Abstract
Meckel's diverticulum (MD) is the most common congenital anomaly of the gastrointestinal (GI) tract, affecting about 2% of the population. Most cases of Meckel's diverticula are asymptomatic. The diagnosis of symptomatic MD is often difficult to make. We report the case of an 8-year-old boy who presented with GI bleeding due to MD. The diagnostic difficulties after an initial negative endoscopic evaluation and the diagnostic value of the various endoscopic procedures are discussed. The patient had suffered from bright red stools for 20 h before hospital admission. GI scintigraphy with 99mTc-Na-pertechnetate was negative for heterotopic gastric tissue in the small bowel area. Colonoscopy performed in order to exclude Crohn's disease was also negative. He was placed on ranitidine at a dose of 6 mg/kg body weight twice daily. The patient remained asymptomatic over a period of 6 months before he was readmitted due to macroscopic rectal bleeding. Upper endoscopy and colonoscopy used to investigate the source of bleeding showed normal macroscopic findings. Radiolabeling of blood constituents with 99mTc on delayed imaging showed radionucleotide concentration in the ascending and transverse colon suggestive of a lesion in the ileocecal area. Further investigation with the use of wireless capsule endoscopy revealed a MD. Wireless capsule endoscopy may thus be indicated for patients with GI blood loss when other diagnostic methods, such as upper and lower endoscopy and colonoscopy, have failed to identify the source of bleeding.
Key words: Meckel's diverticulum, Gastrointestinal bleeding, Children, Wireless capsule endoscopy
Introduction
Meckel's diverticulum (MD) is an embryologic abnormality that is part of a spectrum of anomalies known as yolk stalk or omphalomesenteric duct remnants. It was first described by Fabricius Hildanus in the sixteenth century and later named after Johann Friedrich Meckel, who described the embryological origin of this type of diverticulum in 1809 [1]. MD is the most common congenital abnormality of the small intestine. The prevalence of MD is usually noted to be approximately 2% of the population, but published series range from 0.2 to 4%. MD is a true congenital diverticulum containing all three layers of the bowel wall. It is a vestigial remnant of the omphalomesenteric duct. Although it affects 2–4% of the general population, symptomatic cases are just 4–16%. Over 60% of patients are up to 2 years old and 15% over 4 years of age. Males are more likely to be affected than females (3:1) [2]. The diverticulum arises from the antimesenteric border of the ileum, 40–100 cm from the ileocecal valve. Its length is 2–5 cm and has a diameter of approximately 2 cm [2, 3]. More than 50% of the diverticula harbor heterotopic mucosa.
Most cases of MD are asymptomatic (95–98%) and are usually discovered incidentally. The lifetime probability of a MD becoming symptomatic is 4.2–6.4%. The lifetime risk of complications is estimated to be about 4–40% [4]. Most of the children have their first symptoms when they are less than 5 years of age (median age 2 years). It is rarely seen in older children or adults. The most common complication of MD is gastrointestinal (GI) bleeding. The classic presentation in young children is considered to be painless rectal bleeding. Ectopic tissue is found up to 55% of Meckel's diverticula. Gastric and pancreatic tissues predominate, with corresponding indices of 60–85% and 5–16%.
The diagnosis of MD must be considered in anyone with unexplained abdominal complaints, nausea and vomiting, or intestinal bleeding. It can be asymptomatic or mimic common abdominal disorders such as Crohn's disease, appendicitis, peptic ulcer disease, polyps, hemorrhoids and hemangiomatosis of the intestine [5, 6]. Although MD is the most prevalent congenital abnormality of the GI tract, it is often difficult to diagnose. 99mTc-Na-pertechnetate scintigraphy appears to be the diagnostic study of choice if MD is suspected and the patient is clinically stable. However, alternative diagnostic techniques are in some cases required to overcome the limitations of the standard diagnostic tests and their use is necessary in order to confirm the diagnosis [6, 7].
The choice of diagnostic method depends on symptoms, clinical suspicion and availability of the diagnostic method. 99mTc-pertechnetate scan is the investigation of choice to diagnose Meckel's diverticula. The concentration of 99mTc-Na-pertechnetate by gastric parietal cells has been used effectively to detect the presence of gastric mucosa in these ectopic sites [8]. Pertechnetate is taken up by the mucin cells of the gastric mucosa and ectopic gastric tissue. In children the scan has a sensitivity of 85% and a specificity of 95%, but in adults sensitivity falls to 62.5% and specificity to only 9% [9, 10].
Many conditions can cause a false-positive diagnosis in adults: mucosal hyperemia of any cause, angiomas, urinary tract obstruction, an ectopic kidney or uterine pooling of blood [6]. False-negative results are less common, causative conditions including impaired vascular supply, recent barium GI study, premedication with atropine, a small diverticulum or hemorrhage washing out the isotope [8, 11, 12]. Cimetidine (Tagamet) improves diagnostic accuracy by inhibiting the intraluminal release of technetium, and glucagon does so as an antiperistaltic [8, 13]. Conventional barium studies (small bowel follow-through study, enteroclysis, or retrograde ileal opacification by means of barium enema) have been largely replaced by other imaging techniques for evaluation of patients with acute symptoms [6].
We report the case of an 8-year-old boy who suffered from persistent recurrent GI bleeding. The diagnostic difficulties after an initial negative endoscopic evaluation of the patient are outlined. MD identification was achieved by wireless capsule endoscopy (WCE). The most frequent indications for performing a WCE in the evaluation of obscure GI bleeding are also discussed.
Case Report
The 8-year-old boy had been suffering from bright red loose stools for almost 20 h before hospital admission. He had a family history of Hashimoto's thyroiditis on his mother's side. With regards to his personal medical history he received a diagnosis of cow's milk allergy at the age of 15 months when he had some blood in his stools. The patient's laboratory investigation at that time revealed specific IgE antibodies to cow's milk protein of 6.4 IU/ml (normal <0.35). A milk protein elimination diet was commenced. Reintroduction of cow's milk into the patient's diet was done at the age of 2.5 years after a negative cow's milk protein challenge. At the age of 4 years he was admitted to a hospital due to recurrent abdominal pain. Laboratory investigation at that time showed negative specific IgE antibodies to cow's milk and a positive fecal occult blood test.
On clinical examination he was hemodynamically stable (blood pressure 89/48 mm Hg, heart rate 92/min). Digital rectal examination revealed deep red feces in the rectum. Stomach fluids aspirated by means of a nasogastric tube were normal. Laboratory investigation showed abnormally decreased hemoglobin levels of 7.2 mg/dl, which gradually reached normal levels on the following days, and positive fecal occult blood test. During the first day of hospitalization he was placed on ranitidine at a dose of 6 mg/kg body weight twice daily. On the second day he presented black stools although he was hemodynamically stable (blood pressure 88/47 mm Hg, heart rate 85/min). The bleeding stopped spontaneously on the following day. In order to search for the cause of GI bleeding a number of imaging studies were requested. GI scintigraphy with 99mTc-Na-pertechnetate for detection of MD was negative for heterotopic gastric tissue in the small bowel area, showing no abnormal concentration of the radionucleotide in the abdominal area (negative study for functional heterotopic gastric mucosa) (fig. 1). A small bowel follow-through procedure was indicative of edema of the terminal ileum wall, small nodular filling deficits and abnormal terminal ileum wall border (fig. 2). Colonoscopy performed in order to exclude Crohn's disease showed that the mucosa of the terminal ileum and the colonic mucosa had a nodular appearance while the macroscopic findings of the rest of the colon were normal. No evidence of GI bleeding was present. Biopsy specimens taken during the procedure showed mild cell infiltration of the colonic mucosa. Investigation with upper GI endoscopy showed mild nodular appearance of the duodenal mucosa. The histologic findings obtained during the endoscopy showed chorionic lymphocytes, plasmacytes and eosinophils which were indicative of mild nonspecific enteritis. Biochemical and other laboratory values on days 1, 3 and 14 after admission are shown in table 1.
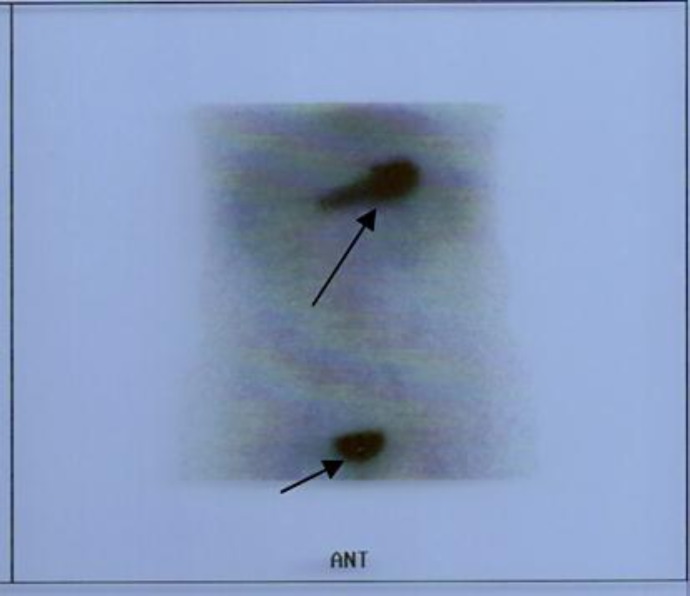
Fig. 1.
GI scintigraphy with 99mTc-Na-pertechnetate for detection of MD, showing no abnormal concentration of the radionucleotide in the abdominal area (negative study for functional heterotopic gastric mucosa) and normal concentration of the radionucleotide in the stomach and bladder (arrows).
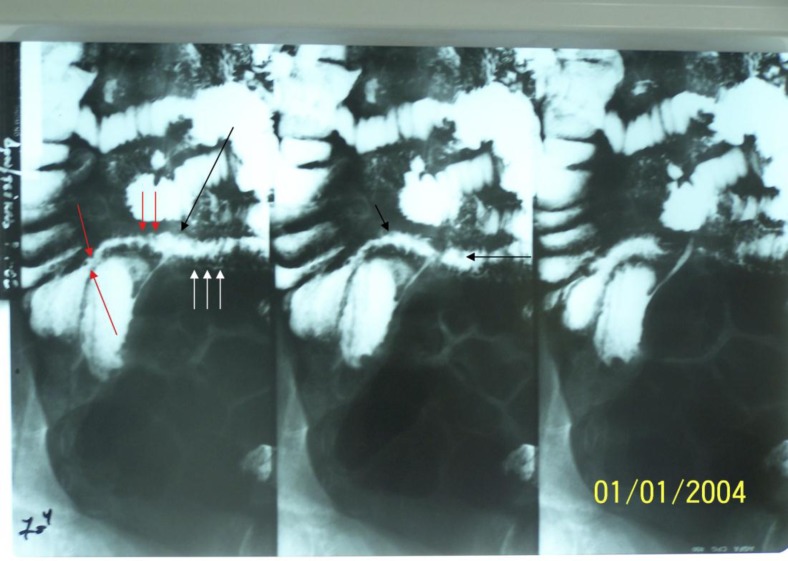
Fig. 2.
Small bowel follow-through showing edema of the terminal ileum wall (white arrows), small nodular filling deficits (black arrows) and abnormal terminal ileum wall border (red arrows).
Table 1.
Laboratory values on days 1, 3 and 14 after admission
| Day after admission | 1st | 3rd | 14th |
|---|---|---|---|
| WBC | 9,100/mm3 | 7,100/mm3 | 6,490/mm3 |
| Hb | 9.5 g/dl | 8.3 g/dl | 10.2 g/dl |
| Hct | 29.7% | 24.5% | 32.0% |
| PLT | 145,000 | 151,000 | 189,000 |
| ESR (1st hour) | 19 | 15 | |
| CRP | <3 mg/dl | <3 mg/dl | <3 mg/dl |
| PT | 11.9 | 12.3 | |
| INR | 0.99 | 1.07 | |
| Fe | 58 | 14 | 22 |
| AST | 17 | 23 | |
| ALT | 11 | 13 | |
| Fecal occult blood test | positive | negative | |
| Stool culture | negative |
Upon discharge the patient was treated with ranitidine and iron therapy of 1 month, and follow-up instructions were given for frequent clinical and laboratory evaluation. The patient remained without microscopic or macroscopic rectal bleeding and achieved hemoglobin levels of 13 g/dl over a period of 6 months before readmission due to abdominal pain and macroscopic fecal blood loss. Blood in the stools was either dark red or black. Upon admission the patient's hemoglobin level was 8.8 g/dl and decreased to 6.6 g/dl over a period of 3 days. This was managed with transfusion of concentrated erythrocytes. After transfusion the hemoglobin level was 11.8 g/dl. Biochemistry of the blood and blood clotting tests were normal. Upper GI endoscopy and colonoscopy used to investigate the source of bleeding showed normal macroscopic findings. Scintigraphy with 99mTc radiolabeled erythrocytes (99mTc-RBC) showed (first 3.5 h) normal radionucleotide concentrations in the abdominal area, making active GI hemorrhage unlikely (fig. 3a). Delayed imaging (20 h) showed radionucleotide concentration in the ascending and transverse colon, not a reliable finding because delayed appearance could be attributed to intestinal bleeding possibly from the ileocecal area (fig. 3b).
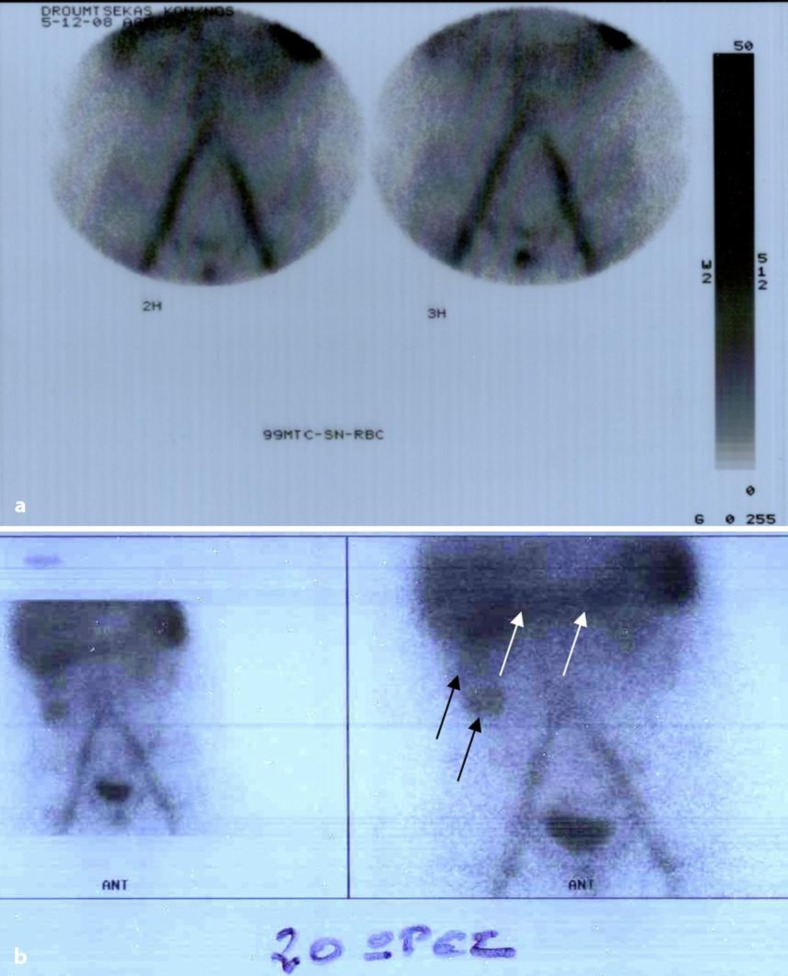
Fig. 3.
Scintigraphy with 99mTc-RBC. a First study period (3.5 h). No abnormal concentrations of the radionucleotide were seen in in the abdominal area (active GI hemorrhage unlikely). b Delayed imaging study (20 h). Radionucleotide concentration in the ascending (black arrows) and transverse colon (white arrows).
WCE was considered medically necessary in order to investigate the obscure GI bleeding suspected to be of small bowel origin. A patency capsule was used for verifying adequate patency of the GI tract prior to administration of WCE. WCE of the small intestine (fig. 4a) revealed a polypoidform lesion of the terminal ileum producing relative stenosis, thus delaying the capsule passage, which was possibly an MD (fig. 4b). The child was admitted to the Pediatric Surgery Unit of the hospital and underwent surgical resection of the MD as well as of the appendix. Biopsies of the diverticulum removed showed intestinal wall with ectopic gastric mucosa. The clinical course of the patient was excellent and no adverse events occurred.
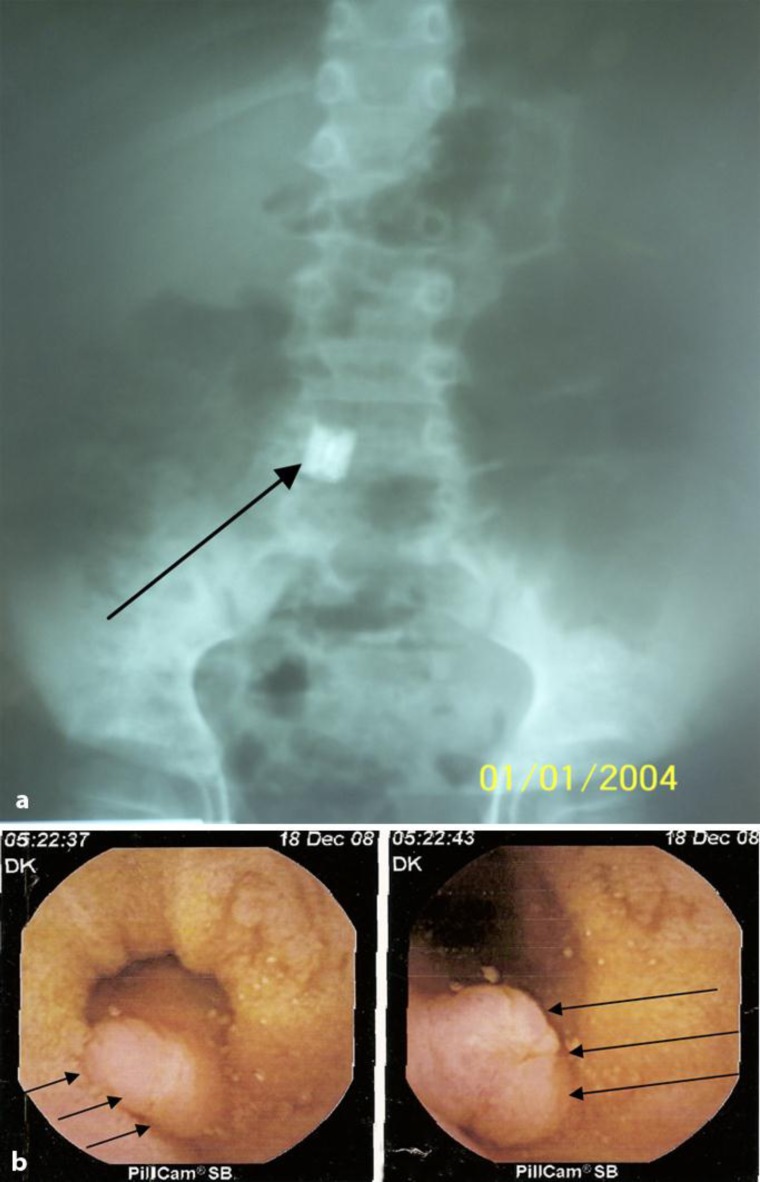
Fig. 4.
WCE. a Abdominal X-ray showing the wireless capsule in the lower abdomen (arrow). b WCE image showing the polypoidform lesion at the terminal ileum lumen (MD) (arrows).
Discussion
MD is one of the most common congenital anomalies of the GI tract. Although most cases in children are discovered incidentally during laparotomy, several complications, such as ectopic mucosal tissue leading to GI bleeding in younger children, require medical attention. The patient already described required blood transfusion when he was admitted for the second time due to GI bleeding. MD is difficult to diagnose. Despite the availability of modern imaging techniques diagnosis is challenging. 99mTc-pertechnetate imaging is a diagnostic procedure of choice to show ectopic gastric mucosa, which is present in most cases of symptomatic MD. GI scintigraphy with 99mTc-Na-pertechnetate in the small bowel area did not detect the anomaly (fig. 1). Upper and lower endoscopy in our case failed to detect the source of bleeding. Scintigraphy with 99mTc-RBC illustrated the lesion. Success in establishing a diagnosis was obtained by WCE.
Gastric mucosa is the most common heterotopic tissue found in MD (>50%) followed in pancreatic tissue (5%). Other ectopic tissues include the ileum and the large intestine [4, 5, 14, 15]. In a recent study heterotopic gastric tissue was found in 68.3% of patients with MD. In our case heterotopic gastric mucosa was found within MD [16]. The highly acidic secretions of gastric tissue can cause ulcerations that often lead to GI bleeding [3, 6]. The alkaline secretions of ectopic pancreatic tissue can also cause ulcerations [17]. Massive or intermittent bleeding may warrant blood transfusions, such as in our case.
Patients with upper GI hemorrhage often present with hematemesis or melena if the hemorrhage is severe. Upper GI endoscopy is the test used most often to look for the cause of GI bleeding. Colonoscopy is the investigation of choice in patients with rectal bleeding (dark or bright red stools). Our patient suffered from rectal bleeding, but upper and lower endoscopy failed to detect the bleeding site. Rectal bleeding may reveal itself as dark red (40%), bright red (35%), dark or bright red (12%). Rectal bleeding can also present as extremely dark stool, ranging in color from deep red to black (melena) (7%). Complications of MD include intestinal obstruction (25–40%), inflammation (diverticulitis 10–20%), volvulus, intussusception, Littré's hernia, intestinal perforation due to inflammation, ulcers, stone formation, peritonitis, omphalitis and neoplasm [16, 18, 19]. Menezes et al. [16] in 2008 reported that MD has various presentations and can be easily misdiagnosed. The records of 71 patients with a diagnosis of MD were retrospectively reviewed. In 8 patients MD was an incidental finding at laparotomy, 35 patients (55.5%) presented with episodes of rectal bleeding, 10 patients (15.8%) had clinical features of peritonitis, 9 patients (14.2%) were diagnosed as intestinal obstruction, and 9 patients (14.2%) had a patent vitello-intestinal duct and presented with umbilical discharge. Intestinal obstruction and diverticulitis are the most frequent complications in adults. In contrast, painless rectal bleeding is a common clinical feature in childhood. Our patient had manifested recurrent painless rectal bleeding before the diagnosis was confirmed.
MD is not often seen on routine barium studies because of its small ostium, filling with intestinal contents, and peristalsis with rapid emptying. Meticulous examination with enteroclysis has been reported to be more sensitive [20]. On barium studies, MD appears as a blind-ending pouch arising from the antimesenteric side of the distal ileum. Filling defects in the diverticulum may suggest gastric mucosa or tumor in adults [18]. MD may be inverted, serving as a lead point for intussusception, and appears as a soft polypoid filling defect [21]. The small bowel follow-through exam performed in our patient was not diagnostic for the anomaly.
The site of bleeding is not always identified easily on plain radiograph. Arteriography is not always diagnostic because the arterial supply of the intestine may not be impaired and it can only detect bleeding of at least 1–2 ml/min. GI scintigraphy should be done as soon as possible after the patient presents for medical care, since active bleeding is more likely at early times and is needed for correct localization. For 99mTc-RBC, if no bleeding site is identified on the initial 60–90 min dynamic images, delayed images may be acquired. These images are optional. Typically delayed images are done at 2–6 h and/or at 18–24 h after the injection of the radiopharmaceutical. Delayed images are useful in showing subsequent bleeding and categorizing the severity but may result in incorrect localization when identifying a bleeding site [22]. Delayed images performed in our patient showed radionucleotide concentration in the ascending and transverse colon, which was attributed to intestinal bleeding from the ileocecal area.
One of the newer technologies that expand the diagnostic capabilities in the small intestine is WCE. The indications for WCE in children are obscure GI bleeding and suspected Crohn's disease, though there are many other indications for the use of WCE (table 2) [23]. The WCE performed in our patient detected a polypoidform malformation in the terminal ileum (fig. 4). Surgical resection subsequently confirmed the diagnosis.
Table 2.
Pediatric indications for the use of WCE [23]
| Intestinal inflammation |
| Crohn's disease |
| Celiac disease |
| Occult or obscure intestinal bleeding |
| Vascular malformations |
| Vasculitis (Henoch-Schönlein purpura) |
| Meckel's diverticulum |
| Protein-losing enteropathies |
| Intestinal lymphangiectasia |
| Miscellaneous |
| Peutz-Jeghers syndrome |
| Familial and nonfamilial polyposis |
| Eosinophilic enteropathy |
| Food allergy |
| Mucosal injury |
| Drugs |
| Chemotherapy |
| Radiotherapy |
| Graft versus host disease |
| Malignancy |
| Chronic abdominal pain |
According to the literature there are very few adult case reports detecting MD after WCE [24, 25, 26]. In a prospective European multicenter study, children aged 1.5–7.9 years were investigated by WCE for obscure intestinal bleeding. WCE revealed 2 cases of Meckel's diverticula among 30 children who suffered from GI bleeding [27]. In conclusion, WCE is a useful tool to investigate obscure GI bleeding in children. In cases with negative prior investigation the cause of intestinal hemorrhage can be detected timely by applying this noninvasive procedure in young children.
References
- 1.Opitz JM, Schultka R, Gobbel L. Meckel on developmental pathology. Am J Med Genet A. 2006;140:115–128. doi: 10.1002/ajmg.a.31043. [DOI] [PubMed] [Google Scholar]
- 2.Satya R, O’Malley JP. Meckel diverticulum with massive bleeding. Radiology. 2005;236:836–840. doi: 10.1148/radiol.2363031026. [DOI] [PubMed] [Google Scholar]
- 3.Williams RS. Management of Meckel's diverticulum. Br J Surg. 1981;68:477–480. doi: 10.1002/bjs.1800680712. [DOI] [PubMed] [Google Scholar]
- 4.Fink AM, Alexopoulou E, Carty H. Bleeding Meckel's diverticulum in infancy: unusual scintigraphic and ultrasound appearances. Pediatr Radiol. 1995;25:155–156. doi: 10.1007/BF02010334. [DOI] [PubMed] [Google Scholar]
- 5.Turgeon DK, Barnett JL. Meckel's diverticulum. Am J Gastroenterol. 1990;85:777–781. [PubMed] [Google Scholar]
- 6.Martin JP, Connor PD, Charles K. Meckel's diverticulum. Am Fam Physician. 2000;61:1037–1042. [PubMed] [Google Scholar]
- 7.Elsayes KM, Menias CO, Harvin HJ, Francis IR. Imaging manifestations of Meckel's diverticulum. AJR Am J Roentgenol. 2007;189:81–88. doi: 10.2214/AJR.06.1257. [DOI] [PubMed] [Google Scholar]
- 8.DiGiacomo JC, Cottone FJ. Surgical treatment of Meckel's diverticulum. South Med J. 1993;86:671–675. doi: 10.1097/00007611-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Daneman A, Lobo E, Alton DJ, Shuckett B. The value of sonography, CT and air enema for detection of complicated Meckel diverticulum in children with nonspecific clinical presentation. Pediatr Radiol. 1998;28:928–932. doi: 10.1007/s002470050502. [DOI] [PubMed] [Google Scholar]
- 10.Cooney DR, Duszynski DO, Camboa E, Karp MP, Jewett TC., Jr The abdominal technetium scan (a decade of experience) J Pediatr Surg. 1982;17:611–619. doi: 10.1016/s0022-3468(82)80121-8. [DOI] [PubMed] [Google Scholar]
- 11.Garretson DC, Frederich ME. Meckel's diverticulum. Am Fam Physician. 1990;42:115–119. [PubMed] [Google Scholar]
- 12.Poulsen KA, Qvist N. Sodium pertechnetate scintigraphy in detection of Meckel's diverticulum: is it usable? Eur J Pediatr Surg. 2000;10:228–231. doi: 10.1055/s-2008-1072364. [DOI] [PubMed] [Google Scholar]
- 13.Sfakianakis GN, Conway JJ. Detection of ectopic gastric mucosa in Meckel's diverticulum and in other aberrations by scintigraphy: I. Pathophysiology and 10-year clinical experience. J Nucl Med. 1981;22:647–654. [PubMed] [Google Scholar]
- 14.Cullen JJ, Kelly KA, Moir CR, Hodge DO, Zinsmeister AR, Melton LJ., 3rd Surgical management of Meckel's diverticulum. An epidemiologic, population-based study. Ann Surg. 1994;220:564–569. doi: 10.1097/00000658-199410000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KL, Persaud TV. 5. Philadelphia: Saunders; 1993. The Developing Human; pp. 255–257. [Google Scholar]
- 16.Menezes M, Tareen F, Saeed A, Khan N, Puri P. Symptomatic Meckel's diverticulum in children: a 16-year review. Pediatr Surg Int. 2008;24:575–577. doi: 10.1007/s00383-007-2094-4. [DOI] [PubMed] [Google Scholar]
- 17.Artigas V, Calabuig R, Badia F, Rius X, Allende L, Jover J. Meckel's diverticulum: value of ectopic tissue. Am J Surg. 1986;151:631–634. doi: 10.1016/0002-9610(86)90576-3. [DOI] [PubMed] [Google Scholar]
- 18.Levy AD, Hobbs CM. From the archives of the AFIP. Meckel diverticulum: radiologic features with pathologic correlation. Radiographics. 2004;24:565–587. doi: 10.1148/rg.242035187. [DOI] [PubMed] [Google Scholar]
- 19.Kusomoto H, Yoshida M, Takahashi I, et al. Complications and diagnosis of Meckel's diverticulum in 776 patients. Am J Surg. 1992;164:382–383. doi: 10.1016/s0002-9610(05)80909-2. [DOI] [PubMed] [Google Scholar]
- 20.Maglinte DD, Elmore MF, Isenberg M, Dolan PA. Meckel diverticulum: radiologic demonstration by enteroclysis. AJR Am J Roentgenol. 1980;134:925–932. doi: 10.2214/ajr.134.5.925. [DOI] [PubMed] [Google Scholar]
- 21.Hori K, Suzuki Y, Fujimori T. Inverted Meckel's diverticulum. Surgery. 2003;133:116–117. doi: 10.1067/msy.2003.89. [DOI] [PubMed] [Google Scholar]
- 22.Ford PV, Bartold SP, Fink-Bennett DM, et al. Procedure guideline for gastrointestinal bleeding and Meckel's diverticulum scintigraphy. J Nucl Med. 1999;40:1226–1232. [PubMed] [Google Scholar]
- 23.Shamir R, Eliakim R. Capsule endoscopy in pediatric patients. World J Gastroenterol. 2008;14:4152–4155. doi: 10.3748/wjg.14.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SM, Chun HJ, Jeen YT, et al. A case of chronic gastrointestinal bleeding from a Meckel's diverticulum detected by wireless capsule endoscopy. Korean J Gastroenterol. 2004;43:125–128. [PubMed] [Google Scholar]
- 25.Mylonaki M, MacLean D, Fritscher-Ravens A, Swain P. Wireless capsule endoscopic detection of Meckel's diverticulum after nondiagnostic surgery. Endoscopy. 2002;34:1018–1020. doi: 10.1055/s-2002-35850. [DOI] [PubMed] [Google Scholar]
- 26.Sokol H, Seksik P, Wendum D, Bellanger J, Parc Y, Cosnes J. Gastrointestinal bleeding diagnosed using video capsule endoscopy. Gut. 2009;58:1206. doi: 10.1136/gut.2008.172155. [DOI] [PubMed] [Google Scholar]
- 27.Fritscher-Ravens A, Scherbakov P, Bufler P, et al. The feasibility of wireless capsule endoscopy in detecting small intestinal pathology in children under the age of 8 years: a multicentre European study. Gut. 2009;58:1467–1472. doi: 10.1136/gut.2009.177774. [DOI] [PubMed] [Google Scholar]