Abstract
A 66-year-old woman was diagnosed with hepatic metastasized carcinoid tumor of the ileocecal junction resulting in elevated plasma chromogranin A levels and urinary 5-hydroxyindoleacetic acid (5-HIAA) levels. Further examination showed right-sided heart failure with severe tricuspid valve regurgitation. Carcinoid tumors produce serotonin which leads to flushing, secretory diarrhea, bronchospasm and hypotension, known as carcinoid syndrome. Serotonin is metabolized to 5-HIAA, which is inactive, in the liver and the lungs. However, hepatic metastases may result in direct exposure of the heart to serotonin, which induces plaque-like deformities on the tricuspid valve, and in turn induces valve regurgitation. This condition is known as carcinoid heart disease. Tricuspid valve regurgitation may induce risk of massive blood loss in case of liver surgery through high-volume backflow in the hepatic veins. This report shows the clinical relevance of carcinoid heart disease in the perioperative setting.
Key words: Carcinoid tumor, Carcinoid heart disease, Serotonin, Tricuspid valve regurgitation
Introduction
Carcinoid tumors are rare neuroendocrine tumors arising from enterochromaffin cells typically located in the lungs or gastrointestinal tract. The incidence of these tumors is 1–2 per 100,000 people in the United States [1]. The tumors release serotonin and other vasoactive substances which are classically associated with carcinoid syndrome, characterized by episodic vasomotor flushing, secretory diarrhea, bronchospasm and hypotension. Upon diagnosis up to 30% have disseminated disease and carcinoid syndrome [1, 2]. Once released, the active serotonin is metabolized by monoamine oxidases in the liver, lungs and brain to metabolically inactive 5-hydroxyindoleacetic acid (5-HIAA), which is renally cleared. When liver metastasis occurs, the heart is exposed to intermittently high levels of vasoactive substances, which is believed to result in endocardial damage. Cardiac involvement is characterized by endocardial plaque-like deposits found predominantly on right-sided heart valves, resulting in thickening, retraction and fixation of the right heart valves, valvular dysfunction and, eventually, right-sided heart failure, determined as carcinoid heart disease. Although serotonin levels in patients with carcinoid heart disease are generally higher than in those without cardiac involvement, it is unclear what factors are involved in the progression of the cardiac lesions. In rare cases, exposure of the heart follows a route through the thoracic duct, bypassing the liver [3]. Carcinoid heart disease occurs in up to 60% of patients with carcinoid syndrome [4]. Right ventricular failure remains a major cause of morbidity and mortality in patients with carcinoid heart disease [5]. Left-sided cardiac involvement can also occur in <10% of patients and is frequently associated with a patent foramen ovale (PFO) [6, 7]. In addition, PFO could be a result of progressive right-sided cardiac dilatation secondary to valve insufficiency. We present a case with severe tricuspid valve regurgitation as a result of persistent serotonin load due to a mid-gut carcinoid tumor metastasized to the liver and illustrate the perioperative risks involving right-sided heart failure during liver surgery.
Case Report
A 66-year-old woman was analyzed for a changed defecation pattern characterized by diarrhea. Her medical history revealed ductal carcinoma in situ for which breast-conserving surgery had been performed 3 years earlier. She was otherwise healthy, physically fit and used no medication. On physical examination there was a holosystolic murmur (grade 4/6) radiating to the right side of the chest, suggestive of tricuspid valve regurgitation. There were no abdominal abnormalities and physical examination was otherwise unremarkable.
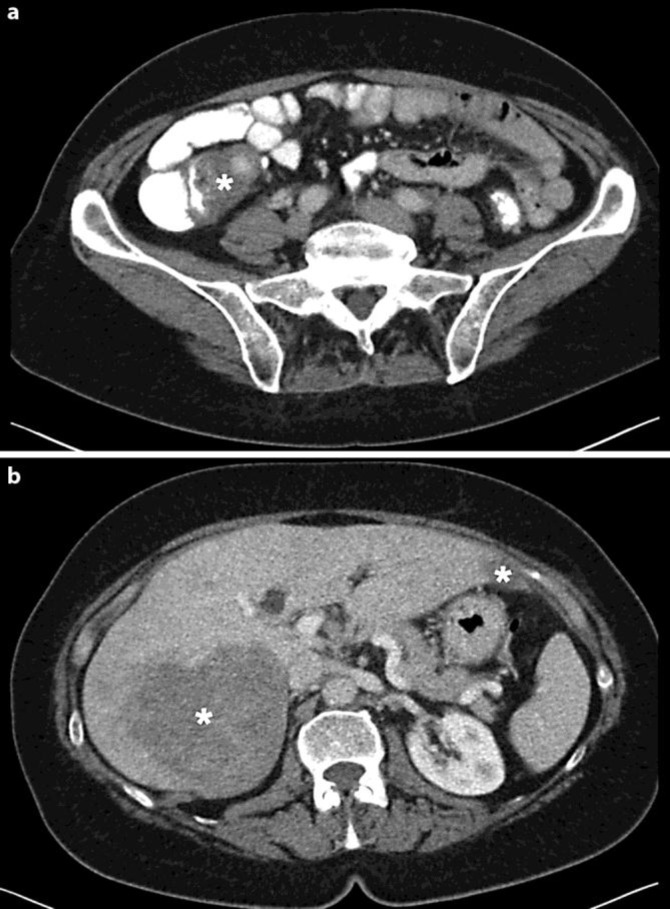
Colonoscopy showed a benign cecal polyp and a focal lesion in the terminal ileum clinically suggestive for neuroendocrine tumor or lipoma. Pathologic analysis of the biopsy samples was inconclusive. Additional computed tomography of the abdomen showed a mass in relation to the terminal ileum with locoregional deposition and hepatic metastasis with a 3 cm lesion in segment 3 and a 12 cm lesion in segment 6 (fig. 1). Laboratory values revealed increased levels of serum chromogranin A (444 μg/l, normal 0–120 μg/l) and urinary 5-HIAA (798.3 μmol/24 h, normal 10.5–47.1 μmol/24 h).
Fig. 1.
Computed tomography image showing primary carcinoid tumor encasing the terminal ileum (asterisk in a) and its hepatic metastasis in segments 3 and 6 (asterisks in b).
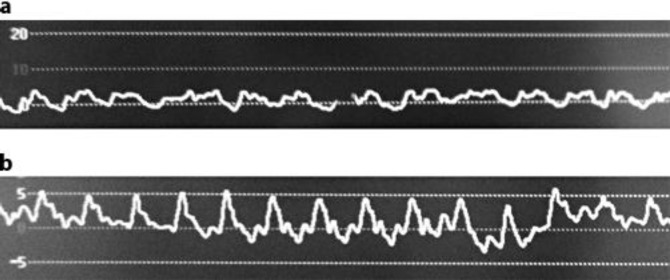
Preoperative cardiac analysis showed severe tricuspid valve regurgitation due to malcoaptation of the valve slips with a pressure gradient of 22 mm Hg and concomitant systolic blood flow reversal in the hepatic veins. The left side of the heart showed normal function. There were no signs of pulmonary hypertension or a PFO. The patient was found eligible for right hemicolectomy and metastasectomy from segments 3 and 6. Despite selective occlusion of arterial and portal inflow of the liver and low central venous pressure, serious blood loss occurred during dissection of the liver due to a pulsatile systolic blood flow reversal in the hepatic veins with concomitant systolic peaks on the central venous pressure curve (fig. 2). Pathologic analysis of the resected tissue showed low-grade carcinoid with liver metastasis with clear margins.
Fig. 2.
a Normal central venous pressure curve. b Central venous pressure curve of our patient with systolic peaks due to tricuspid valve regurgitation. These peaks in pressure where responsible for the pulsatile blood flow in the hepatic veins noticed during liver resection.
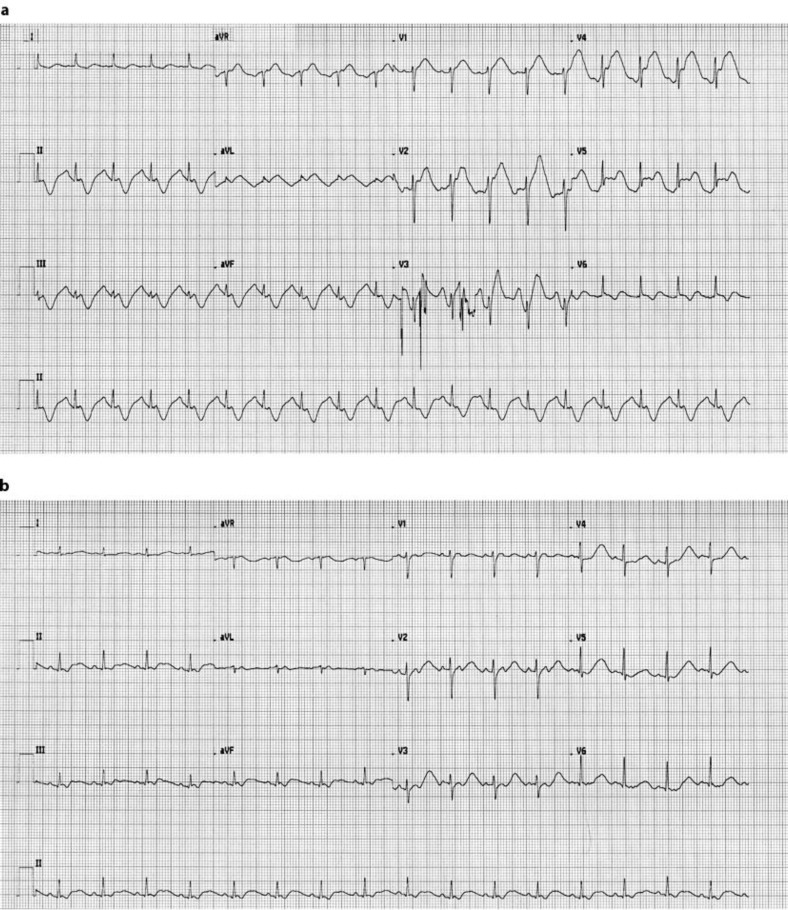
Postoperatively the patient developed electrocardiography (ECG) changes in relation to sinus tachycardia consisting of diffuse ST elevation and negative T waves in the inferior leads (fig. 3). Echocardiography at that moment showed no changes in myocardial motility and troponin-I levels remained negative. ECG changes normalized in parallel with a spontaneously decreasing pulse rate under 100 bpm.
Fig. 3.
a Postoperative ECG of our patient showing diffuse ST elevations and negative T waves in the inferior leads V1–3. These findings are consistent with coronary spasm mimicking acute coronary syndrome. b Recovery from ECG changes in relation to a lowered heart rate.
The patient recovered without complications after surgery and was discharged from hospital after 8 days. The tumor as well as the metastases turned out to be resected completely. At follow-up clinical manifestations of carcinoid syndrome had resolved completely. Echocardiography done at follow-up 3 months postoperatively showed significant recovery of the tricuspid valve regurgitation and recovery from right-sided heart failure.
Discussion
In our patient marked tricuspid valve regurgitation was noticed preoperatively by echocardiography. These findings are in line with the known literature, where echocardiographic features include thickening of valve leaflets that become retracted and eventually immobile, resulting in a combination of valvular regurgitation and stenosis [6]. In a report describing clinical and echocardiographic features in a group of patients with carcinoid heart disease, tricuspid valve involvement was most common, with 97% of patients. The pulmonary valve appeared thickened, retracted and immobile in 49% of patients. However, clinical presentation was less common. In addition, Doppler examination of the hepatic veins may also show a systolic flow reversal, consistent with severe tricuspid regurgitation as shown in our patient. The severity of pulmonary stenosis may be underestimated because of a low cardiac output and severe tricuspid regurgitation [7]. Furthermore, PFO is an important marker for carcinoid disease progression and is advised to be systematically assessed during echocardiography [8]. Tricuspid valve regurgitation is shown to be reversed if exposure of the heart to high serotonin levels is discontinued. This phenomenon was already seen 3 months postoperatively with echocardiography during follow-up in our patient. In this respect, resection of the hepatic metastasis is the primary treatment for tricuspid valve regurgitation, whereas valve replacement should only be considered after insufficient valve recovery.
The event in our patient, noticed by marked ECG findings, seems to be rare, with only two reports describing ECG changes in relation to serotonin-producing carcinoid tumors [9, 10]. It is believed that serotonin may affect coronary spasms, mimicking acute coronary syndrome with typical ECG changes, which may even lead to coronary stent placement and balloon dilatation. However, in our patient these ECG changes only occurred in the direct aftermath of the surgery, which could be caused by temporarily occurring coronary vasospasm due to high circulating serotonin levels caused by manipulation of the tumor and the metastases during surgery. The absence of elevated serum troponin-I levels, normal myocardial function and complete spontaneous recovery of ECG changes are supporting evidence for this hypothesis (fig. 3).
Metastasized carcinoid tumors have a relatively benign prognosis since these tumors grow slowly. However, when carcinoid heart disease is involved, median survival is only 1–4 years, referring to the latest numbers [11]. Combinations of cytotoxic agents, somatostatin analogues or hepatic artery embolization have neither been shown to be effective in patients with metastatic mid-gut carcinoid tumors, nor have these modalities been shown to have any effect on carcinoid heart disease [12]. The only effective treatment known is hepatic resection, which is associated with decreased progression and an improved prognosis in patients with carcinoid heart disease. Hence, resection of the primary tumor as well as hepatic metastases should be considered as primary treatment if preoperative evaluation reveals that over 90% of primary and regional disease can be resected [13, 14].
Conclusion
The primary treatment for metastasized mid-gut carcinoid tumors is surgery. However, a potential hazard for surgery and anesthesia is right-sided cardiac impairment, which is highly related to hepatic metastases. Therefore, patients with high carcinoid burden who are planned for surgical resection should be analyzed systematically for their cardiac condition. Adequate preparations should be planned in order to avoid peri- as well as postoperative cardiac complications.
Disclosure Statement
The authors declare no financial or non-financial competing interests.
References
- 1.Mollin IM, Sandor A. An analysis of 8,305 cases of carcinoid tumors. Cancer. 1997;79:813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Kulke MH, Mayer RJ. Carcinoid tumors. N Engl J Med. 1999;340:858–868. doi: 10.1056/NEJM199903183401107. [DOI] [PubMed] [Google Scholar]
- 3.Bernheim AM, Connolly HM, Pellikka PA. Carcinoid heart disease in patients without liver metastasis. Am J Cardiol. 2007;99:292–294. doi: 10.1016/j.amjcard.2006.07.092. [DOI] [PubMed] [Google Scholar]
- 4.Anderson AS, Krauss D, Lang R. Cardiovascular complications of malignant carcinoid disease. Am Heart J. 1997;134:693–702. doi: 10.1016/s0002-8703(97)70053-x. [DOI] [PubMed] [Google Scholar]
- 5.Palaniswamy C, Frishman WH, Aronow WS. Carcinoid heart disease. Cardiol Rev. 2012;20:167–176. doi: 10.1097/CRD.0b013e31824c866e. [DOI] [PubMed] [Google Scholar]
- 6.Pellikka PA, Tajik AJ, Khandheria BK, et al. Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Circulation. 1993;87:1188–1196. doi: 10.1161/01.cir.87.4.1188. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharyya S, Toumpanakis C, Burke M, et al. Features of carcinoid heart disease identified by 2- and 3-dimensional echocardiography and cardiac MRI. Circ Cardiovasc Imaging. 2010;3:103–111. doi: 10.1161/CIRCIMAGING.109.886846. [DOI] [PubMed] [Google Scholar]
- 8.Mansencal N, Mitry E, Forissier JF, et al. Assessment of patent foramen ovale in carcinoid heart disease. Am Heart J. 2006;151:1129.e1–1129.e6. doi: 10.1016/j.ahj.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Vohra HA, Alzetani A, Guha T, Rosin MD. A thymic carcinoid mimicking acute aortic dissection. Cardiovasc Surg. 2003;11:96–98. doi: 10.1016/s0967-2109(02)00141-2. [DOI] [PubMed] [Google Scholar]
- 10.Bourgault C, Bergeron S, Bogaty P, Poirier P. A most unusual acute coronary syndrome. Can J Cardiol. 2006;22:429–432. doi: 10.1016/s0828-282x(06)70930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller JE, Pellikka PA, Bernheim AM, et al. Prognosis of carcinoid heart disease: analysis of 200 cases over two decades. Circulation. 2005;112:3320–3327. doi: 10.1161/CIRCULATIONAHA.105.553750. [DOI] [PubMed] [Google Scholar]
- 12.Moller JE, Connolly HM, Rubin JR, Seward JB, Modesto K, Pellikka PA. Factors associated with progression of carcinoid heart disease. N Engl J Med. 2003;348:1005–1015. doi: 10.1056/NEJMoa021451. [DOI] [PubMed] [Google Scholar]
- 13.Bernheim AM, Connolly HM, Rubin J, et al. Role of hepatic resection for patients with carcinoid heart disease. Mayo Clin Proc. 2008;83:142–150. doi: 10.4065/83.2.143. [DOI] [PubMed] [Google Scholar]
- 14.Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]