Abstract
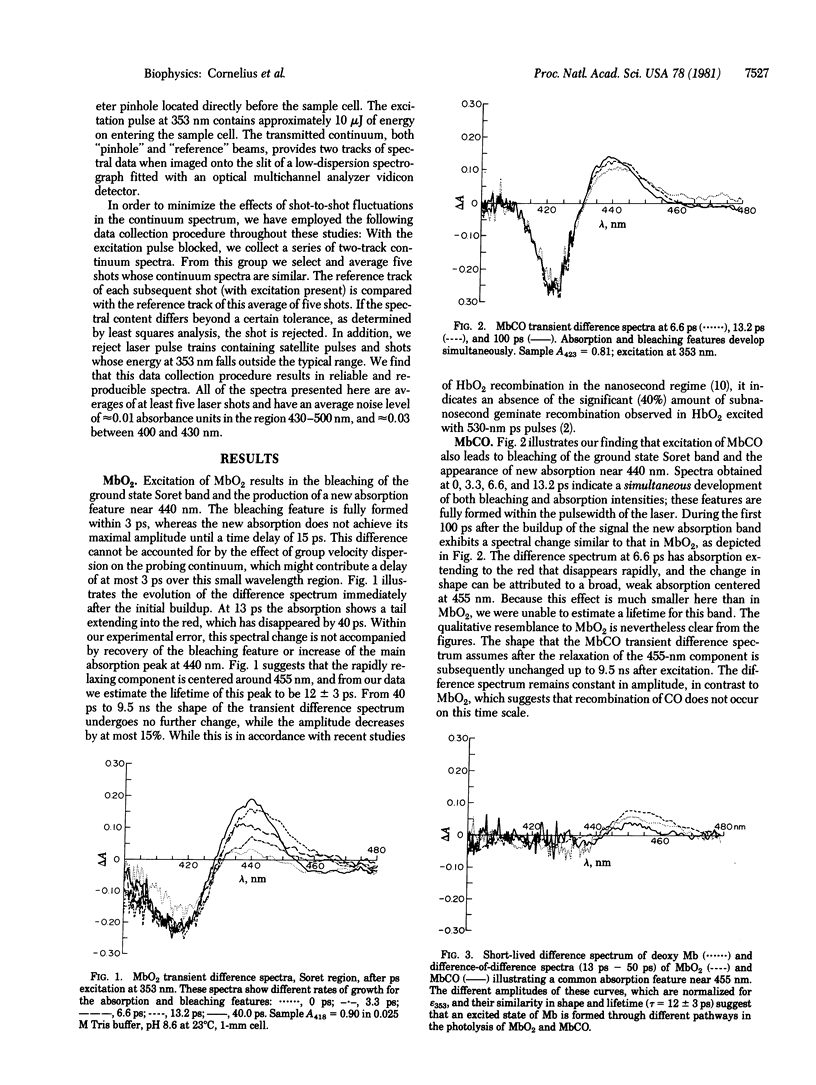
Picosecond transient absorption spectra of Mb, MbCO, and MbO2 have been studied at time delays of up to 10 ns after excitation at 353 nm. Particular attention has been paid to the rapid spectral changes that occur in the Soret region during the first 50 ps in MbCO and MbO2. In MbCO both the bleaching of the Soret peak (feature I) and the appearance of new deoxy-like absorption (feature II) occur instantaneously, whereas in MbO2 feature II is delayed with respect to feature I. A short-lived (approximately 12 ps) feature near 455 nm (feature III) was much more intense in MbO2 than in MbCO and was also identified in the transient spectrum of Mb. No evidence of subnanosecond geminate recombination was found in either MbCO or MbO2. These observations are consistent with a scheme in which MbO2 photodissociates through an excited state of Mb, whereas MbCO under the same conditions produces ground state Mb directly. The results and conclusions are compared with those of previous picosecond studies on these molecules and related hemoglobin derivatives.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert B., Banerjee R., Lindqvist L. The kinetics of conformational changes in hemoglobin, studied by laser photolysis. Proc Natl Acad Sci U S A. 1974 Feb;71(2):558–562. doi: 10.1073/pnas.71.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Chernoff D. A., Hochstrasser R. M., Steele A. W. Geminate recombination of O2 and hemoglobin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5606–5610. doi: 10.1073/pnas.77.10.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddell D. A., Morris R. J., Richards J. T. Nanosecond laser photolysis of aqueous carbon monoxy- and oxyhaemoglobin. Biochim Biophys Acta. 1980 Jan 24;621(1):1–8. doi: 10.1016/0005-2795(80)90056-2. [DOI] [PubMed] [Google Scholar]
- Eisert W. G., Degenkolb E. O., Noe L. J., Rentzepis P. M. Kinetics of carboxymyoglobin and oxymyoglobin studied by picosecond spectroscopy. Biophys J. 1979 Mar;25(3):455–464. doi: 10.1016/S0006-3495(79)85315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. M., Lyons K. B. Transient Raman study of CO-haemoprotein photolysis: origin of the quantum yield. Nature. 1980 Apr 10;284(5756):570–572. doi: 10.1038/284570a0. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., AINSWORTH S. Photosensitivity of haem compounds. Nature. 1957 Dec 21;180(4599):1416–1417. doi: 10.1038/1801416b0. [DOI] [PubMed] [Google Scholar]
- Greene B. I., Hochstrasser R. M., Weisman R. B., Eaton W. A. Spectroscopic studies of oxy- and carbonmonoxyhemoglobin after pulsed optical excitation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5255–5259. doi: 10.1073/pnas.75.11.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. H., Rand S. D., Rentzepis P. M. Mechanisms for excited state relaxation and dissociation of oxymyoglobin and carboxymyoglobin. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2292–2296. doi: 10.1073/pnas.78.4.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothgeb T. M., Gurd F. R. Physical methods for the study of myoglobin. Methods Enzymol. 1978;52:473–486. doi: 10.1016/s0076-6879(78)52052-1. [DOI] [PubMed] [Google Scholar]
- Shank C. V., Ippen E. P., Bersohn R. Time-resolved spectroscopy of hemoglobin and its complexes with subpicosecond optical pulses. Science. 1976 Jul 2;193(4247):50–51. doi: 10.1126/science.935853. [DOI] [PubMed] [Google Scholar]
- Terner J., Stong J. D., Spiro T. G., Nagumo M., Nicol M., El-Sayed M. A. Picosecond resonance Raman spectroscopic evidence for excited-state spin conversion in carbonmonoxy-hemoglobin photolysis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1313–1317. doi: 10.1073/pnas.78.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]



