Abstract
Buckwheat (Fagopyrum esculentum Moench. cv Jianxi), which shows high Al resistance, accumulates Al in the leaves. The internal detoxification mechanism was studied by purifying and identifying Al complexes in the leaves and roots. About 90% of Al accumulated in the leaves was found in the cell sap, in which the dominant organic acid was oxalic acid. Purification of the Al complex in the cell sap of leaves by molecular-sieve chromatography resulted in a complex with a ratio of Al to oxalic acid of 1:3. A 13C-nuclear magnetic resonance study of the purified cell sap revealed only one signal at a chemical shift 164.4 ppm, which was assigned to the Al-chelated carboxylic group of oxalic acid. A 27Al-nuclear magnetic resonance analysis revealed one major signal at the chemical shift of 16.0 to 17.0 ppm, with a minor signal at the chemical shift of 11.0 to 12 ppm in both the intact roots and their cell sap, which is consistent with the Al-oxalate complexes at 1:3 and 1:2 ratios, respectively. The purified cell sap was not phytotoxic to root elongation in corn (Zea mays). All of these results indicate that Al tolerance in the roots and leaves of buckwheat is achieved by the formation of a nonphytotoxic Al-oxalate (1:3) complex.
Al3+ is toxic to most plant species, but some can accumulate Al without showing any toxicity. Tea and hydrangea are well-known Al accumulators. Old leaves of tea can accumulate Al up to 30,000 mg kg−1 on a dry-weight basis (Matsumoto et al., 1976), and the Al content in the leaves of hydrangea plants with blue-sepaled flowers can become higher than 3,000 mg kg−1 (Ma et al., 1997a). Some trees in tropical cloud forests (such as Richeria grandis) have also been reported to accumulate high levels of Al (more than 1,000 mg kg−1; Cuenca et al., 1990). Recently, several plants (Melastoma malabathricum and Vaccinium macrocarpon; Osaki et al., 1997) adapted to low-pH soils were found to accumulate high Al in either roots or leaves. Although localization of Al in the cells of these plants has not yet been well identified, several reports have suggested the presence of Al in the symplasm in a soluble form (Cuenca et al., 1990). At the pH of symplasmic solution (>7.0), the concentration of free Al3+ is decreased to less than 10−10 m due to the formation of insoluble Al(OH)3 (Martin, 1988), but this does not imply that such low concentrations are biologically inert (Taylor, 1991). Because of the high affinity of Al for O2-donor compounds such as Pi, nucleotides, RNA, DNA, proteins, carboxylic acids, phospholipids, polygalacturonic acids, heteropolysaccharides, lipopolysaccharides, flavonoids, anthocyanins, etc. (Haug, 1984; Martin, 1986), very low concentrations of free Al in the symplasm are potentially phytotoxic (Taylor, 1991). For example, Al3+ binds almost 107 times more strongly to ATP than does Mg2+; therefore, less than nanomolar amounts of Al3+ can compete with Mg for the P sites (Martin, 1988). These facts suggest that Al-accumulating plants must possess effective mechanisms for detoxifying Al internally. However, these mechanisms have not been well documented.
The buckwheat (Fagopyrum esculentum Moench.) is an important economic crop in Asia. The cv Jianxi, which is cultivated in the acid-soil area of southern China, was found to show high resistance to Al toxicity (Zheng et al., 1998a, 1998b), and one of the mechanisms responsible for the high Al resistance in this cultivar was the secretion of oxalic acid, a strong Al chelator, by the roots (Ma et al., 1997b; Zheng et al., 1998b). This response was very rapid (occurring within 30 min after exposure to Al solution) and was specific to Al stress; neither P deficiency nor other toxic metals such as La could induce the secretion of oxalic acid. Furthermore, we found that cv Jianxi accumulated Al in the leaves.
Following 10 d of intermittent treatment with 50 μm Al, the Al concentration of the buckwheat leaves reached about 450 mg Al kg−1 on a dry-weight basis, in contrast to other species such as wheat, oat, radish, and rape, which contained less than 50 mg Al kg−1 after the same treatment (Ma et al., 1997b). However, the Al concentration in buckwheat roots was less than that of other species. Based on a 27Al-NMR study, the form of Al in the buckwheat leaves has been suggested to be an Al-oxalate complex at a 1:3 ratio (Ma et al., 1997b). In the present study the Al complex in the leaves was purified and identified using 13C-NMR, and the form of Al in the roots was also examined using 27Al-NMR. The results indicate that accumulation of Al as Al-oxalate (1:3) complex, a nonphytotoxic form, is also responsible for high Al resistance in buckwheat.
MATERIALS AND METHODS
Plant Materials and Al Treatment
Seedlings of buckwheat (Fagopyrum esculentum Moench. cv Jianxi) were prepared as described elsewhere (Zheng et al., 1998b). Ten-day-old seedlings were exposed to 0.5 mm CaCl2 solution containing 0 or 50 μm Al at pH 4.5 every other day. The seedlings were grown in the nutrient solution on the other days. The purpose for this intermittent Al treatment was to avoid interaction between Al and other nutrients such as P. After a 10-d repeated treatment, plants were harvested and separated into roots and leaves. Leaves were stored at −80°C for cell sap extraction. To observe Al formation in the roots after a short exposure to Al solution, root samples for 27Al-NMR measurement and cell sap extraction were prepared by exposing 20-d-old seedlings to 50 μm Al in 0.5 mm CaCl2 solution at pH 4.5 for 20 h. The plants were grown in a growth cabinet (TGE-9 h-S, TABAI Espec, Hiroshima, Japan) at 25/20°C and 14-/10-h day/night cycles, 40 W m−2 light intensity, and 70% RH.
Extraction and Purification of the Cell Sap
Frozen samples were ground by hand and then placed on filters in centrifuge tubes (Centricut U-50, Mrcutoff 50,000, Biofield, Tokyo, Japan). Before completely thawing at room temperature, the samples were centrifuged at 10,000g for 20 min to obtain the cell sap (Ma et al., 1997a). The Al complex in the cell sap was purified by applying the freshly prepared cell sap to a 1.6- × 170-cm column of Sephadex G-10 as described previously (Ma et al., 1997a). Distilled water was used as the eluant after the pH was adjusted to 4.6 using HClO4 and passed with a peristaltic pump at a flow rate of 0.72 mL min−1. The concentration of Al in each fraction was determined by graphite furnace atomic absorption spectrophotometry (model Z-9000, Hitachi, Tokyo, Japan). Organic acids in each fraction were determined by HPLC equipped with an ion-exclusion column (Shimpack SCR-102H, 0.8 × 30 cm, Shimadzu, Kyoto, Japan; Ma et al., 1997a). Detection was conducted at 425 nm after reaction with bromthymol blue. Fractions containing Al were concentrated using a rotary evaporator at 40°C and purified four times by Sephadex G-10 column chromatography.
13C- and 27Al-NMR Measurement
13C-NMR spectra of purified cell sap and Al-oxalate (1:25) complex (similar ratio as that in the crude cell sap) were recorded on a 150.8-MHz spectrometer (JNM-α-600) under the following conditions: frequency range, 40.65 kHz; data points, 16,384; acquisition time, 0.20 s; and scans, 40,000. The Al-oxalate (1:25) complex was prepared by mixing an equal volume of 4 mm AlCl3 and 100 mm Na2C2O4, and then the pH was adjusted to 4.6 using 0.1 m HCl.
27Al-NMR spectra were obtained at 156.3 MHz (JNM-α-600 spectrometer, JEOL). The roots were cut with scissors and then placed in NMR tubes 10 mm in diameter. Solution samples were analyzed using NMR tubes 5 mm in diameter. The parameters used were: frequency range, 62.5 kHz; data points, 33,000; acquisition time, 0.52 s. AlCl3 (0.2 mm in 0.1 m HCl) was used as an external reference for calibration of the chemical shift (0 ppm).
Complexes of Al-oxalate at a molar ratio of 1:1, 1:2, and 1:3 were prepared by mixing an equal volume of 4 mm AlCl3 and 4, 8, and 12 mm Na2C2O4. After the solution pH was adjusted to 4.6, the complexes were subjected to 27Al-NMR measurement as described above.
Bioassay of Al Toxicity
To examine the toxicity of Al complex purified from cell sap of buckwheat leaves, the effect on root elongation of corn (Zea mays L. cv Golden Cross Bantam) was investigated. Seedlings were prepared as described previously (Zheng et al., 1998b) and subjected to the following treatments in 100 μm CaCl2 solution at pH 4.5: (a) −Al (control, no Al addition), (b) +Al (addition of 20 μm AlCl3), and (c) +Sap (purified cell sap containing 20 μm Al from the buckwheat leaves as described above). The treatment period was 20 h. Root length was measured with a ruler before and after treatment.
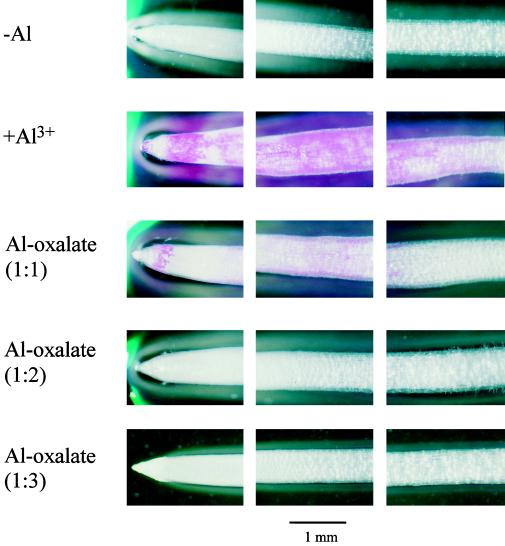
To examine different binding of Al-oxalate complexes with different molar ratios of Al to oxalic acid, staining patterns of roots with Eriochrome Cyanine R were compared using an Al-sensitive cultivar of wheat (Tritium aestivum L. cv Scout 66). Seeds were soaked in distilled water for 10 h and then germinated on a net tray in the dark at 25°C. After 2 d the tray was put in a plastic container containing 100 μm CaCl2 solution, pH 4.5. After a further 2 d seedlings selected for uniform size were exposed to 100 μm CaCl2 solutions, pH 4.5, without Al (−Al) or with 20 μm AlCl3 (+Al3+) or 20 μm Al-oxalate complex at a 1:1, 1:2, or 1:3 molar ratio. After 20 h the roots were placed in distilled water for 5 min and then stained with a 0.1% aqueous solution of Eriochrome Cyanine R solution (Sigma) for 10 min. The excess dye was removed by washing with distilled water and then the roots were observed with a light microscope (model B061, Olympus). The root length was measured before and after treatment.
RESULTS
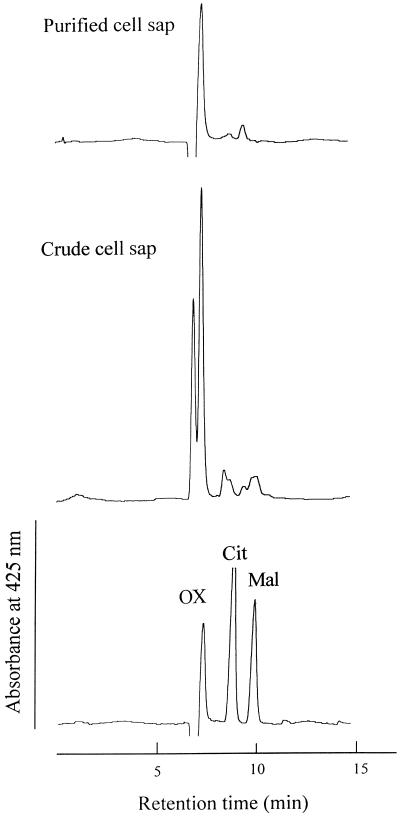
Buckwheat leaves accumulated as much as 2.01 mmol Al kg−1 fresh weight following 10 d of intermittent treatment with 50 μm Al (Table I), and the roots contained 3.45 mmol Al kg−1 fresh weight after 20 h of exposure to Al solution. Roots intermittently treated with Al for 10 d contained a similar Al concentration (data not shown). About 90% of the Al in the leaves was found in the cell sap, where the concentration was higher than 2 mm (Table I). About 60% of Al in the roots was extracted in the cell sap. The major organic acid was oxalic acid (Fig. 1), at a concentration of about 50 mm in the cell sap of leaves with or without Al treatment (Table I).
Table I.
Al and oxalic acid concentrations in the leaves, roots, and cell sap of buckwheat treated with or without Al
| Leaves or Roots
|
Cell Sap
|
||||
|---|---|---|---|---|---|
| Al | H2O | Al | Oxalic acid | ||
| mmol kg−1 fresh wt | % | mm | %a | mm | |
| Leaves | |||||
| +Al | 2.01 | 87.5 | 2.03 | 88.4 | 51.08 |
| −Al | 0.02 | 87.4 | 0.01 | – | 46.79 |
| Roots | |||||
| +Al | 3.45 | 94.7 | 2.09 | 57.3 | 8.80 |
Percent of total Al.
Figure 1.
HPLC profile of ligands in the Al complex in the crude and purified cell sap of buckwheat leaves on an ion-exclusion column. Buckwheat was intermittently exposed to 50 μm Al in 0.5 mm CaCl2, pH 4.5, for 10 d. The crude cell sap was purified four times by Sephadex G-10 column chromatography. Detection was at 425 nm. OX, Oxalic acid; Cit, citric acid; and Mal, malic acid.
The cell sap from the leaves was purified by molecular-sieve chromatography (Sephadex G-10). After four purification procedures, a pure peak having the same retention time as oxalic acid was obtained (Fig. 1), suggesting the separation of the Al-oxalate complex from other compounds; 67% of Al in the crude cell sap was recovered (Table II). The ratio of oxalic acid to Al decreased from about 25 to nearly 3. At the fourth round of purification, the ratio of oxalic acid to Al in each fraction was approximately 3 (Fig. 2). All of these findings indicate the presence of the Al-oxalate (1:3) complex in the leaves.
Table II.
Amount of Al and oxalic acid in the crude and purified cell sap of buckwheat leaves
| Sample | Al | Oxalic Acid | Oxalic Acid/Al |
|---|---|---|---|
| μmol | |||
| Crude cell sap | 8.10 | 204.32 | 25.22 |
| Sephadex G-10 | |||
| First (fractions 30–42) | 8.74 | 139.29 | 15.94 |
| Second (fractions 34–41) | 6.07 | 54.75 | 9.02 |
| Third (fractions 36–39) | 5.52 | 25.94 | 4.70 |
| Fourth (fractions 34–38) | 5.43 | 16.33 | 3.01 |
Figure 2.
Molecular-sieve Sephadex G-10 chromatography (fourth) of the cell sap of buckwheat leaves. The crude cell sap was prepared from the leaves of buckwheat intermittently exposed to 50 μm Al in 0.5 mm CaCl2, pH 4.5, for 10 d. Dilute HClO4 solution, pH 4.6, was used as the eluant, and fractions (3 mL each) were collected at a flow rate of 0.72 mL min−1. □, Al; ⋄, oxalic acid.
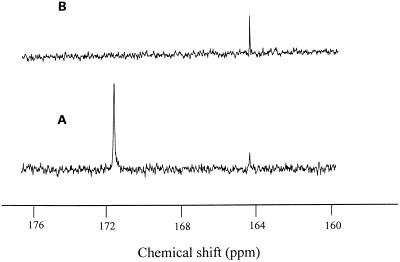
To further identify the structure of the purified Al complex from the leaves, the purified cell sap was subjected to 13C-NMR. The complex of Al-oxalate at 1:25 gave two peaks at a chemical shift of 164.4 and 171.6 ppm at 13C-NMR spectra (Fig. 3A). However, only one signal at a chemical shift of 164.4 ppm was observed in purified cell sap (Fig. 3B). The signal at a chemical shift of 171.6 was assigned to the free carboxylic group of oxalic acid, whereas the signal at 164.4 ppm was assigned to the Al-chelated carboxylic group of oxalic acid, confirming that the Al complex in the purified cell sap was Al-oxalate at 1:3. Furthermore, the complex in the purified cell sap was separated into the ligand and Al by passage through a cation-exchange resin column, followed by passage through an anion-exchange resin column. Negative-ion fast-atom-bombardment MS of the anionic fraction exhibited the pseudomolecular ion peak [M-H]− at m/z 89 (data not shown), indicating that the ligand chelated with Al in buckwheat leaves is oxalic acid.
Figure 3.
13C-NMR spectra of Al-oxalate (1:25) complex (A) and the purified cell sap of buckwheat leaves (B). Buckwheat was intermittently exposed to 50 μm Al in 0.5 mm CaCl2, pH 4.5, for 10 d. The crude cell sap was purified four times by Sephadex G-10 column chromatography. Spectra were measured at 150.8 MHz. See Methods for the purification process and measurement conditions.
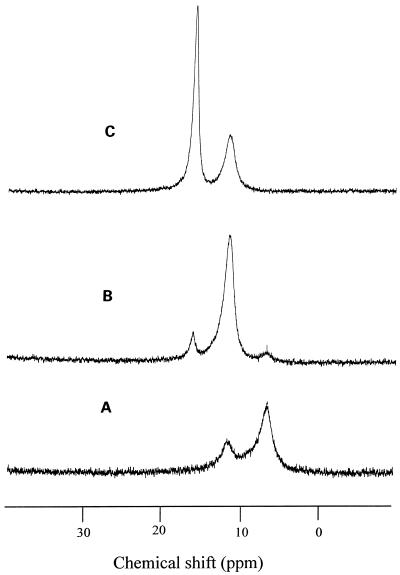
To investigate the form of Al in the intact roots, the 27Al-NMR spectra were measured. No signal was observed in the roots without Al treatment (Fig. 4A), but one major signal at the chemical shift of 16.0 to 17.0 ppm with a minor signal at the chemical shift of 11.0 to 12.0 ppm was observed in the intact roots exposed to the Al solution for 20 h (Fig. 4B). Crude cell sap of the roots gave a similar chemical shift of 27Al as that in intact tissues (Fig. 4C), suggesting that the Al form in the intact roots and in their cell sap was the same and that the Al was in a hexacoordinated complex (Haraguchi and Fujiwara, 1969). The chemical shift of major signal in the roots was similar to that observed in the leaves (Ma et al., 1997b). Since the chemical shift suggested that the Al may be chelated with organic acids, the organic acids in the cell sap were analyzed by HPLC. The major organic acid was oxalic acid at a concentration of 8.8 mm in the cell sap of the roots (Table I).
Figure 4.
27Al-NMR spectra of intact roots exposed to 0 (A) or 50 μm (B) Al for 20 h or the cell sap (C) extracted from the roots exposed to 50 μm Al for 20 h. Spectra were measured at 156.3 MHz. See Methods for measurement conditions.
Because oxalic acid can form a complex with Al at an Al:oxalic acid molar ratio of 1:1, 1:2, or 1:3, the chemical shifts for these three Al complexes were compared under the same pH as the cell sap (pH 4.6). Signals were observed at chemical shifts of 6.5, 11.6, and 16.1 ppm (Fig. 5), which are assigned to the 1:1, 1:2, and 1:3 Al-oxalate complex, respectively (Kerven et al., 1995). This suggests that the Al in buckwheat roots is present in the form of Al-oxalate complexes at a molar ratio of 1:3 and 1:2.
Figure 5.
27Al-NMR spectra of Al-oxalate complexes with a 1:1 (A), 1:2 (B), or 1:3 (C) molar ratio of Al to oxalic acid. The pH of the solution was 4.6. Spectra were measured at 156.3 MHz.
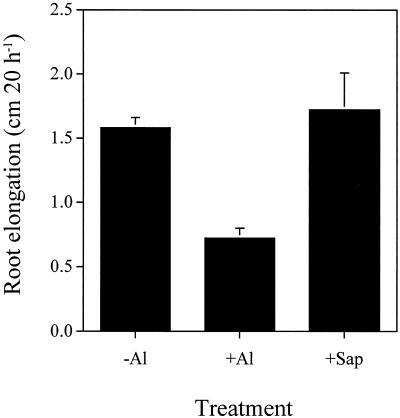
The primary response of plants to Al toxicity is the inhibition of root elongation, which occurs within several hours after Al treatment (Ryan et al., 1993). The toxicity of the purified cell sap was tested by investigating the effect on the root elongation of corn. A 20-h treatment with 20 μm AlCl3 in 100 μm CaCl2 solution inhibited the elongation of corn roots by 50%, whereas the purified cell sap containing the same concentration of Al (20 μm) did not inhibit root elongation (Fig. 6). This suggests that the purified Al complex is not phytotoxic.
Figure 6.
Effect of the purified cell sap of buckwheat leaves on the root elongation of corn. Roots were exposed to 100 μm CaCl2 solution containing 0 (−Al) or 20 μm AlCl3 (+Al) or 20 μm Al complex purified from the cell sap of buckwheat leaves. The treatment period was 20 h. Values are means ± sd of 10 replicates.
The toxicity mechanism of Al is still unknown, but the binding of Al to cellular components such as the cell wall, plasma membrane, DNA, enzymes, ATP, and others is considered to be the initial step in Al toxicity (Kochian, 1995). Binding of Al-oxalate complexes with different ratios of Al to oxalic acid to roots was investigated by staining with Eriochrome Cyanine R using cv Scout 66, an Al-sensitive cultivar of wheat. Heavy staining was observed in the root apex treated with Al3+, but no staining was observed in the roots treated with 1:2 and 1:3 Al-oxalate complexes even though roots treated with 1:1 Al-oxalate were also stained (Fig. 7). The 1:3 complex did not inhibit the root elongation at all, but a 20-h exposure to 20 μm AlCl3 inhibited it by 90% (data not shown). This suggests that oxalic acid prevents binding of Al to the cellular components, thereby detoxifying Al.
Figure 7.
Staining patterns of wheat cv Scout 66 roots exposed to Al-oxalate complexes with different molar ratios of Al to oxalic acid. Roots were exposed to 100 μm CaCl2 solution containing 0 (−Al) or 20 μm AlCl3 (+Al) or 20 μm 1:1, 1:2, or 1:3 Al-oxalate complexes. After 20 h the roots were stained with Eriochome Cyanine R. Pink color shows the binding of Al to the root apex.
DISCUSSION
Several potential mechanisms of internal tolerance for Al have been suggested, including chelation in the cytosol, compartmentation in the vacuole, Al-binding proteins, evolution of Al-tolerant enzymes, and elevated enzyme activity (Taylor, 1991; Kochian, 1995). However, there is still little evidence supporting these mechanisms. Because Al3+ has a high affinity for cellular components, forms other than Al3+ must be present in Al-tolerant plants. However, very little is known about the Al form in plants. In tea leaves most of the Al was proposed to be bound to catechins, based on the observed signal on the 27Al-NMR spectrum (Nagata et al., 1992). However, such a complex has not been isolated or identified. In the vacuoles of the mycorrhizal basidiomycete Laccaria bicolor, Al was present in the form of polyphosphate complexes (Martin et al., 1994). Previously, we found that more than two-thirds of the Al in hydrangea leaves was present in the cell sap in a soluble form, and this Al form has been identified as Al-citrate complex at a 1:1 molar ratio of Al to citric acid (Ma et al., 1997a). We therefore suggest that internal detoxification of Al in hydrangea leaves is achieved by the formation of the Al-citrate complex, a nonphytotoxic form.
Because of the high binding ability of Al3+ with cellular components of roots, Al is not usually translocated to the upper parts of plants even when the root growth is severely inhibited. The high Al concentration in buckwheat leaves suggests that this cultivar accumulated Al in the leaves (Table I). It is noteworthy that about 90% of the Al was present in the cell sap in a soluble form and that the Al concentration in the cell sap was as high as 2 mm. An 27Al-NMR study revealed a sharp signal at chemical shift 16.0 to 17.0 ppm in both the intact leaves and extracted cell sap (Ma et al., 1997b), which was apparently different from those observed in hydrangea leaves (broad signal at chemical shift 11–12 ppm) but was consistent with that of Al-oxalate complex at 1:3 ratio. Four purifications by Sephadex G-10 resulted in a complex with a molar ratio of Al to oxalic acid of 1:3 (Table II; Fig. 2). The ligand chelated with Al had the same retention time as oxalic acid on HPLC (Fig. 1). A 13C-NMR study revealed that the chemical shift of purified Al complex was consistent with that of a 1:3 Al-oxalate complex (Fig. 3). All of these results suggested that the Al in buckwheat leaves was complexed with oxalic acid at a molar ratio of 1:3.
Oxalic acid was the dominant organic acid in buckwheat leaves; the concentration in the cell sap was 50 mm (Table I). Although it is the simplest dicarboxylic acid, oxalic acid can form a bidentate complex through its two carboxyl functional groups. Formation of a stable five-ring structure with Al results in its strong chelation (Hue et al., 1986). Oxalic acid can form three species of complexes with Al at an Al to oxalic acid molar ratio of 1:1, 1:2, and 1:3, but 1:3 Al-oxalate complex is the most stable, with a stability constant of 12.4 (Nordstrom and May, 1996). This stability constant is much higher than that of Al-citrate (8.1) or Al-ATP (10.9; Martin, 1988), meaning that formation of a 1:3 Al-oxalate complex can prevent binding of Al to cellular components, thereby detoxifying Al.
The detoxifying effect of Al by oxalic acid has been reported (Hue et al., 1986), and we suggest here that the extent of Al detoxification depends on the molar ratio of Al to oxalic acid. Two times more oxalic acid than Al did not inhibit root elongation of cotton, but complexes with less than this ratio inhibited the root elongation (Hue et al., 1986). The Al-oxalate complex at 1:2 did not inhibit root elongation of corn at all (Zheng et al., 1998b). In the present study the purified cell sap containing a 1:3 Al-oxalate complex did not show toxicity affecting elongation in corn roots (Fig. 6). The staining results clearly showed that the 1:1 Al-oxalate complex was bound to the root apex of an Al-sensitive cultivar of wheat (Scout 66), whereas no binding of Al to the roots was observed upon exposure to an Al-oxalate complex with a molar ratio of oxalic acid to Al higher than 2 (Fig. 7). All of these findings indicate that the 1:3 Al-oxalate complex is nontoxic. The difference in Al-oxalate complexes with different ratios in detoxifying Al can be attributable to their different stability constants, which result in different activities of free Al3+.
Oxalic acid is a widely occurring natural product of plants, but little is known about its metabolism and function (Libert and Franceschi, 1987). Because there was no big difference in the concentration of oxalic acid in the cell sap between leaves treated with Al and those not treated with Al (Table I), it is unlikely that de novo biosynthesis of oxalic acid was induced by Al. Al was also mainly present in the form of a 1:3 complex in buckwheat roots based on the chemical shift of 27Al (Fig. 4). Thus, the Al complex in the leaves may be translocated from the roots, although the form of translocation remains to be identified.
Buckwheat roots responded to Al stress by rapid secretion of oxalic acid (Ma et al., 1997b). One possibility of Al entering the roots is that secreted oxalic acid chelates with external Al, and then the Al-oxalate is taken up by the roots. However, we found that more Al was accumulated in the leaves when the roots were exposed to the AlCl3 solution compared with roots exposed to the Al-oxalate complex at 1:3 (data not shown). This result suggests that some of Al3+ may be directly transported into the cell, which then forms a complex with internal oxalic acid. Further research is needed to confirm this.
Buckwheat shows a high Al resistance; relatively long-term treatment (10 d) with 50 μm Al in a 0.5 mm CaCl2 solution, pH 4.5, did not affect the growth of the root or shoot of buckwheat, but the same treatment significantly inhibited the root growth of wheat (cvs Atlas 66 and Scout 66), oat (Avena sativa L. cvs Tochiyutaka and Heoats), and oilseed rape (Brassica rapus L. cvs 94008 and H166; Zheng et al., 1998a). Short-term Al treatment (16 h) also showed that buckwheat had higher resistance to Al compared with an Al-tolerant cultivar of wheat, Atlas 66 (Zheng et al., 1998b). One of the mechanisms responsible for this high resistance in buckwheat is proposed to be rapid and specific secretion of oxalic acid by the roots (Ma et al., 1997b; Zheng et al., 1998b). The secretion of oxalic acid can prevent Al3+ from entering the roots. However, recent studies indicate that a fraction of the Al enters the root symplasm fairly rapidly (Lazof et al., 1994) and is thought to interact at many cellular sites (Kochian, 1995). This suggests that internal tolerance mechanisms are also required for high Al resistance. Internal detoxification of Al by the formation of a 1:3 Al-oxalate complex in both the roots and leaves is also responsible for high Al resistance in buckwheat.
In conclusion, buckwheat accumulated Al in the form of a 1:3 Al-oxalate complex, a nonphytotoxic form, in both roots and shoots. Secretion of oxalic acid from the roots and accumulation of Al in a nontoxic form in the leaves indicate that both internal and external Al-detoxification mechanisms contribute to the high Al resistance seen in buckwheat.
ACKNOWLEDGMENT
We are grateful to Dr. Hideo Naoki (Suntory Institute for Bioorganic Research, Osaka, Japan) for fast-atom bombardment-MS measurement.
Footnotes
This study was supported in part by a grant-in-aid for Scientific Research, for Encouragement of Young Scientists, for Creative Basic Research, and for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan, by a Sunbor grant, and by the Ohara Foundation for Agricultural Sciences.
LITERATURE CITED
- Cuenca G, Herrera R, Medina E. Aluminum tolerance in trees of a tropical cloud forest. Plant Soil. 1990;125:169–175. [Google Scholar]
- Haraguchi H, Fujiwara S. Aluminum complexes in solution as studied by aluminum-27. J Phys Chem. 1969;73:3467–3473. [Google Scholar]
- Haug A. Molecular aspects of aluminum toxicity. CRC Crit Rev Plant Sci. 1984;1:345–373. [Google Scholar]
- Hue NV, Craddock GR, Adams F. Effect of organic acids on aluminum toxicity in subsoils. Soil Sci Soc Am J. 1986;50:28–34. [Google Scholar]
- Kerven GL, Larsen PL, Bell LC, Edwards DG. Quantitative 27Al NMR spectroscopic studies of Al(III) complexes with organic acid ligands and their comparison with GEOCHEM predicated values. Plant Soil. 1995;171:35–39. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Lazof DB, Goldsmith JG, Rufty TW, Linton RW. Rapid uptake of aluminum into cells of intact soybean root tips. Plant Physiol. 1994;106:1107–1114. doi: 10.1104/pp.106.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert B, Franceschi VR. Oxalate in crop plants. J Agric Food Chem. 1987;35:926–938. [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H. Internal detoxification mechanism of Al in hydrangea. Identification of Al form in the leaves. Plant Physiol. 1997a;113:1033–1039. doi: 10.1104/pp.113.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Hiradate S, Matsumoto H. Detoxifying aluminum with buckwheat. Nature. 1997b;390:569–570. [Google Scholar]
- Martin F, Rubini P, Cote R, Kottke I. Aluminum polyphosphate complexes in the mycorrhizal basidiomycete Laccaria bicolor: a 27Al-nuclear magnetic resonance study. Planta. 1994;194:241–246. [Google Scholar]
- Martin RB. The chemistry of aluminum as related to biology and medicine. Clin Chem. 1986;32:1797–1806. [PubMed] [Google Scholar]
- Martin RB (1988) Bioinorganic chemistry of aluminum. In H Sigel, A Sigel, eds, Metal Ions in Biological Systems: Aluminum and Its Role in Biology, Vol 24. Marcel Dekker, New York, pp 1–57
- Matsumoto H, Hirasawa E, Morimura S, Takahashi E. Localization of aluminum in tea leaves. Plant Cell Physiol. 1976;17:627–631. [Google Scholar]
- Nagata T, Hayatsu M, Kosuge N. Identification of aluminum forms in tea leaves by 27Al NMR. Phytochemistry. 1992;31:1215–1218. [Google Scholar]
- Nordstrom DK, May HM. Aqueous equilibrium data for mononuclear aluminum species. In: Sposito G, editor. Environment Chemistry of Aluminum. Boca Raton, FL: CRC Press; 1996. pp. 39–80. [Google Scholar]
- Osaki M, Watanabe T, Tadano T. Beneficial effect of aluminum on growth of plants adapted to low pH soils. Soil Sci Plant Nutr. 1997;43:551–563. [Google Scholar]
- Ryan PR, DiTomaso JM, Kochian LV. Aluminum toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot. 1993;44:437–446. [Google Scholar]
- Taylor GJ. Current views of the aluminum stress response: the physiological basis of tolerance. Curr Top Plant Biochem Physiol. 1991;10:57–93. [Google Scholar]
- Zheng SJ, Ma JF, Matsumoto H (1998a) Continuous secretion of organic acid is related to aluminum resistance in relatively long-term exposure to aluminum stress. Physiol Plant (in press)
- Zheng SJ, Ma, JF, Matsumoto H (1998b) High aluminum resistance in buckwheat. I. Aluminum-induced specific secretion of oxalic acid from root tips. Plant Physiol 117: 745–751 [DOI] [PMC free article] [PubMed]