre-clinically) to reliably predict drug efficacy in treating pain and discomfort associated with IBS has proven inconsistent. Fedotozine (κ opioid receptor agonist) reduced visceral hyperalgesia (Sengupta et al., 1996), but clinical trials were discontinued due to lack of efficacy (Callahan, 2002). Alosetron (5-HT3 receptor antagonist) was effective in some models of visceral hypersensitivity (Mori et al., 2004; Miranda et al., 2006) but produced conflicting results clinically in colorectal barostat studies in d-IBS patients (Mayer and Bradesi, 2003). Thus, the extent to which improvements in colorectal distention hypersensitivity is predictive of drug treatment efficacy is not clear (Izquierdo et al., 2005).
The molecular mechanism for the in vivo differences in loperamide and MuDelta are unknown at present but may be interpreted in light of the novel pharmacology of engaging µ and δ opioid receptor heterodimers. It is recognized the δ opioid receptor heterodimerizes with other opioid receptor subtypes, which determines their pharmacology (Jordan and Devi, 1999). There is extensive co-expression of µ and δ opioid receptors in mouse and rat ileum myenteric neurons (Gray et al., 2006; Poole et al., 2011). If endogenous heterodimer formation occurs in enteric neurones expressing µ and δ opioid receptors, similar to spinal cord membranes (Gomes et al., 2004), then heterodimerized receptors could be engaged by MuDelta as follows. Activation of µ opioid receptors alone on heterodimers activates β-arrestin 2 signalling, but a combination of µ and δ opioid receptor agonists/antagonists drives signalling through the G-protein (Rozenfeld and Devi, 2007). Morphine-induced inhibition of colonic propulsion is attenuated in β-arrestin 2 knockout mice (Raehal and Bohn, 2005), although tolerance to µ opioid receptor agonists occurs in the colon of β-arrestin2 knockout but not wild-type mice (Maguma et al., 2012). Therefore, MuDelta differentiation from loperamide may be due to µ/δ opioid receptor signalling through G-protein, which would reduce the likelihood of constipation associated with β-arrestin 2 signalling. Subsequent to generating these preclinical data, MuDelta was assessed in a phase II clinical trial in ∼800 IBS-d patients. MuDelta met the primary and a number of secondary end points; and, in a summary of adverse events, constipation was not reported (FuriexPharmaceuticals, 2011).
Acknowledgments
We acknowledge the δ opioid receptor immunohistochemistry and quantification performed by Holburn Group, Bowmanville Ontario, Canada. We appreciate the support of Patricia Andrade-Gordon and guidance of early development by John Moyer (formerly J&J PRD).
Glossary
- DAMGO
Tyr-DAla-Gly-[NMePhe]-NH(CH2)2
- d-IBS
diarrhoea-predominant irritable bowel syndrome
- DPDPE
cyc[DPen2, DPen5]enkephalin
- EFS
electrical field stimulation
- GC
geometric centre
- GI
gastrointestinal
- Isc
short-circuit current
- NorBNI
nor-binaltorphimine
- OD
optical density
- RT
room temperature
- SNC80
(+)-4-[(aR)-a-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide
Conflict of interest
All authors were J&J employees and have no Conflict of Interest to declare.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awouters F, Megens A, Verlinden M, Schuurkes J, Niemegeers C, Janssen PA. Loperamide. Survey of studies on mechanism of its antidiarrheal activity. Dig Dis Sci. 1993;38:977–995. doi: 10.1007/BF01295711. [DOI] [PubMed] [Google Scholar]
- Bagnol D, Mansour A, Akil H, Watson SJ. Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience. 1997;81:579–591. doi: 10.1016/s0306-4522(97)00227-3. [DOI] [PubMed] [Google Scholar]
- Beubler E, Badhri P, Schirgi-Degen A. Antisecretory activities of orally administered loperamide and loperamide oxide on intestinal secretion in rats. J Pharm Pharmacol. 1993;45:803–806. doi: 10.1111/j.2042-7158.1993.tb05689.x. [DOI] [PubMed] [Google Scholar]
- Breslin HJ, Cai C, Miskowski TA, Coutinho SV, Zhang SP, Hornby P, et al. Identification of potent phenyl imidazoles as opioid receptor agonists. Bioorg Med Chem Lett. 2006;16:2505–2508. doi: 10.1016/j.bmcl.2006.01.082. [DOI] [PubMed] [Google Scholar]
- Breslin HJ, Diamond C, Kavash R, Cai C, Dyatkin A, Miskowski TA, et al. Identification of a dual delta OR antagonist/muOR agonist as a potential therapeutic for diarrhea-predominant Irritable Bowel Syndrome. Bioorg Med Chem Lett. 2012;22:4869–4872. doi: 10.1016/j.bmcl.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Broccardo M, Improta G, Tabacco A. Central effect of SNC80, a selective and systemically active delta-opioid receptor agonist, on gastrointestinal propulsion in the mouse. Eur J Pharmacol. 1998;342:247–251. doi: 10.1016/s0014-2999(97)01470-2. [DOI] [PubMed] [Google Scholar]
- Burks TF, Fox DA, Hirning LD, Shook JE, Porreca F. Regulation of gastrointestinal function by multiple opioid receptors. Life Sci. 1988;43:2177–2181. doi: 10.1016/0024-3205(88)90410-9. [DOI] [PubMed] [Google Scholar]
- Callahan MJ. Irritable bowel syndrome neuropharmacology. A review of approved and investigational compounds. J Clin Gastroenterol. 2002;35(1) Suppl.:S58–S67. doi: 10.1097/00004836-200207001-00011. [DOI] [PubMed] [Google Scholar]
- Camilleri M. Novel pharmacology: asimadoline, a kappa-opioid agonist, and visceral sensation. Neurogastroenterol Motil. 2008;20:971–979. doi: 10.1111/j.1365-2982.2008.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS) Dig Dis Sci. 1984;29:239–247. doi: 10.1007/BF01296258. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non-NMDA receptors. Brain Res. 1996;736:7–15. doi: 10.1016/0006-8993(96)00661-0. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Burgos LC, Cassel JA, Daubert JD, DeHaven RN, Mansson E, et al. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J Pharmacol Exp Ther. 1999;289:494–502. [PubMed] [Google Scholar]
- Delvaux M, Wingate D. Trimebutine: mechanism of action, effects on gastrointestinal function and clinical results. J Int Med Res. 1997;25:225–246. doi: 10.1177/030006059702500501. [DOI] [PubMed] [Google Scholar]
- Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham DJ, Lambert DG. Simultaneous targeting of multiple opioid receptors: a strategy to improve side-effect profile. Br J Anaesth. 2009;103:38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- Feng P, Rahim RT, Cowan A, Liu-Chen LY, Peng X, Gaughan J, et al. Effects of mu, kappa or delta opioids administered by pellet or pump on oral Salmonella infection and gastrointestinal transit. Eur J Pharmacol. 2006;534:250–257. doi: 10.1016/j.ejphar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Fickel J, Bagnol D, Watson SJ, Akil H. Opioid receptor expression in the rat gastrointestinal tract: a quantitative study with comparison to the brain. Brain Res Mol Brain Res. 1997;46:1–8. doi: 10.1016/s0169-328x(96)00266-5. [DOI] [PubMed] [Google Scholar]
- Foxx-Orenstein AE, Jin JG, Grider JR. 5-HT4 receptor agonists and delta-opioid receptor antagonists act synergistically to stimulate colonic propulsion. Am J Physiol. 1998;275((5 Pt 1)):G979–G983. doi: 10.1152/ajpgi.1998.275.5.G979. [DOI] [PubMed] [Google Scholar]
- FuriexPharmaceuticals. 2011. MuDelta (mu opioid receptor agonist and delta opioid receptor antagonist). http://www.furiex.com/pipeline/discoverydevelopment-pipeline/mu-delta/
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AC, Coupar IM, White PJ. Comparison of opioid receptor distributions in the rat ileum. Life Sci. 2006;78:1610–1616. doi: 10.1016/j.lfs.2005.07.048. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Gardner CJ, Little PJ, Hicks GA, Dehaven-Hudkins DL. Preclinical studies of opioids and opioid antagonists on gastrointestinal function. Neurogastroenterol Motil. 2004;16(Suppl. 2):46–53. doi: 10.1111/j.1743-3150.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- Grider JR, Makhlouf GM. Identification of opioid receptors on gastric muscle cells by selective receptor protection. Am J Physiol. 1991;260((1 Pt 1)):G103–G107. doi: 10.1152/ajpgi.1991.260.1.G103. [DOI] [PubMed] [Google Scholar]
- Holt JD, Watson MJ, Chang JP, O'Neill SJ, Wei K, Pendergast W, et al. DPI-221 [4-((alpha-s)-alpha-((2s,5r)-2,5-dimethyl-4-(3-fluorobenzyl)-1-piperazinyl)benzyl)-N,N-diethylbenzamide]: a novel nonpeptide delta receptor agonist producing increased micturition interval in normal rats. J Pharmacol Exp Ther. 2005;315:601–608. doi: 10.1124/jpet.105.090498. [DOI] [PubMed] [Google Scholar]
- Izquierdo S, Rey E, Garcia Alonso M, Almansa C, Diaz-Rubio M. Has the identification of rectal hypersensitivity any implication in the clinical outcome of irritable bowel syndrome? Rev Esp Enferm Dig. 2005;97:223–228. doi: 10.4321/s1130-01082005000400002. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur JF, Miller RJ, Field M. Control of guinea pig intestinal electrolyte secretion by a delta-opiate receptor. Proc Natl Acad Sci U S A. 1980;77:2753–2756. doi: 10.1073/pnas.77.5.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball ES, Palmer JM, D'Andrea MR, Hornby PJ, Wade PR. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1266–G1273. doi: 10.1152/ajpgi.00444.2004. [DOI] [PubMed] [Google Scholar]
- Kimball ES, Prouty SP, Pavlick KP, Wallace NH, Schneider CR, Hornby PJ. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol Motil. 2007;19:390–400. doi: 10.1111/j.1365-2982.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- Kuemmerle JF, Makhlouf GM. Characterization of opioid receptors in intestinal muscle cells by selective radioligands and receptor protection. Am J Physiol. 1992;263((2 Pt 1)):G269–G276. doi: 10.1152/ajpgi.1992.263.2.G269. [DOI] [PubMed] [Google Scholar]
- Law PY, McGinn TM, Wick MJ, Erikson LJ, Evans C, Loh HH. Analysis of delta-opioid receptor activities stably expressed in CHO cell lines: function of receptor density? J Pharmacol Exp Ther. 1994;271:1686–1694. [PubMed] [Google Scholar]
- Maguma HT, Dewey WL, Akbarali HI. Differences in the characteristics of tolerance to mu-opioid receptor agonists in the colon from wild type and beta-arrestin2 knockout mice. Eur J Pharmacol. 2012;685:133–140. doi: 10.1016/j.ejphar.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Bradesi S. Alosetron and irritable bowel syndrome. Expert Opin Pharmacother. 2003;4:2089–2098. doi: 10.1517/14656566.4.11.2089. [DOI] [PubMed] [Google Scholar]
- McKnight AT, Corbett AD, Marcoli M, Kosterlitz HW. The opioid receptors in the hamster vas deferens are of the delta-type. Neuropharmacology. 1985;24:1011–1017. doi: 10.1016/0028-3908(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Mihara S, North RA. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986;88:315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981;6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, McLean PG, Sengupta JN. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. Pain. 2006;126:54–63. doi: 10.1016/j.pain.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Mori T, Kawano K, Shishikura T. 5-HT3-receptor antagonist inhibits visceral pain differently in chemical and mechanical stimuli in rats. J Pharmacol Sci. 2004;94:73–76. doi: 10.1254/jphs.94.73. [DOI] [PubMed] [Google Scholar]
- Mosberg H, Purington L, Traynor JR, Pogozheva ID, Sobczyk-Kojiro K. Development and in vitro characterization of a novel bifunctional mu-agonist/delta-antagonist opioid tetrapeptide. ACS Chem Biol. 2011;6:1375–1381. doi: 10.1021/cb200263q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei K, Iwanaga T. Localization of Met-enkephalin-Arg6-Gly7-Leu8-like immunoreactivity in the gastrointestinal tract of rat and pig. J Histochem Cytochem. 1985;33:1001–1006. doi: 10.1177/33.10.3900192. [DOI] [PubMed] [Google Scholar]
- Parkhill AL, Bidlack JM. Several delta-opioid receptor ligands display no subtype selectivity to the human delta-opioid receptor. Eur J Pharmacol. 2002;451:257–264. doi: 10.1016/s0014-2999(02)02241-0. [DOI] [PubMed] [Google Scholar]
- Pol O, Puig MM. Expression of opioid receptors during peripheral inflammation. Curr Top Med Chem. 2004;4:51–61. doi: 10.2174/1568026043451519. [DOI] [PubMed] [Google Scholar]
- Pol O, Ferrer I, Puig MM. Diarrhea associated with intestinal inflammation increases the potency of mu and delta opioids on the inhibition of gastrointestinal transit in mice. J Pharmacol Exp Ther. 1994;270:386–391. [PubMed] [Google Scholar]
- Pol O, Valle L, Puig MM. Antisense oligodeoxynucleotides to mu- and delta-opioid receptor mRNA block the enhanced effects of opioids during intestinal inflammation. Eur J Pharmacol. 2001;428:127–136. doi: 10.1016/s0014-2999(01)01281-x. [DOI] [PubMed] [Google Scholar]
- Poole DP, Pelayo JC, Scherrer G, Evans CJ, Kieffer BL, Bunnett NW. Localization and regulation of fluorescence-labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology. 2011;141:982–991. doi: 10.1053/j.gastro.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MM, Pol O. Peripheral effects of opioids in a model of chronic intestinal inflammation in mice. J Pharmacol Exp Ther. 1998;287:1068–1075. [PubMed] [Google Scholar]
- Quock RM, Burkey TH, Varga E, Hosohata Y, Hosohata K, Cowell SM, et al. The delta-opioid receptor: molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol Rev. 1999;51:503–532. [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7:E587–E591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Liu HC, Loh HH. mu-Opioid receptor-knockout mice: the role of mu-opioid receptor in gastrointestinal transit. Brain Res Mol Brain Res. 1998;56:281–283. doi: 10.1016/s0169-328x(98)00051-5. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Su X, Gebhart GF. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology. 1996;111:968–980. doi: 10.1016/s0016-5085(96)70064-1. [DOI] [PubMed] [Google Scholar]
- Shahbazian A, Heinemann A, Schmidhammer H, Beubler E, Holzer-Petsche U, Holzer P. Involvement of mu- and kappa-, but not delta-, opioid receptors in the peristaltic motor depression caused by endogenous and exogenous opioids in the guinea-pig intestine. Br J Pharmacol. 2002;135:741–750. doi: 10.1038/sj.bjp.0704527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Jr, Bunnett NW, von Zastrow M, et al. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci U S A. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Holper EM, Hammond DL. Delta antagonist and kappa agonist activity of Naltriben: evidence for differential kappa interaction with the delta 1 and delta 2 opioid receptor subtypes. Life Sci. 1994;55:PL79–PL84. doi: 10.1016/0024-3205(94)00738-1. [DOI] [PubMed] [Google Scholar]
- Talley NJ. Pharmacologic therapy for the irritable bowel syndrome. Am J Gastroenterol. 2003;98:750–758. doi: 10.1111/j.1572-0241.2003.07306.x. [DOI] [PubMed] [Google Scholar]
- Tammpere A, Brusberg M, Axenborg J, Hirsch I, Larsson H, Lindstrom E. Evaluation of pseudo-affective responses to noxious colorectal distension in rats by manometric recordings. Pain. 2005;116:220–226. doi: 10.1016/j.pain.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Townsend DT, Portoghese PS, Brown DR. Characterization of specific opioid binding sites in neural membranes from the myenteric plexus of porcine small intestine. J Pharmacol Exp Ther. 2004;308:385–393. doi: 10.1124/jpet.103.058016. [DOI] [PubMed] [Google Scholar]
- Wood J, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl. 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]


