Abstract
BACKGROUND AND PURPOSE
Conditioned gaping reactions reflect nausea-induced behaviour in rats. Cannabinoid 1 receptor (CB1) agonists interfere with the establishment of nausea-induced conditioned gaping; however, it is not known if their effects are mediated by an action at peripheral or central CB1 receptors.
EXPERIMENTAL APPROACH
We utilized the conditioned gaping model of nausea to evaluate the effect of peripheral and central administration of the peripherally restricted CB1 agonist, CB13, on the establishment of LiCl-induced gaping in rats. We further evaluated the ability of HU-210 administered to the gustatory insular cortex (GIC) or visceral insular cortex (VIC) to interfere with LiCl-induced conditioned gaping and determined if this effect was mediated by CB1 receptors.
KEY RESULTS
Central, but not peripheral, CB13 suppressed LiCl-induced conditioned gaping. Central administration of the potent CB1 agonist, HU-210, delivered to the VIC, but not the GIC, suppressed the establishment of LiCl-induced gaping reactions, but not LiCl-induced suppression of hedonic reactions or conditioned taste avoidance. This pattern of results suggests that HU-210 delivered to the VIC prevented LiCl-induced nausea, but not learning per se. The suppression of LiCl-induced conditioned gaping by HU-210 was mediated by CB1 receptors because it was prevented by co-administration of CB1 antagonist/inverse agonist, AM-251, into the VIC. A high dose of AM-251 (20 µg) administered alone into the VIC did not produce conditioned gaping reactions.
CONCLUSIONS AND IMPLICATIONS
The nausea-relieving effects of CB1 agonists, but not the nausea-inducing effects of CB1 inverse agonists, are mediated, at least in part, by their action at the VIC in rats.
Keywords: nausea, insular cortex, cannabinoid, taste reactivity, gaping, CB1
Introduction
The neurobiology of nausea is not well understood. One limitation to the study of nausea has been the lack of a reliable animal model. Although rats are incapable of vomiting, there is considerable evidence that conditioned disgust reactions (most reliably gaping: rapid, large amplitude opening of the mandible with retraction of the corners of the mouth) elicited by an illness-paired flavour (e.g. Grill and Norgren, 1978a; Parker et al., 2008) represent such a model. Conditioned gaping reactions are measured in the taste reactivity (TR) test (Grill and Norgren, 1978a), during which an illness-paired flavour is intraorally infused across their tongues. Unlike the traditionally employed consumption test measure of conditioned taste avoidance (CTA), conditioned gaping is exclusively produced by emetic treatments, but not by rewarding drugs (Parker, 1995; Parker et al., 2008). Furthermore, anti-emetic treatments selectively interfere with the establishment of conditioned gaping, but not CTA (e.g. Grant, 1987; Limebeer and Parker, 2000; Tuerke et al., 2011). Therefore, conditioned gaping in rats, unlike CTA, represents nausea-induced behaviour (Parker et al., 2008). At low doses, cannabinoid 1 receptor (CB1) agonists and drugs that enhance the activity of the endogenous cannabinoid system reduce not only vomiting (Darmani, 2001; Van Sickle et al., 2001; Parker et al., 2004) but also conditioned disgust in rats in a manner that suggests they reduce nausea-like behaviours (Parker and Mechoulam, 2003; Parker et al., 2003; Cross-Mellor et al., 2007; Sticht et al., 2011).
As there is evidence that both central and peripheral CB1 receptors may be involved in the control of emetic reactions (Van Sickle et al., 2001; Darmani and Johnson, 2004), it is unclear whether the regulation of nausea by agonists of these receptors is peripherally or centrally mediated. Recently, a relatively peripherally restricted CB1/CB2 agonist, CB13, has been developed (Dziadulewicz et al., 2007). The experiments reported here evaluated the effect of peripheral and i.c.v. administration of CB13 to prevent conditioned gaping in rats. As only i.c.v. administration of CB13 reduced LiCl-induced conditioned gaping, the CB1 agonist HU-210 was microinfused into the visceral insular cortex (VIC) and gustatory insular cortex (GIC), and the effect on LiCl-induced conditioned gaping was evaluated. The insular cortex is a forebrain region that has been shown to be involved in the generation of human disgust (e.g. Calder et al., 2001; 2007), conditioned disgust in rats (Kiefer and Orr, 1992) and nausea in humans and other animals (Kaada, 1951; Penfield and Faulk, 1955; Fiol et al., 1988; Contreras et al., 2007; Catenoix et al., 2008). Finally, the potential of intracranial AM-251 delivered to the VIC to interfere with HU-210-induced attenuation of conditioned gaping was evaluated.
Methods
Animals
A total of 171 male Sprague-Dawley rats (Charles River Lab, St Constant, QC, Canada) weighing 300–380 g on the day of conditioning (11 rats were removed from final data analysis due to inaccurate cannula placement). The rats were individually housed in Plexiglas cages (48 × 26 × 20 cm) in the colony room at an ambient temperature of 21°C with a 12/12 reverse light/dark schedule (lights off at 07:00 h). All experimental manipulations were conducted during the dark phase of the cycle. Rats were provided with two clean paper towels (replenished during weekly cage changes) and a soft plastic container that was 14 cm long and 12 cm in diameter that remained in the home cage. They were maintained on food [Highland Rat Chow (8640)] and water ad libitum throughout the experiments. All procedures were approved by the Animal Care Committee of the University of Guelph and adhered to the guidelines of the Canadian Council of Animal Care. All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
Drugs
LiCl was prepared in a 0.15 M solution with sterile water and was administered i.p. at a volume of 20 mL·kg−1 (127.2 mg·kg−1). Peripherally administered CB13 and Δ9-tetrahydrocannabinol (THC) were prepared in a vehicle of 45% 2-hydroxypropyl-β-cyclodextrin (2-HPβCD) with sterile water. Various groups were injected i.p. with CB13 at doses of 0.3 (prepared as 0.3 mg·mL−1), 1 (prepared as 1.0 mg·mL−1) and 3 mg·kg−1 (prepared as 1.5 mg·mL−1). At doses of 3 mg·kg−1 or less, CB13 has been shown to have anti-hyperalgesic efficacy in a rat model of neuropathic mechanical hyperalgesia that is blocked by N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methylpyrazole-3-carboxamide (SR141716A) with no indication of CNS effects as measured by catalepsy (a typical centrally mediated CB1 agonist response). Furthermore, a 3 mg·kg−1 dose had a Cmax of 1.13 µM at 1 h post-delivery and a maximal brain concentration of 0.24 µmol·kg−1 at 4 h post-dose (Dziadulewicz et al., 2007). THC (3.0 mg·kg−1, i.p.) was used as a positive control for the peripheral restriction of CB13 due to the ability of THC to cross the blood–brain barrier at this dose. Centrally (i.c.v.) administered CB13 was prepared in 100% dimethyl sulfoxide (DMSO) vehicle at concentrations of 0.3, 1.0 and 3.0 µg·µL−1 and delivered at a rate of 1 µL·min−1. For Experiment 2, HU-210 for intracranial (VIC and GIC) administration was prepared in a vehicle of 45% HPβCD at concentrations of 0.001 and 0.01 µg·µL−1 and was delivered at a rate of 1 µL·min−1. For Experiment 3, HU-210 was prepared in 45% HPβCD at a concentration of 0.02 µg·µL−1 and AM-251 was prepared in 100% DMSO vehicle at a concentration of 2 µg·µL−1. Both drugs were delivered at a rate of 0.5 µL·min−1, with AM-251 being delivered 5 min before HU-210. For Experiment 4, AM-251 was prepared in 100% DMSO vehicle at concentrations of 0.1, 1.0, 10.0 and 20.0 µg·µL−1 and was delivered at a rate of 1 µL·min−1. All microinfusions were of 1 min duration with the injector left in place for 1 min post-infusion, before removal from the guide cannula. The obdurator was replaced after each microinfusion. For Experiments 2a, 2b, 3 and 4, the microinfusions were delivered bilaterally. Our drug/molecular target nomenclature conforms to BJP's Guide to Receptors and Channels (Alexander et al., 2011).
Surgery
In all experiments, the rats were surgically implanted with an intraoral cannula, as described in detail by Limebeer et al. (2010). In Experiments 1b-4, the rats were anaesthetized with isoflurane gas and prepared for intracranial surgery by shaving a strip of skin between the ears (2.5–3 cm long), cleaning the skin and injecting Carprofen (5 mg·kg−1, i.p.) and a topical anaesthetic (0.1 mL, s.c.) on either side of the skull (Marcaine; Hospira, Montreal, QC, Canada). Verification of a surgical plane of anaesthesia in the rat was done by demonstration of a lack of a withdrawal reflex response in the hindlimb, decreased muscle tone and a slow, regular respiratory response. They were then stabilized in the flat skull position (according to Paxinos and Watson, 1998) in the stereotaxic frame and the skull was exposed. In Experiment 1b, the rats had a unilateral indwelling guide cannula surgically implanted in the lateral ventricle. The stainless steel guide cannula (22 G, 6 mm below pedestal) was lowered into either the left or the right lateral ventricle (counterbalanced among groups) using the following coordinates from Bregma: −1.0 mm anteroposterior (AP), ±1.4 mm lateromedial (LM), −3.6 mm dorsoventral (DV) (Erb et al., 2003). In Experiments 2a-4 (VIC) and 2b (GIC), the rats had bilateral indwelling guide cannulae implanted into the VIC (arms set at divergent 10° angle, relative to Bregma: AP −0.5; LM ± 5.0; DV −4.5 from the skull) or the GIC (relative to Bregma: AP +1.5, LM ± 5.0, DV −4.5 from the skull). The guide cannulae were stabilized by six screws secured in the skull and dental cement. Once the dental cement had hardened, a stainless steel obdurator was inserted into the guide cannulae to maintain patency. Following removal from the stereotaxic frame, the rats were surgically implanted with an intraoral cannula. They were then placed in a heated recovery area and monitored until they were ambulatory, at which time they were returned to the colony room. The rats were allowed to recover for 5 days following surgery and were monitored daily as previously described (Limebeer et al., 2010).
Histology
Verification of cannula placement was determined by histological evaluation of tissue. The rats were deeply anaesthetized using an 85 mg·kg−1 injection of Euthansol (Intervet Canada Corp., Kirkland, QC, Canada), followed by transcardial perfusion with PBS (0.1 M) and 4% formalin. The brains were removed and stored at 4°C in 4% formalin solution for 24–48 h, after which they were placed in a 20% sucrose solution overnight at room temperature. The brains were then sliced in 60 µm sections using a CM1850 Leica cryostat and relevant sections were mounted on gelatin-subbed glass microscope slides. The tissue was stained with thionin 24 h later and examined for accurate cannula placement using a Leica MZ6 Stereomicroscope with a Leica DFC420 Digital Camera and Leica Application Suite software (Leica Microsystems Inc., Concord, ON, Canada). Any rat with improper cannula placement was excluded from the behavioural analysis.
Apparatus
Conditioning and testing was conducted in a square Plexiglas TR chamber (26.5 × 26.5 × 12 cm) placed on a glass-topped table, with a mirror set at 45° below the glass top to facilitate videotaping the ventral surface of the rat while in the chamber. The rat's intraoral cannula was attached to the infusion pump (KDS100; KD Scientific Inc., Holliston, MA, USA) using a length of PE160 tubing fitted over the intraoral cannula that ran through a hole at the top of the chamber and connected to the pump. Orofacial responses were captured directly by the computer using the Roxio Videowave Premiere Suite 8 video capture program (Corel Corporation, Ottawa, ON, Canada) and a Sony DCR-HC28 Handycam camera (Sony Corporation, Toronto, ON, Canada).
For central administration of solutions, microinfusions were conducted using a 28 G injector that extended 2 mm beyond the guide cannula tip and was attached to a microinfusion pump (KDS101; KD Scientific Inc.) using Tygon tubing with a 0.1905 mm inner diameter (Cole-Parmer, Vernon Hills, IL, USA).
Procedure
Experiment 1: potential of systemic CB13 (Experiment 1a) or i.c.v. CB13 (Experiment 1b) to attenuate LiCl-induced conditioned gaping
Five days after recovering from surgery, the rats received an adaptation trial, during which they were individually placed in the TR chamber with their cannula attached to the infusion pump for fluid delivery. After 3 min in the TR chamber, water was infused into their intraoral cannula over a period of 3 min at a rate of 1 mL·min−1. Twenty-four hours later, the rats received a conditioning trial. In Experiment 1a, they were randomly assigned to pretreatment groups on the basis of dose of CB13 (with all ns in each experiment reported only for correct cannula placements): group vehicle (VEH; n= 6), group 0.3 mg·kg−1 CB13 (n= 7), group 1 mg·kg−1 CB13 (n= 8), group 3 mg·kg−1 CB13 (n= 8) and group 3.0 mg·kg−1 THC (n= 7). Thirty minutes before the conditioning trial, the rats were injected i.p. with the appropriate pretreatment drug. In Experiment 1b, the rats were randomly assigned to groups on the basis of pretreatment drug: VEH (n= 7), 10 µg CB13 (n= 7) or 30 µg CB13 (n= 8). Immediately before the conditioning trial, 1 µL of the pretreatment drug was microinfused and the injector was left in place for 1 min following the microinfusion. In both Experiments 1a and 1b, the rats were then placed in the TR chamber and 3 min later were intraorally infused with 0.1% saccharin for 3 min at a rate of 1 mL·min−1. Immediately following the saccharin infusion, the rats were injected with 20 mL·kg−1 of LiCl and returned to their home cage. Seventy-two hours later, the rats received the drug-free TR test trial, during which they were placed in the TR chamber for 3 min before receiving an intraoral infusion of 0.1% saccharin solution over a period of 3 min (1 mL·min−1), while their orofacial reactions were recorded from the mirror beneath the chamber. On the following day, after being deprived of water for 16 h, the rats received a test of CTA. The test was a single-bottle test because the single-bottle test has been shown to be more sensitive to between-group differences than two-bottle choice tests, with water as an alternative (Batsell and Best, 1993). The two-bottle test is highly sensitive to the detection of even very weak CTA, producing floor effects that may mask between-group differences. The rats were given a single bottle of saccharin to consume for 30 min and the amount consumed was measured.
Experiment 2: potential of intracranial administration of HU-210 into the VIC (Experiment 2a) or the GIC (Experiment 2b) to attenuate LiCl-induced conditioned gaping
Experiments 2a and 2b were conducted similar to Experiment 1, except as indicated. Rats received adaptation training to the TR procedure exactly as in Experiment 1 on the sixth day following surgery. Unlike Experiments 1a and 1b, the groups received two conditioning trials (separated by 72 h) in Experiments 2a and 2b before TR testing. In Experiment 2a (VIC infusions), the rats were randomly assigned to the various groups: VEH (n= 6), 0.001 µg HU-210 (n= 6) and 0.01 µg HU-210 (n= 8). In Experiment 2b (GIC), they were randomly assigned to the following groups: VEH (n= 6), 0.001 µg HU-210 (n= 6) and 0.01 µg HU-210 (n= 7). On each of the two conditioning trials, the rats were bilaterally microinfused with 1 µL of the pretreatment drug at a rate of 1 µL·min−1, with the injector left in place for 1 min. Immediately following the microinfusion, the rats were placed in the TR chamber and after 3 min, they received a 3 min intraoral infusion of 0.1% saccharin solution at a rate of 1 mL·min−1, with their orofacial reactions videotaped. They were all injected with LiCl immediately upon removal from the TR chamber. The drug-free TR test occurred 72 h following the second conditioning trial. The rats received a CTA test on the following day as in Experiments 1a and 1b.
Experiment 3: potential of AM-251 to reverse the suppression of conditioned gaping by HU-210, both delivered to the VIC
Experiment 3 aimed to determine if the suppression of LiCl-induced nausea by HU-210 delivery to the VIC was CB1-mediated. The procedures of Experiment 3 were identical to those of Experiment 2a, except that the rats received two pretreatment microinfusions into the VIC immediately before each of the saccharin-LiCl pairings on the conditioning trials according to the following random group assignment: VEH-VEH (n= 6), AM2-51-VEH (n= 7), VEH-HU210 (n= 7) and AM-251-HU210 (n= 7). The microinfusions were delivered at a rate of 0.5 µL·min−1 for 1 min and the injector was left in place for 1 min, making the total volume of liquid delivered to the VIC 1 µL.
Experiment 4: potential of AM-251 delivered to the VIC to produce conditioned gaping
CB1 inverse agonists produce nausea in humans (Janero and Makriyannis, 2009) and conditioned gaping in rats (McLaughlin et al., 2005) when explicitly paired with saccharin solution. Experiment 4 aimed to determine if the nausea produced by AM-251 is mediated by its action in the VIC. In Experiment 4, the rats were treated as in Experiment 2a, except they did not receive pretreatment drugs or LiCl. Instead, on each conditioning trial immediately following the intraoral infusion of saccharin solution, the rats received VIC microinfusions of VEH (n= 8), 0.1 µg AM-251 (n= 5), 1.0 µg AM-251 (n= 8), 10 µg AM-251 (n= 8) or 20 µg AM-251 (n= 7). Because the two-bottle preference test is more sensitive to the detection of a weak CTA than the one-bottle test (Batsell and Best, 1993), following 16 h of water deprivation, on the day following the TR test trial, the rats were given a graduated tube with saccharin and one with water for a total of 6 h to detect a CTA with measures taken at 30, 120 and 360 min.
Behavioural measures
The video recordings obtained were scored using the Observer (Noldus Information Technology, Sterling, VA, USA) event recording programme for the following behaviours: the number of gaping reactions (rapid, large amplitude opening of the mandible with retraction of the corners of the mouth) was counted during the 3 min infusions. The number of seconds that the rats displayed hedonic reactions of mouth movements (movement of the lower mandible without opening the mouth) and tongue protrusions (extensions of the tongue out of the mouth) was also measured and the total of the two measures was used as the hedonic score. The mean amount of saccharin consumed during the 30 min CTA test in Experiments 1−3 served as a measure of CTA. The mean saccharin preference ratios [amount of saccharin consumed/(amount of saccharin + water consumed)] at each interval of testing served as a measure of CTA in Experiment 4.
Data analysis
In each experiment, the mean number of gapes and periods (measured in s) of hedonic reactions displayed by the rats on the conditioning trial(s) and on the test trial were entered into mixed-factor anovas with the between-group factor of pretreatment condition and the within-group factor of trial. Bonferroni post hoc comparison tests were conducted for statistically significant effects. The amount of saccharin solution consumed during the 30 min CTA test in each of Experiments 1–3 were entered into a one-way anova and the mean saccharin preference ratio in Experiment 4 was analysed as a 5 (dose) by 3 (time) mixed-factor anova. Statistical significance was defined as P < 0.05.
Results
Experiment 1: effect of systemic (1a) or i.c.v. (1b) CB13 on LiCl-induced conditioned gaping
Systemic administration of the peripherally restricted CB1 and CB2 agonist, CB13, failed to attenuate the establishment of LiCl-induced conditioned gaping reactions, suppression of hedonic reactions or CTA. Systemic administration of THC attenuated LiCl-induced conditioned gaping reactions but did not interfere with suppression of hedonic reactions or CTA. Figure 1 presents the mean (±SEM) number of gapes (upper graph) and mean duration (s; ± SEM) of hedonic reactions (lower graph) displayed by each pretreatment group during the conditioning trial and the drug-free TR test trial in Experiment 1. The 5 × 2 mixed-factor anova of the gaping data revealed a significant trial effect, F(1,31) = 141.0; P < 0.001 and a significant trial by pretreatment interaction, F(4,31) = 5.7; P= 0.001. Simple main effect analysis of the interaction revealed that rats displayed gaping only on the test trial, F(4,31) = 11.43; P < 0.001. Subsequent Bonferroni post hoc comparisons revealed that rats pretreated with THC expressed significantly fewer conditioned gaping reactions than rats in any other pretreatment group, which did not differ from each other (P < 0.025). Analysis of the hedonic reaction score also revealed only an effect of trials, F(1, 31) = 199.6; P < 0.001, with rats showing fewer hedonic reactions during the test than during conditioning. The single-factor anova of the mean amount consumed in the CTA test revealed no significant pretreatment group effect, F(4,31) = 0.3 (data not shown).
Figure 1.
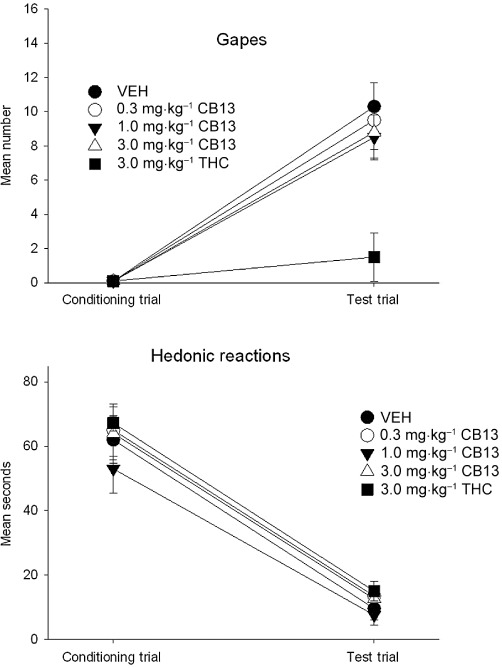
The mean (±SEM) number of gaping responses (upper graph) and duration (s) of hedonic reactions (lower graph) expressed during the conditioning and test trials following systemic administration of CB13 or THC. The peripherally restricted CB1/CB2 agonist and THC did not alter hedonic reactions during the conditioning trial and did not modify suppressed hedonic reactions at test. THC, but not CB13, suppressed LiCl-induced conditioned gaping reactions at test.
When administered i.c.v. to the lateral ventricle, a dose of 30 µg, but not 10 µg, of CB13 interfered with the establishment of LiCl-induced conditioned gaping; however, no dose of CB13 attenuated suppressed hedonic reactions or CTA. Figure 2 presents the mean (±SEM) number of gapes (upper graph) and duration (s; ± SEM) of hedonic reactions (lower graph) displayed by the various groups in Experiment 1b during conditioning and testing. The 3 x 2 mixed factors ANOVA of the number of gapes revealed a significant pretreatment x trials interaction, F (2, 19) = 4.5; P= 0.026. Subsequent LSD comparison tests revealed that on the TR test trial rats pretreated with 30 µg CB13 displayed fewer gapes during testing than either other group (P < 0.05). The 3 x 2 ANOVA for the hedonic reaction scores revealed only a significant effect of trials, F(1, 19) = 161.4; P < 0.001. Regardless of pretreatment conditions, rats showed fewer hedonic reactions during the TR test trial than during conditioning. The single factor ANOVA of the mean amount consumed in the CTA test revealed no significant effect of pretreatment group, F(2, 19) = 0.2 (data not depicted). Figure 3 presents a representative photomicrograph of the track of an i.c.v. cannula.
Figure 2.
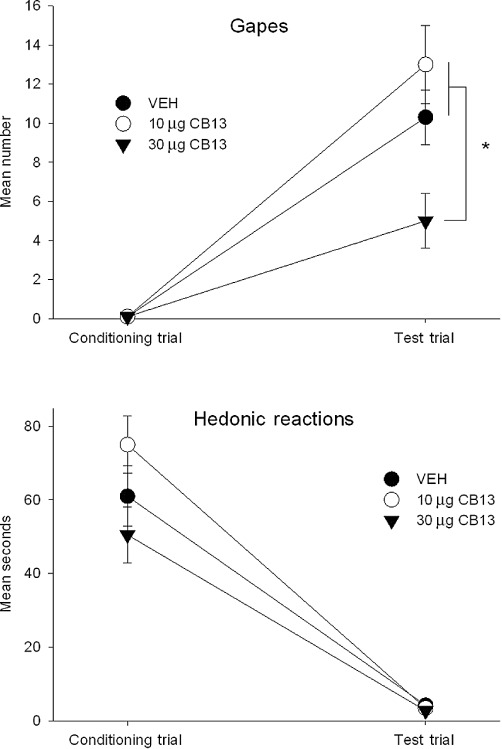
The mean (+SEM) number of gaping responses (upper graph) and duration (s) of hedonic reactions (lower graph) expressed during the conditioning and test trials following central (i.c.v.) administration of CB13. The peripherally restricted CB1/CB2 agonist attenuated LiCl-induced conditioned gaping reactions in a dose-dependent manner; the 30 µg group expressed significantly fewer gaping responses than the vehicle or 10 µg groups (*P < 0.05). Centrally administered CB13 did not alter hedonic reactions during the conditioning trial and failed to attenuate suppressed hedonic responding during the test trial.
Figure 3.
Representative photomicrograph of an i.c.v. cannula track in Experiment 1B.
Experiment 2: effect of intracranial administration of HU-210 to the VIC (2a) and the GIC (2b) on LiCl-induced conditioned gaping
When administered to the VIC, but not to the GIC, HU-210 blocked the establishment of LiCl-induced conditioned gaping at both 0.001 and 0.01 µg. Figure 4 presents the mean (±SEM) number of gapes displayed by the groups pretreated with VEH, 0.001 or 0.01 µg of HU-210 in Experiment 2a (VIC) and Experiment 2b (GIC). The 3 × 3 mixed-factor anova of gaping in Experiment 2a revealed significant effects of pretreatment, F(1,17) = 4.0; P= 0.038, trial, F(2,34) = 27.0; P < 0.001 and pretreatment × trial, F(4,34) = 4.3; P= 0.006. Subsequent Bonferroni post hoc comparison tests revealed that in the test trial only, the rats pretreated with VEH displayed significantly more gaping reactions than the rats pretreated with either 0.001 or 0.01 µg HU-210 (P < 0.05). The anova for the mean number of gapes displayed in Experiment 2b (GIC) revealed only a significant trial effect, F(2,32) = 30.2; P < 0.001; across all pretreatment groups, the rats displayed significantly more gaping across trials.
Figure 4.
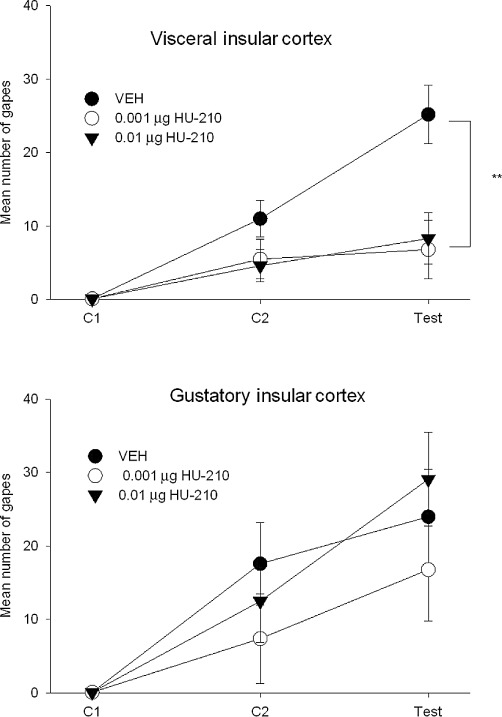
The mean (±SEM) number of gaping responses expressed during the conditioning and test trials following microinfusion of HU-210 to the VIC (upper graph) or GIC (lower graph). This potent CB1 agonist attenuated conditioned gaping responses at both doses (**P < 0.01) only when microinfused into the VIC. HU-210 did not interfere with LiCl-induced gaping responses at either dose when administered to the GIC.
Whether administered to the VIC or the GIC, HU210 did not reduce LiCl-induced suppression of hedonic reactions or CTA. Figure 5 presents the mean (±SEM) number of seconds that the rats displayed hedonic reactions in Experiments 2a (VIC) and 2b (GIC). The 3 × 3 mixed-factor anovas of hedonic reaction scores revealed only a significant effect of trials for Experiment 2a (VIC), F(2,34) = 52.6; P < 0.001, and for Experiment 2b (GIC), F(2,32) = 116.9; P < 0.001. Regardless of the pretreatment condition, the interaction was not significant. The single-factor anovas of the mean amount of saccharin consumed in the CTA tests in Experiments 2a (VIC) and 2b (GIC) revealed that the pretreatment groups did not have a significantly different score for CTA [Experiment 2a: F(2,17) = 1.9; Experiment 2b: F(2,16) = 0.5] (data not depicted).
Figure 5.
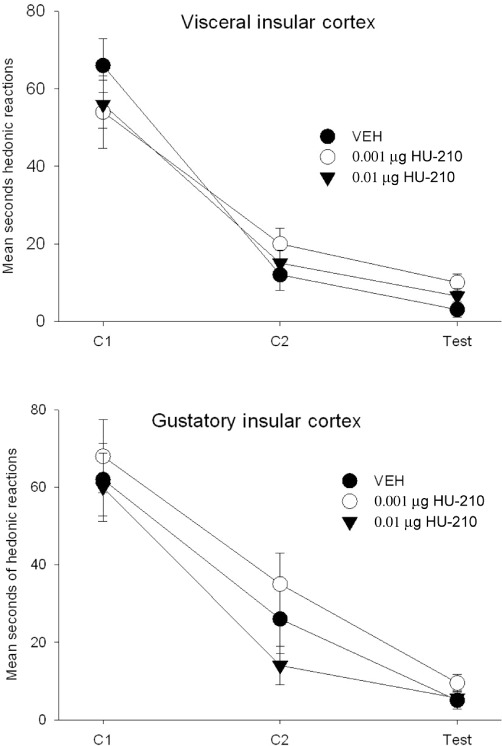
The mean (±SEM) duration (s) of hedonic reactions expressed during the conditioning and test trials following microinfusion of HU-210 to the VIC (upper graph) and GIC (lower graph). HU-210 did not alter hedonic reactions during the first conditioning trial and failed to attenuate suppressed hedonic reactions during the second conditioning or test trials when administered to either the VIC or the GIC.
Experiment 3: effect of co-administration of AM-251 and HU-210 in the VIC on LiCl-induced conditioned gaping
Central administration of AM-251 to the VIC before HU-210 prevented the suppression of LiCl-induced conditioned gaping; however, as in Experiments 1 and 2, no pretreatment condition modified suppressed hedonic reactions or CTA. Figure 6 presents the mean (±SEM) number of gapes (upper graph) and the mean (±SEM) number of seconds that the rats displayed hedonic reactions (lower graph) in Experiment 3. The 4 × 3 mixed-factor anova of gaping revealed significant effects of group, F(3,23) = 3.6; P= 0.03, trials, F(2,46) = 39.3; P < 0.001, and group × trials, F(6, 46) = 2.4; P= 0.04. Subsequent Bonferroni post hoc comparison tests revealed that on the test trial only, group VEH-HU210 displayed significantly fewer gapes than any other group (P < 0.05) with no other significant effects. The 4 × 3 mixed-factor anova of hedonic reactions revealed only a significant effect of trials, F(2,46) = 139.0; P < 0.001. The single-factor anova of the CTA test revealed no group differences in the strength of the CTA, F (3,23) = 1.1 (data not depicted).
Figure 6.
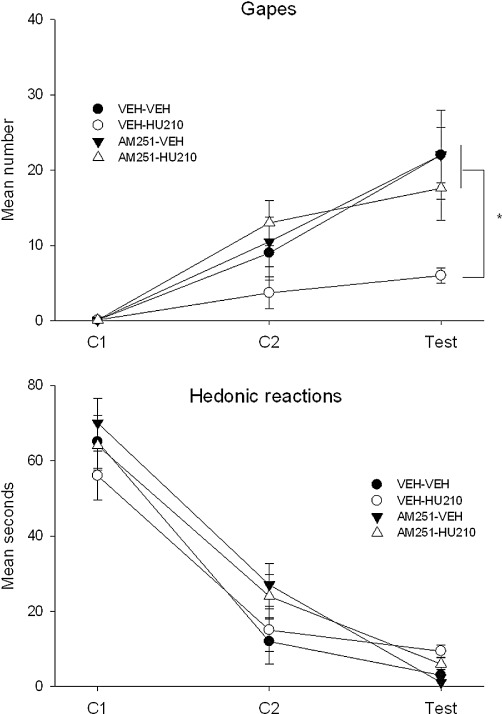
The mean (+SEM) number of gaping responses (upper graph) and duration (s) of hedonic reactions (lower graph) expressed during the conditioning and test trials following microinfusion of the CB1 antagonist/inverse agonist, AM-251, and the CB1 agonist, HU-210, to the VIC. HU-210 attenuated LiCl-induced conditioned gaping reactions (*P < 0.05) and this effect was blocked by pretreatment with AM-251. No pretreatment altered hedonic reactions during the first conditioning trial or attenuated suppressed hedonic reactions during the second conditioning or test trials.
Experiment 4: does VIC administration of AM-251 produce nausea?
When AM-251 was centrally administered to the VIC, it did not produce conditioned gaping reactions, suppress hedonic reactions or CTA at doses as high as 20 µg. Figure 7 presents the mean (±SEM) number of gapes (upper graph) and the mean (±SEM) number of seconds that the rats displayed hedonic reactions (lower graph) in Experiment 4. The 5 × 3 mixed-factor anova of gaping and hedonic reactions revealed only a significant effect of trial [gaping: F (2,62) = 3.4; P= 0.04; hedonic reactions: F(2, 62) = 4.0; P= 0.023] with gaping higher and hedonic reactions lower on the test trial than the other trials. The mean (±SEM) preference ratios at 30, 120 and 360 min for each group are depicted in Figure 8. The 5 × 3 mixed-factor anova revealed only a significant effect of time, F(2,62) = 35.3; P < 0.001; the rats had a higher saccharin preference ratio at the 360 min measure than at the earlier measure, but this did not differ among the groups.
Figure 7.
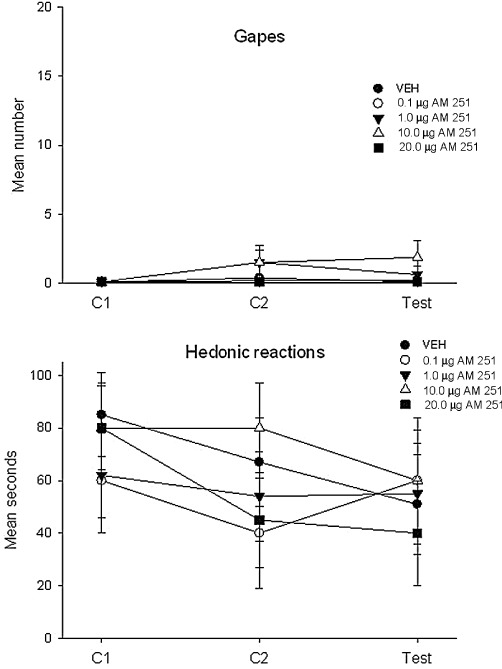
The mean (±SEM) number of gaping responses (upper graph) and duration (s) of hedonic reactions (lower graph) expressed during the conditioning and test trials following microinfusion of AM-251 to the VIC subsequent to intraoral infusion of saccharin. There was no effect of drug treatment.
Figure 8.
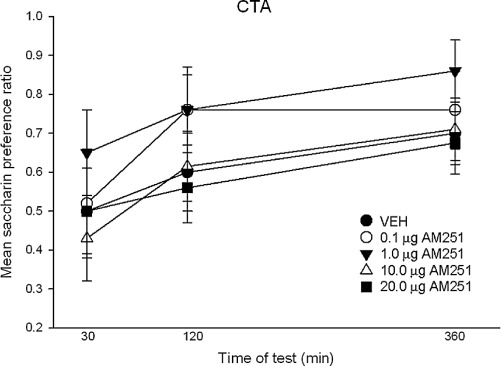
The mean (+SEM) saccharin preference ratio expressed by the groups during the two-bottle test at 30, 120 and 360 min. There were no group differences.
Discussion
There is considerable evidence indicating that CB1 agonists are involved in the regulation of both nausea and vomiting in animal models (for complete review, see Parker et al., 2011). Here, we provide evidence that the potent CB1 agonist, HU-210, acts at the visceral region of the insular cortex to relieve the nausea-inducing effect of systemically administered LiCl. This effect was not simply the result of HU-210 interfering with the association between saccharin and LiCl because neither LiCl-induced suppressed hedonic reactions nor CTA (which are not selectively produced by nausea, see Grant, 1987; Parker et al., 2008) were attenuated by this pretreatment. Indeed, only the reaction of gaping that is specifically produced by the nausea induced by LiCl was blocked by pretreatment with HU-210. Specifically, microinfusion of HU-210 into the VIC selectively attenuated the nausea produced by LiCl, an effect that was reversed by AM-251 and is therefore mediated by CB1 receptors.
The finding that the peripherally restricted compound CB13 interfered with conditioned gaping when delivered i.c.v., but not when administered systemically, confirms that the reduction of LiCl-induced nausea was centrally regulated. Indeed, at a dose of 3 mg·kg−1, the centrally active THC, but not the peripherally restricted CB13, suppressed conditioned gaping. However, the finding that AM-251 did not produce nausea on its own when infused directly into the VIC was puzzling. Nevertheless, this latter finding is consistent with our recent report that systemic administration of AM-251 potentiates LiCl-induced nausea, but i.c.v. administration of AM-251 (at a dose as high as 1/10 the effective systemic dose) does not enhance LiCl-induced gaping (Limebeer et al., 2010). Apparently, the production of nausea by CB1 inverse agonists (Parker et al., 2003; McLaughlin et al., 2005) originates peripherally. Interestingly, however, newly developed CB1 receptor antagonists devoid of inverse agonist effects, including AM4113 (Sink et al., 2008), AM6527 (Limebeer et al., 2010) and AM6545 (Cluny et al., 2010; Limebeer et al., 2010) neither produce conditioned gaping reactions nor potentiate LiCl-induced conditioned gaping, suggesting that the nausea produced by SR141716 (Parker et al., 2003) and AM-251 (McLaughlin et al., 2005; Limebeer et al., 2010) is mediated by inverse agonism of the CB1 receptor. Other investigators have reported that CB1 agonists decrease intestinal motility via peripheral CB1 receptors (e.g. Pertwee, 2001), but act either centrally in the nucleus of the solitary tract in the brainstem (Van Sickle et al., 2001; 2003) or peripherally (Darmani and Johnson, 2004) to attenuate emesis.
The IC receives visceral input in the granular region, VIC, and gustatory input in the adjacent dorsal agranular region (GIC; e.g. Lundy and Norgren, 2004; Saper, 2004). Therefore, the IC is an important site for taste-illness associations, which may lead to conditioned disgust reactions. Both electrolytic and neurochemical lesions of the GIC have been shown to interfere with the establishment of LiCl-induced CTA (e.g. Braun et al., 1972; Bermudez-Rattoni and McGaugh, 1991; Cubero et al., 1999; Roman and Reilly, 2007). Lesions that fall within the VIC do not interfere with LiCl-induced CTA (Mackey et al., 1986; Yamamoto, 1993; Yamamoto et al., 1995; Gedes et al., 2008) even though inactivation of the VIC attenuates LiCl-induced malaise (Contreras et al., 2007), as measured by them lying flat on their stomachs (Parker et al., 1984; Bernstein et al., 1992), which is also attenuated by area postrema lesions (Bernstein et al., 1992). Using the conditioned disgust reaction measure, Kiefer and Orr (1992) found that ablation of the entire IC eliminated the establishment of LiCl-induced conditioned disgust reactions (without modifying the reaction to bitter quinine), but not taste avoidance. Our results suggest that the region of importance in these ablations was the VIC.
Penfield and Faulk (1955) observed that electrical stimulation of the insula in conscious patients undergoing surgery produced sensations of nausea. In addition, functional MRI research has demonstrated that the insula is engaged when a human views pictures of disgusting food (Calder et al., 2007) or is exposed to disgusting smells (Heining et al., 2003; Wicker et al., 2003). Interestingly, the amygdala does not appear to be involved in processing conditioned disgust reactions in rats (Rana and Parker, 2008) or disgust reactions in humans (Calder et al., 2007); instead, it appears to be more selectively activated in response to fear-evoking stimuli in humans (Calder et al., 2007) and, in the case of the basolateral amygdala, CTA in rats (for review, see Reilly and Bornovalova, 2005), which may reflect conditioned fear rather than conditioned nausea (Parker et al., 2008). Forebrain regions necessary for the production of the conditioned disgust reactions in rats are also necessary for the generation of nausea.
The establishment of these nausea-induced conditioned gaping reactions relies on an intact forebrain (Grill and Norgren, 1978b); that is, decerebrate rats display unconditional gaping reactions to a bitter taste, but do not display conditioned gaping reactions when exposed to a taste previously paired with a nausea-inducing treatment. Forebrain 5-HT may play a role in the generation of nausea because lesions of the dorsal and median raphe, that reduce the amount of 5-HT available in the forebrain, interfere with the establishment of LiCl-induced conditioned gaping, but not taste avoidance (Limebeer et al., 2004). Most recently, it has been demonstrated that selective 5,7-DHT lesions of the insular cortex that reduced 5-HT levels by 76% dramatically interfered with LiCl-induced conditioned gaping reactions (Tuerke et al., 2010). Further, the classical anti-emetic agent, ondansetron, which attenuates LiCl-induced conditioned gaping reactions (and malaise-induced lying-on the belly; Tuerke et al., 2011) when administered systemically (Limebeer and Parker, 2000), also attenuated these reactions when microinfused directly into the VIC, but not the GIC (Tuerke et al., 2010). Therefore, the effects of ondansetron and HU-210 reported here on conditioned gaping reactions are equivalent. Although CB1 agonists have been demonstrated to inhibit the function of 5-HT3 receptors (Fan, 1995; Barann et al., 2002; Racz et al., 2008; Xiong et al., 2011) as does ondansetron, it is unlikely that such a mechanism is responsible for the results reported here because the reduction of nausea by HU-210 was reversed by AM-251, suggesting a CB1 mechanism. It is, however, likely that the endocannabinoid system may modulate the release of 5-HT in the VIC to regulate nausea in the normal functioning system. Indeed, considerable evidence points to an interaction between these two systems (Morales and Bloom, 1997; Hermann et al., 2002; Morales and Bäckman, 2002; Morales et al., 2004; Häring et al., 2007). Future research is needed to elucidate how these interactions within the VIC may serve to regulate nausea.
Acknowledgments
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC-92057) to LAP, a scholarship from NSERC to EMR, and the Israel Science Foundation and the National Institute of Drug Abuse, U.S. (DA-9789) to RM. Please send reprint request to parkerl@uoguelph.ca.
Glossary
- 2-HPβCD
2-hydroxypropyl-β-cyclodextrin
- CB1
cannabinoid 1 receptor
- CB13
naphthalene-1-y-(4-pentyloxynaphthalen-1-yl)methanone
- CTA
conditioned taste avoidance
- GIC
gustatory insular cortex
- HU-210
3-(1,1′-dimethylheptyl)-6aR,7,10,10aR-tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol
- THC
Δ9-tetrahydrocannabinol
- TR
taste reactivity
- VIC
visceral insular cortex
Conflict of interest
There is no conflict of interest on behalf of all the authors.
References
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edn. Br J Pharmacol. 2011;164(Suppl 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barann M, Molderings G, Brüss M, Bönisch H, Urban BW, Göthert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsell WR, Jr, Best MR. One bottle too many? Method of testing determines the detection of overshadowing and retention of taste aversions. Anim Learn Behav. 1993;21:154–158. [Google Scholar]
- Bermudez-Rattoni F, McGaugh JL. Insular cortex and amygdala differentially effect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Bernstein IL, Chavez M, Allen D, Taylor EM. Area postrema mediation of physiological and behavioral effects of lithium chloride in the rat. Brain Res. 1992;575:132–137. doi: 10.1016/0006-8993(92)90432-9. [DOI] [PubMed] [Google Scholar]
- Braun JJ, Slick TB, Lorden JF. Involvement of gustatory neocortex in learning of taste aversions. Physiol Behav. 1972;9:637–641. doi: 10.1016/0031-9384(72)90023-6. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Calder AK, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Catenoix H, Isnard J, Guénot M, Petit J, Remy C, Mauguiére F. The role of the anterior insular cortex in ictal vomiting: a stereotactic electroencephalography study. Epilepsy Behav. 2008;13:560–563. doi: 10.1016/j.yebeh.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M, Ceric F, Torrealba F. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- Cross-Mellor SK, Ossenkopp KP, Piomelli D, Parker LA. Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in rats. Psychopharmacology (Berl) 2007;190:135–143. doi: 10.1007/s00213-006-0589-7. [DOI] [PubMed] [Google Scholar]
- Cubero I, Thiele TE, Bernstein IL. Insular cortex lesions and taste aversion learning: effects of conditioning method and timing of lesion. Brain Res. 1999;839:323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- Darmani NA. The cannabinoid CB1 receptor antagonist SR 141716A reverses the antiemetic and motor depressant actions of WIN 55, 212-2. Eur J Pharmacol. 2001;430:49–58. doi: 10.1016/s0014-2999(01)01355-3. [DOI] [PubMed] [Google Scholar]
- Darmani NS, Johnson CJ. Central and peripheral mechanisms contribute to the antiemetic actions of delta-9-tetrahydrocannabinol against 5-hydroxytryptophan-induced emesis. Eur J Pharmacol. 2004;488:201–212. doi: 10.1016/j.ejphar.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Dziadulewicz EK, Bevan SJ, Brain CT, Coote PR, Culshaw AJ, Davis AJ, et al. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J Med Chem. 2007;50:3851–3856. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Prior, repeated exposure to cocaine potentiates locomotor responsivity to central injections of corticotrophin-releasing factor (CRF) in rats. Psychopharmacology (Berl) 2003;170:383–389. doi: 10.1007/s00213-003-1556-1. [DOI] [PubMed] [Google Scholar]
- Fan P. Cannabinoid agonists inhibit the activation of 5-HT3 receptors in rat nodose ganglion neurons. J Neurophysiol. 1995;73:907–910. doi: 10.1152/jn.1995.73.2.907. [DOI] [PubMed] [Google Scholar]
- Fiol M, Leppik IE, Mireless R, Maxwell R. Ictus emeticus and the insular cortex. Epilepsy Res. 1988;2:127–131. doi: 10.1016/0920-1211(88)90030-7. [DOI] [PubMed] [Google Scholar]
- Gedes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci. 2008;122:1038–1050. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant VL. Do conditioned taste aversions result from activation of emetic mechanisms? Psychopharmacology (Berl) 1987;93:405–416. doi: 10.1007/BF00207227. [DOI] [PubMed] [Google Scholar]
- Grill HC, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978b;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978a;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Häring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Heining M, Young AW, Ioannou G, Andrew CM, Brammer MJ, Gray JA, et al. Disgusting smells activate human anterior inusla and ventral striatum. Ann N Y Acad Sci. 2003;1000:380–384. doi: 10.1196/annals.1280.035. [DOI] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Janero DR, Makriyannis A. Cannabinoid receptor antagonists: pharmacological opportunities, clinical experience, and translational prognosis. Expert Opin Emerg Drugs. 2009;14:1–23. doi: 10.1517/14728210902736568. [DOI] [PubMed] [Google Scholar]
- Kaada BR. Somato-motor, autonomic and electrocorticographic responses to electrical stimulation of rhinencephalic and other structures in primates, cat, and dog; a study of responses from the limbic, subcallosal, orbito-insular, piriform and temporal cortex, hippocampus-fornix and amygdala. Acta Physiol Scand Suppl. 1951;24:1–262. [PubMed] [Google Scholar]
- Kiefer SW, Orr MS. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106:140–146. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA. The anti-emetic drug, ondansetron, interferes with lithium-induced conditioned rejection reactions, but not lithium-induced conditioned taste avoidance. J Exp Psychol Anim Behav Process. 2000;26:371–384. doi: 10.1037//0097-7403.26.4.371. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Parker LA, Fletcher P. 5,7-Dihydroxytryptamine lesions of the dorsal and median raphe nuclei interfere with lithium-induced conditioned gaping, but not conditioned taste avoidance, in rats. Behav Neurosci. 2004;118:1391–1399. doi: 10.1037/0735-7044.118.6.1391. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Vemuri VK, Bedard H, Lang ST, Ossenkopp KP, Makriyannis A, et al. Inverse agonism of cannabinoid CB1 receptors potentiates LiCl-induced nausea in the conditioned gaping model in rats. Br J Pharmacol. 2010;161:336–349. doi: 10.1111/j.1476-5381.2010.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy RF, Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. 3rd edn. San Diego, CA: Elsevier; 2004. pp. 890–922. [Google Scholar]
- Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversion induced by morphine. Pharmacol Biochem Behav. 1986;24:71–78. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid antagonist AM-251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 2005;180:286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Morales M, Bäckman C. Coexistence of serotonin 3 (5-HT3) and CB1 cannabinoid receptors in interneurons of hippocampus and dentate gyrus. Hippocampus. 2002;12:756–764. doi: 10.1002/hipo.10025. [DOI] [PubMed] [Google Scholar]
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci. 1997;17:3157–3167. doi: 10.1523/JNEUROSCI.17-09-03157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Wang SD, Diaz-Ruiz O, Jho DH. Cannabinoid CB1 receptor and serotonin 3 receptor subunit A (5-HT3A) are co-expressed in GABA neurons in the rat telencephalon. J Comp Neurol. 2004;468:205–216. doi: 10.1002/cne.10968. [DOI] [PubMed] [Google Scholar]
- Parker LA. Rewarding drugs produce taste avoidance, but not taste aversion. Neurosci Biobehav Rev. 1995;19:143–151. doi: 10.1016/0149-7634(94)00028-y. [DOI] [PubMed] [Google Scholar]
- Parker LA, Mechoulam R. Cannabinoid agonists and an antagonist modulate lithium-induced conditioned gaping in rats. Integr Physiol Behav Sci. 2003;38:134–146. doi: 10.1007/BF02688831. [DOI] [PubMed] [Google Scholar]
- Parker LA, Hills K, Jensen K. Behavioral CRs elicited by lithium- or an amphetamine-paired contextual test chamber. Anim Learn Behav. 1984;12:307–315. [Google Scholar]
- Parker LA, Mechoulam R, Schlievert C, Abbott LA, Fudge ML, Burton P. Cannabinoid agonists attenuate and a cannabinoid antagonist potentiates lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology. 2003;166:156–162. doi: 10.1007/s00213-002-1329-2. [DOI] [PubMed] [Google Scholar]
- Parker LA, Kwiatkowska M, Burton P, Mechoulam R. Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew) Psychopharmacology. 2004;171:156–161. doi: 10.1007/s00213-003-1571-2. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rana SA, Limebeer CL. Conditioned nausea in rats: assessment by conditioned disgust reactions, rather than conditioned taste avoidance. Can J Exp Psychol. 2008;62:198–209. doi: 10.1037/a0012531. [DOI] [PubMed] [Google Scholar]
- Parker LA, Rock EM, Limebeer CL. Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol. 2011;163:1411–1422. doi: 10.1111/j.1476-5381.2010.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain. 6th edn. New York: Academic Press; 1998. [Google Scholar]
- Penfield W, Faulk ME. The insula: further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz I, Bilkei-Gorzo A, Markert A, Stamer F, Gothert M, Zimmer A. Anandamide effects on 5HT(3) receptors in-vivo. Eur J Pharmacol. 2008;596:98–101. doi: 10.1016/j.ejphar.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Rana SA, Parker LA. Differential effects of neurotoxic lesions of the basolateral amygdala and the central nucleus of the amygdala on lithium-induced conditioned disgust reactions and conditioned taste avoidance. Behav Brain Res. 2008;189:284–297. doi: 10.1016/j.bbr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: a critical review. Neurosci Biobehav Rev. 2005;29:1067–1088. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Roman C, Reilly S. Effects of insular cortex lesions on conditioned taste aversion and latent inhibition in the rat. Eur J Neurosci. 2007;26:2627–2632. doi: 10.1111/j.1460-9568.2007.05872.x. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic systems. In: Paxinos G, editor. The Rat Nervous System. 3rd edn. San Diego, CA: Elsevier; 2004. pp. 761–795. [Google Scholar]
- Sink KS, McLaughlin PJ, Brown C, Fan P, Vemuri V, Keran V, et al. The novel cannabinoid CB1 receptor neutral antagonist, AM4113, suppresses food intake and food-reinforced behaviour but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:1–10. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sticht MA, Long JZ, Rock EM, Limebeer CL, Mechoulam R, Cravatt BF, et al. The MAGL inhibitor, JZL184, attenuates LiCl-induced vomiting in the Suncus murinus and 2AG attenuates LiCl-induced nausea-like behavior in rats. Br J Pharmacol. 2011;165:2425–2435. doi: 10.1111/j.1476-5381.2011.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerke KJ, Limebeer CL, Chambers J, Fletcher PJ, Parker LA. 2010. Reduced serotonin availability in the insular cortex produces a regionally specific dissociation between gaping and taste avoidance. Poster presented at the Society for Neuroscience meetings, San Diego, CA.
- Tuerke KJ, Winters BD, Parker LA. Ondansetron interferes with unconditioned lying-on belly and acquisition of conditioned gaping induced by LiCl as models of nausea-induced behaviors in rats. Physiol Behav. 2011;105:856–860. doi: 10.1016/j.physbeh.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JJ, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Δ9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly P, Royet JR, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Xiong W, Koo BN, Morton R, Zhang L. Psychotropic and nonpsychotropic cannabis derivatives inhibit human 5-HT(3A) receptors through a receptor desensitization-dependent mechanism. Neuroscience. 2011;184:28–37. doi: 10.1016/j.neuroscience.2011.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. Neural mechanisms of taste aversion learning. Neurosci Res. 1993;16:181–185. doi: 10.1016/0168-0102(93)90122-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y, Shimura T, Sakai N. Conditioned taste aversion in the rat with excitotoxic brain lesions. Neurosci Res. 1995;22:31–49. doi: 10.1016/0168-0102(95)00875-t. [DOI] [PubMed] [Google Scholar]


