Abstract
Prostate cancer (PCa) is the second leading cause of cancer related death in men in the United States, suggesting that novel molecular targets as well as the development of agents that could deregulate such targets would become newer therapeutic approach for the treatment of castrate resistant prostate cancer (CRPC) especially the metastatic CRPC (mCRPC). In search for novel targets, microRNAs (miRNAs) are becoming an emerging area because miRNAs function as regulators of gene expression in human cancers including PCa. Previous studies from our laboratory have shown that the expression of miR-34a is significantly down-regulated in human PCa specimens consistent with PCa cell lines with aggressive characteristics, and that the silencing of miR-34a expression was in part due to hypermethylation of its promoter. There are several genes that are direct targets of miR-34a, and in the current study we investigated the cellular consequence of miR-34a over-expression and under-expression in the regulation of androgen receptor (AR) and Notch-1 in PCa cells. We found that over-expression of miR-34a led to reduced expression of AR, PSA and Notch-1. We also found that over-expression of miR-34a significantly inhibited the growth of PCa cells. Moreover, over-expression of miR-34a resulted in decreased self-renewal capacity of PCa cells, and conversely inactivation of miR-34a led to increased self-renewal capacity, which is an indication of tumor cell aggressiveness. These findings suggest that the loss of miR-34a is directly linked with up-regulation of AR and Notch-1 both of which are highly expressed in PCa, and thus finding innovative approaches by which miR-34a expression could be up-regulated will have a huge impact on the treatment of PCa especially for the treatment of mCRPC.
Keywords: miR-34a, AR, Notch-1, prostate cancer, self-renewal
Introduction
Prostate cancer (PCa) is the second leading cause of cancer related death in men in the United States [1], suggesting that innovative treatment strategies are required. Prostate gland and the PCa are sensitive to androgen, mediated through the activation of androgen receptor (AR) signaling. However, patients treated with androgen ablation therapy (also known as androgen deprivation therapy) eventually develops resistance known as castrate resistant prostate cancer (CRPC), which subsequently lead to metastatic CRPC (mCRPC) for which there is no curative therapy. However, AR signaling is still known to be functional and active in mCRPC although the androgen-AR targeting agents becomes ineffective [2]. One of the reasons why AR may be deregulated and highly expressed causing CRPC or mCRPC is due to the epigenetic silencing of the miR-34a promoter activity [3]. We have found that the expression of miR-34a is silenced in PCa due to promoter hyper-methylation, but this could be easily reversed by treatment of PCa cells or PCa patients with a novel agent called BR-DIM. This agent resulted in the re-expression of miR-34a; thereby it may inhibit cancer progression by suppressing AR expression and activity [3]. Finding ways to up-regulate miR-34a may lead to new treatment for PCa patients and could possibly prevent the transformation of PCa to CRPC or mCRPC. In addition, recently it has been shown that deregulated Notch-1 (a transmembrance receptor that plays a role in cell development, differentiation, proliferation, and survival) signaling plays important role in the development of cancer and subsequent metastasis, which is in part due to the acquisition of epithelial-to-mesenchymal transition (EMT) phenotype [4,5]. Studies have shown that the silencing or inhibition of Notch-1 signaling leads to a significant reduction in cell proliferation and invasiveness of tumor cells, and increased apoptotic cell death [5]. Therefore, Notch-1 may also serve as another promising target for the treatment for PCa patients.
Emerging evidence suggests that the expression of genes such as AR or Notch-1 among many other genes could be regulated by microRNAs (miRNAs). The miRNAs are small, 18-24 nucleotide RNAs that showed to have a wide range of post-transcriptional regulation of genes in in many cellular processes such as cell proliferation, differentiation, migration, and apoptosis [6]. The miRNAs down-regulates protein expression by means of binding to the 3’UTR region in their respective target mRNAs and preventing translation or the degradation of the mRNAs [6]. Previous studies have shown that miR-34a could regulate the expression of AR and p53, and thereby leads to the inhibition of cell growth and proliferation [7]. In this way, miR-34a acts as a tumor suppressor gene. However, studies have shown that the expression of miR-34a is reduced or lost in many cancers such as ovarian, leukemia, pancreatic and colon cancers including PCa [8]. In prostate cancer, the expression of miR-34a appears to be epigenetically silenced due to hyper-methylation of miR- 34a promoter, although other factors such as disruption of the p53 pathway may also be the cause of marked reduction in miR-34a expression [3]. It has also been shown that CD44 (+) prostate cancer cells which are enriched in prostate cancer stem cells (CSCs), under-express miR-34a. When miR-34a was over-expressed, prostate CSCs were inhibited indicating the valuable role miR-34a in PCa [9].
Previous studies from our laboratory have shown that the expression of miR-34a is significantly down-regulated in human PCa specimens consistent with PCa cell lines with aggressive characteristics, and that the silencing of miR-34a expression was in part due to hypermethylation of its promoter [3]. In the current study, we have deregulated the expression of miR-34a by transfection in human PCa cell lines (C4-2B, CWR22rv1, LNCaP, and VCaP cells) and assessed the cellular consequence in the expression of AR, PSA, and Notch-1 as well as investigated the cellular morphology, cell growth, and self-renewal capacity. We found that over-expression of miR-34a led to reduced expression of AR, PSA and Notch-1. We also found that over-expression of miR-34a significantly inhibited the growth of PCa cells. Moreover, over-expression of miR-34a resulted in decreased self-renewal capacity of PCa cells, and conversely inactivation of miR-34a led to increased self-renewal capacity. These findings suggest that the loss of miR-34a is directly linked with up-regulation of AR, PSA and Notch-1, and thus finding therapeutic avenues by which miR-34a expression could be restored would become a newer therapy for the treatment of PCa in general and mCRPC in particular for which novel therapeutics are urgently needed.
Materials and methods
Cell culture and conditions
All four cell lines (LNCaP, VCaP, C4-2B, and CWR22rv1) were maintained in a 5% CO2-humidified atmosphere at 37°C. The media used for cell culture consisted of RPMI 1640 (Invitrogen, Carlsbad, CA) along with 10% fetal bovine serum (FBS). In addition, the media contained 50 units/ml Penicillin and 50 μg/ml Streptomycin. All four cell lines were maintained using the same media conditions except LNCaP cell media which included 10nM testosterone. When seeding the cells in preparation for transfection, the cells were maintained in antibiotic-free RPMI 1640 media.
Transfection of miRNAs
Cells were seeded in 6 well culture plates. After 24 hours of incubation, the cells were transfected with 30 nM pre-miR34a and/or a miR-34a inhibitor (anti-miR-34a) along with their respective controls (Ambion, Austin, TX) using DharmaFECT3 transfection reagent (DHARMACON, Lafayette, CO). The cells were retransfected after three days of transfection. After six days of first transfection, the cells were harvested. The total RNAs and proteins from the cells were extracted for RT-PCR analysis and for Western blot analysis.
Real-time RT-PCR
RT-PCR was performed in order to evaluate the expression of miR-34a in respective cell lines in addition to looking at specific mRNA expressions. First, total RNA extraction was done using the miRNeasy Kit (Qiagen) along with removing the DNA using RNase-free DNAase (Qiagen) according to the manufacturer’s instruction. Then, a miRNA assay was done using 1ng of total RNA which was reverse transcribed into cDNA, followed by real time PCR. Taqman and specific miR-34a primers (Applied Biosystems) were utilized in order to perform RT-PCR and to quantify miR-34a expression. The amount of miR-34a was normalized to the expression of RNU48. When looking for mRNA expression, one microgram of total RNA was reverse transcribed to cDNA using SYBR Green PCR reagents and specific primers (AR, PSA, Notch-1, and GAPDH-control), and subsequently used for real time PCR according to manufacturer’s instructions. The relative amount of mRNA was normalized to the expression of GAPDH expression.
Western blot analysis
Protein extraction was first done by lysing the cells in RIPA buffer followed by the BCA protein assay in order to determine protein concentration. Western blotting was performed as previously described [10], with the only change being the antibodies used. The proteins were tested with the following primary antibodies: AR, PSA, Notch-1, and GAPDH, all of which were purchased from Santa Cruz (Santa Cruz, CA). After washing the membrane, the respective secondary antibodies were applied, and the signal was detected as described earlier [10].
Cell proliferation assay by MTT
PCa cells were seeded in 96 well culture plates. After 24 hours of incubation, the cells were transfected with 30 nM pre-miR34a and/or a miR-34a inhibitor (anti-miR-34a) along with their respective controls as described earlier. After six days of first transfection, the cells were subjected to MTT assay as described previously [11]. The growth inhibition of PCa cells after miRNA transfection was calculated using GraphPad Prism software (GraphPad Software Inc).
Cell renewal capacity assay
C4-2B and CWR22rv1 cells were seeded in 6 well culture plates. After 24 hours of incubation, the cells were transfected with 30 nM premiR34a or 30 nM miR-34a inhibitor along with their respective controls and re-transfected after 3 days as described earlier. After six days of first transfection, the effects of miR-34a on sphere-forming ability of the transfected cells were detected by self renewal capacity assay. Single cell suspensions of transfected cells were plated in the wells of a 6-well ultra-low adherent plate at 2000 cells/well in DMEM/F12 supplemented with B27 and N2 (Invitrogen). Fresh medium was added after 3 days of seeding. After 6 days of seeding, the numbers of prostaspheres were counted. Prostaspheres were also photographed under a phase contrast microscope and the size of the prostaspheres diameter was measured.
Statistical methods
RT-PCR data for both miR-34a expression and for mRNA expression were quantified through means of the comparative Ct method. We compared the relative amount of the target genes to GAPDH which served as reference control. The results in the graphs shown are as relative values compared to GAPDH.
Results
The miR-34a down-regulates AR expression in PCa cells
Prostate cancer (PCa) cells and their stem cells have been shown to have a marked reduction in the expression of miR-34a, which is in part responsible for the up-regulation of AR expression [3,12]. In the current study, we chose to overe-xpress miR-34a and under-express it by transfection in order to investigate the subsequent effects on AR expression in four different PCa cell lines such as C4-2B, CWR22rv1, LNCaP and VCaP. Figure 1 shows the relative amount of miR-34a in each cell line after six days of transfection. As shown in Figure 1, the transfection was effective in increasing the levels of miR-34a, and further experiments in the down-regulation of miR-34a expression showed decreasing the expression of miR-34a after transfecting the cells with miR-34a inhibitor (anti-miR-34a). After a 6-day transfection with miR-34a mimetic, the C4-2B and CWR22rv1 cell lines showed a reduction in mRNA and protein expression of AR (Figures 2A, 2D, 3A, and 3C) consistent with increased expression of miR-34a. This reduction in the expression of AR was also consistent with the down-regulation of AR downstream gene, prostate specific antigen (PSA) in C4-2B cell line (Figure 2C). VCAP cell line also showed similar reduction in the amount of AR and PSA transcripts after only a 3-day transfection of miR-34a (Figure 4A and 4B). This finding confirms that AR is a direct target of miR-34a. However, transfection with a miR-34a inhibitor in order to under-express miR-34a had no significant effects on AR in all four cell lines, which could indeed be due to low basal level expression of miR-34a in PCa cells.
Figure 1.
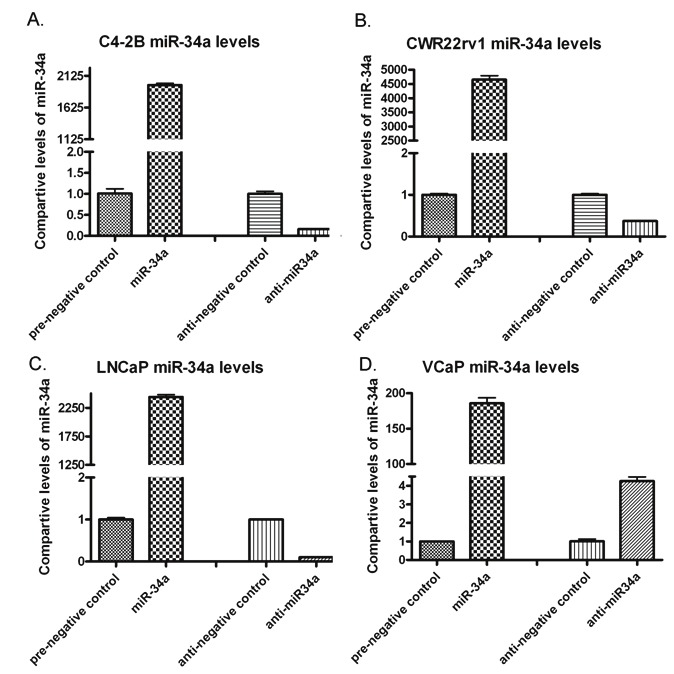
Comparative levels of miR-34a after 6-days of transfection of pre-miR-34a in C4-2B (A), CWR22rv1 (B), LNCaP (C), and VCaP (D) cell lines.
Figure 2.
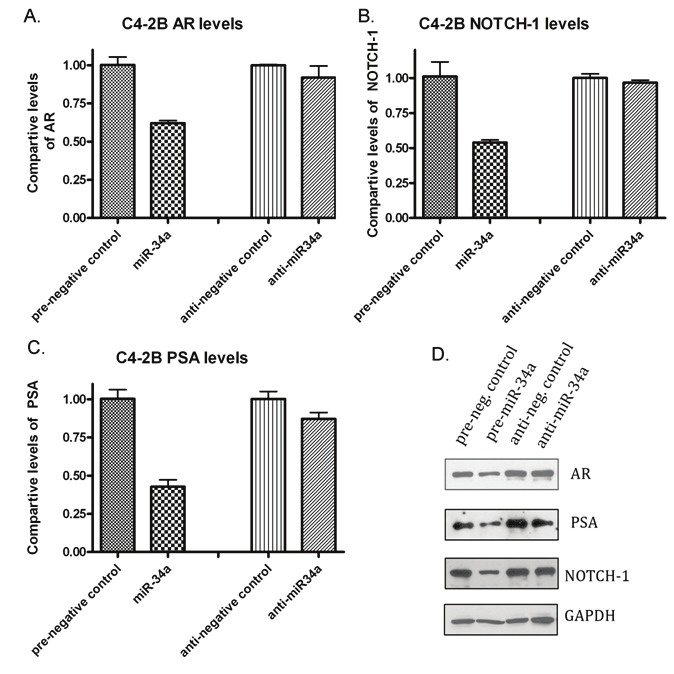
Effects of over-expression of miR-34a in the C4-2B PCa cell line. A) miR-34a led to a decrease in the expression of AR mRNA compared to the respective negative control; however, inhibition of miR-34a seemed to have no effects on AR expression. B) Over-expression of miR-34a significantly decreased the expression of Notch-1 mRNA compared to the control. C) PSA levels were also decreased after transfection with pre-miR-34a. D) Western blot analysis showed a relative decrease in the protein expression of AR, PSA, and Notch-1 compared to respective controls in C4-2B cells with over-expression of miR-34a.
Figure 3.
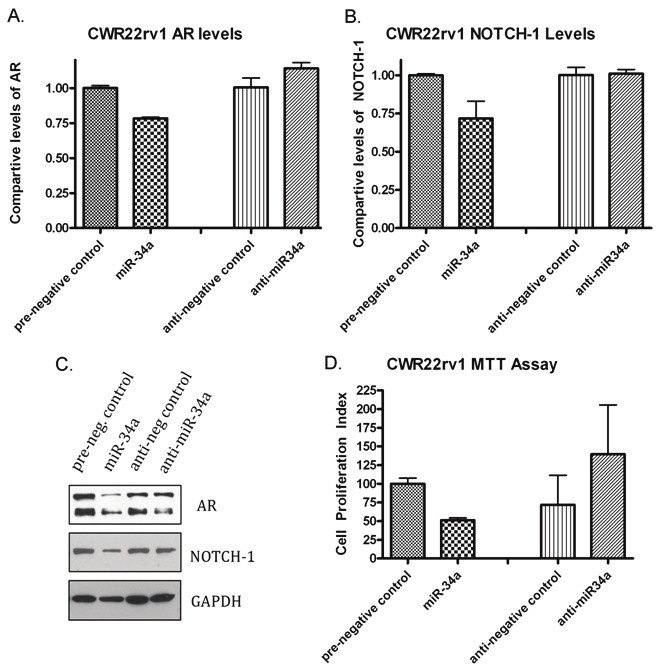
RT-PCR and Western blot analysis after over-expression of miR-34a in the CWR22rv1 cell line. A) Data from RT-PCR showing down-regulation of AR in cells transfected with pre-miR-34a, and showed an increase in the expression of AR when miR-34a was under-expressed with miR-34a inhibitor (anti-miR-34a) transfection. B) RT-PCR data also showing a decrease in the level of the Notch-1 transcripts in cells over-expressing miR-34a compared to the respective negative control. C) Western blot analysis showing a marked reduction in the protein expression of AR and Notch-1 when compared to their respective controls. D) MTT Assay showing decreased cell growth after transfection with pre-miR-34a, and an increase in cell growth after inhibiting miR-34a expression.
Figure 4.
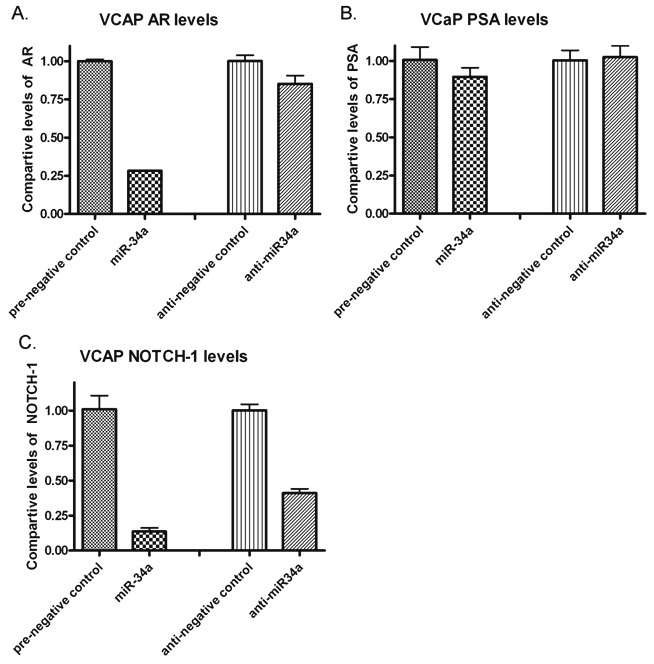
Effects of miR-34a in the VCaP cell line after only a 3 day transfection. A) After a 3-day transfection of pre-miR-34a, RT-PCR showed a significant decrease in the level of the AR transcript with respect to the negative control. B) miR-34a transfected VCaP cells also showed a slight decrease in the production of PSA, whereas the miR-34a inhibitor caused a little increase in the PSA levels. C) RT-PCR data also showed a decrease in the level of the Notch-1 transcripts in cells over-expressing miR-34a compared to the respective negative control.
Notch-1 was down-regulated by miR-34a
Studies have shown that high level of Notch-1 expression is prevalent in PCa. After transfecting all four cell lines with miR-34a, the expression of Notch-1 showed a marked reduction in its expression. This finding indicates that miR-34a down-regulates Notch-1 expression at the mRNA levels. RT-PCR for mRNA expression analysis of Notch-1 shows a decrease in Notch-1 mRNA compared to the negative control in the C4-2B and CWR22rv1 cell lines (Figures 2B and 3B). This data was correlated with western blot analysis showing decreased expression of Notch-1 protein (Figures 2D and 3B). However, transfection with the miR-34a inhibitor showed no effects on Notch-1 expression when compared to the respective negative control.
Re-expression of miR-34a decreased cell proliferation and altered cellular morphology
The MTT Assay was performed uisng CWR22rv1 cell line after a 6-day transfection with miR-34a in order to assess whether miR-34a could have any effect on cell growth and viability. The data showed that miR-34a caused reduced cell growth and also showed reduced number of cells whereas the miR-34a inhibitor caused an increase in the number of cells (Figure 3D). Not only did miR-34a showed reduced cell viability and growth, but it also led to a marked alterations in the cellular morphology. The cells after a 3-day transfection appeared to be undergoing apoptosis as evident by the changes in the cell shape. C4-2B cells normally show fibroblast-type morphology but after transfection with miR-34a, the cells showed more rounded phenotype. In addition, we observed lots of cell death when compared to control cells (Figure 5A). VCAP cells were also affected similarly after transfection of miR-34a (Figure 5B).
Figure 5.
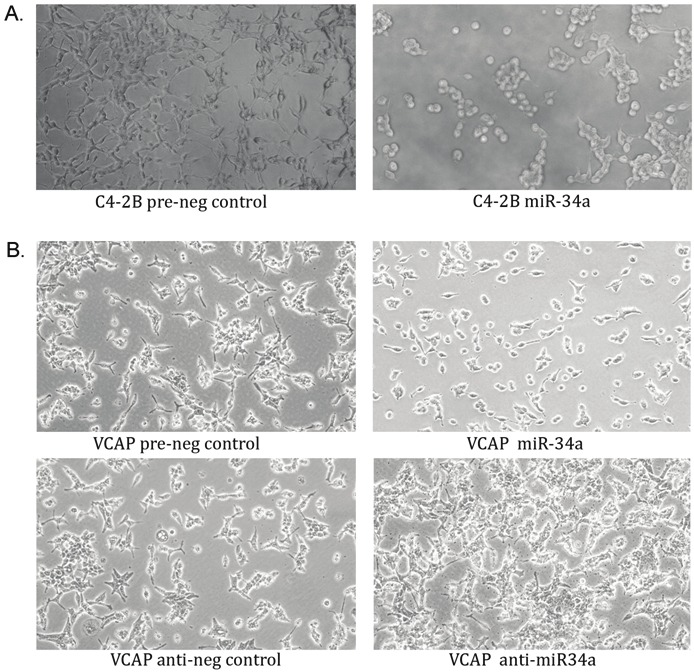
The images of C4-2B (A) and VCAP (B) cells after only a 3-day transfection with pre-miR-34a. A) C4-2B cells showed distinct changes in their morphology with pre-miR-34a transfection compared to its respective control. There was a reduction in cell number as well. B) VCAP cells also showed a change in morphology to a smaller size and rounded shape. Inhibiting miR-34a (anti-miR34a) caused an increase in cell number as evident compared to the negative inhibitor control.
Re-expression of miR-34a inhibited self-renewal capacity of PCa cells
The self-renewal capacity assay was conducted on pre-miR-34a and negative control miRNA transfected C4-2B and CWR22rv1 cells to investigate whether miR-34a could have any changes in the tumor cell aggressiveness as assessed by sphere-forming capacity (Prostaspheres) of PCa cells. The data showed that miR-34a significantly reduced the number of prostaspheres compared to control in C4-2B cells (Figure 6A) and CWR22rv1 cells (Figure 6B). Moreover, the size of prostaspheres formed in miR-34a transfected PCa cells was much smaller than that in the control miRNA transfected PCa cells (Figure 6C and 6D). Furthermore, miR-34a inhibitor transfected PCa cells showed increased number and size of prostaspheres compared to control cells (Figure 6), suggesting that re-expression of miR-34a could inhibit self-renewal capacity of PCa cells, and thus reduced tumor cell aggressiveness.
Figure 6.
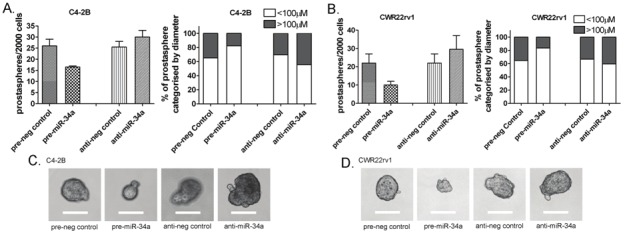
The effects of miR-34a on sphere-forming ability of C4-2B and CWR22rv1 cells. The self-renewal capacity assay showed that miR-34a significantly reduced the number of prostaspheres in C4-2B (A) and CWR22rv1 (B) cells. The size of prostaspheres formed in pre-miR-34a transfected PCa cells was much smaller compared to control (C and D) while miR-34a inhibitor transfected PCa cells showed increased number and size of prostaspheres (A, B, C, and D; Bar: 100μM).
Discussion
Prostate cancer (PCa) is one of the more difficult cancers to treat especially when it becomes hormone resistant such as CRPC and subsequent mCRPC, and thus finding novel targets that could be exploited for development of novel agents would become important for the treatment of mCRPC. Previous studies from our laboratory and others [3,6-9] have shown the importance of miR-34a in the regulation of many cellular processes, and that the loss of miR-34a expression is associated with PCa aggressiveness [3]. Therefore, further investigation on the role of miR-34a expression in PCa became our objective for the present study. A recent study has shown that miR-34a is almost always under-expressed in PCa causing the up-regulation of AR [12]. It is well known that the activation of AR signaling contributes to the development and progression of PCa [13]. Therefore, in this study, we chose to over-express and unde-rexpress miR-34a in different PCa cell lines in order to evaluate its effects on cell growth, expression of miR-34a downstream target (AR and Notch-1) and cellular aggressiveness as assessed by sphere-forming ability of PCa cells.
We found that over-expression of miR-34a led to reduced expression of AR, PSA and Notch-1. We also found that over-expression of miR-34a significantly inhibited the growth of PCa cells (C4-2B, CWR22rv1, LNCaP, and VCAP), suggesting that AR and Notch-1 are direct target of miR-34a. Moreover, over-expression of miR-34a resulted in decreased self-renewal capacity of PCa cells, and conversely inactivation of miR-34a led to increased self-renewal capacity, which is an indication of tumor cell aggressiveness. These findings suggest that the loss of miR-34a is directly linked with up-regulation of AR and Notch-1 both of which are highly expressed in PCa, and thus finding innovative approaches by which miR-34a expression could be up-regulated will have a huge impact on the treatment of PCa especially for mCRPC. These results are consistent with previous reports from our laboratory and others showing that the loss of miR-34a expression is associated with PCa aggressiveness [3,6-9].
Notch signaling is an important signaling pathway which is activated in PCa [14]. In this study, we investigated whether there is a direct correlation between miR-34a and Notch-1. Notch-1 signaling has also been shown to be involved with “stemness” (consistent with cancer stem cell characteristics), which is also linked with the acquisition of EMT phenotype that are critically involved with PCa metastasis, and thus inactivation of Notch-1 signaling is an important consideration for the treatment of PCa especially mCRPC [4,15]. Our findings showed that miR-34a causes the down-regulation of Notch-1, which is very significant because now we have found a novel way to target Notch-1 and, thereby, we could inhibit the development and progression of PCa. Our findings also demonstrate that Notch-1 is another direct target of miR-34a.
Importantly, we found that re-expression of miR-34a significantly inhibited cell growth of PCa cells. The inhibition of PCa cell growth by miR-34a transfection was mediated by the down-regulation of AR and Notch-1 signaling, suggesting the importance of miR-34a as a tumor suppressor in PCa. Both AR and Notch signaling are cell survival signaling and they are involved in the development and progression of PCa [16-19]. Therefore, direct targeting of AR and Notch-1 by miR-34a re-expression would effectively inhibit PCa cell growth and survival. More importantly, we found that miR-34a also significantly inhibited the self-renewal capacity of PCa cells. This inhibition could be mediated by the down-regulation of Notch signaling by miR-34a because Notch signaling has been known to regulate cancer stemness and promote stem cell self-renewal [20,21]. By direct targeting Notch-1, miR-34a could inactivate Notch signaling and, thus, eliminate PCa stem cells, leading to the inhibition of PCa recurrence and progression; however, one must find a newer agent that could re-express miR-34a instead of transfection of pre-miR-34a, and such strategy would become a novel therapeutic strategy for the treatment of PCa in general and mCRPC in particular for which newer treatment is urgently needed.
Acknowledgements
Support was provided by National Cancer Institute, NIH (5R01CA083695, 5R01CA108535, and 1R01CA164318 to FHS). We also thank Guido and Puschelberg Foundation for their generous contribution for the completion of this study.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ryan CJ, Tindall DJ. Androgen receptor rediscovered: the new biology and targeting the androgen receptor therapeutically. J. Clin. Oncol. 2011;29:3651–3658. doi: 10.1200/JCO.2011.35.2005. [DOI] [PubMed] [Google Scholar]
- 3.Kong D, Heath E, Chen W, Cher M, Powell I, Heilbrun L, Li Y, Ali S, Sethi S, Hassan O, Hwang C, Gupta N, Chitale D, Sakr WA, Menon M, Sarkar FH. Epigenetic silencing of miR-34a in human prostate cancer cells and tumor tissue specimens can be reversed by BR-DIM treatment. Am J Transl Res. 2012;4:14–23. [PMC free article] [PubMed] [Google Scholar]
- 4.Hu YY, Zheng MH, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol. 2012;727:186–198. doi: 10.1007/978-1-4614-0899-4_14. [DOI] [PubMed] [Google Scholar]
- 5.Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ, He LY. Silencing Notch-1 induces apoptosis and increases the chemosensitivity of prostate cancer cells to docetaxel through Bcl-2 and Bax. Oncol Lett. 2012;3:879–884. doi: 10.3892/ol.2012.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen F, Hu SJ. Effect of microRNA-34a in cell cycle, differentiation, and apoptosis: a review. J Biochem Mol Toxicol. 2012;26:79–86. doi: 10.1002/jbt.20412. [DOI] [PubMed] [Google Scholar]
- 7.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 8.Peurala H, Greco D, Heikkinen T, Kaur S, Bartkova J, Jamshidi M, Aittomaki K, Heikkila P, Bartek J, Blomqvist C, Butzow R, Nevanlinna H. MiR-34a expression has an effect for lower risk of metastasis and associates with expression patterns predicting clinical outcome in breast cancer. PLoS One. 2011;6:e26122. doi: 10.1371/journal.pone.0026122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65:6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Kelnar K, Vlassov AV, Brown D, Wang J, Tang DG. Distinct microRNA Expression Profiles in Prostate Cancer Stem/Progenitor Cells and Tumor-Suppressive Functions of let-7. Cancer Res. 2012;72:3393–3404. doi: 10.1158/0008-5472.CAN-11-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attard G, Richards J, de Bono JS. New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clin Cancer Res. 2011;17:1649–1657. doi: 10.1158/1078-0432.CCR-10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villaronga MA, Bevan CL, Belandia B. Notch signaling: a potential therapeutic target in prostate cancer. Curr Cancer Drug Targets. 2008;8:566–580. doi: 10.2174/156800908786241096. [DOI] [PubMed] [Google Scholar]
- 15.Alison MR, Lin WR, Lim SM, Nicholson LJ. Cancer stem cells: in the line of fire. Cancer Treat Rev. 2012;38:589–598. doi: 10.1016/j.ctrv.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Bin HB, Adhami VM, Asim M, Siddiqui IA, Bhat KM, Zhong W, Saleem M, Din M, Setaluri V, Mukhtar H. Targeted knockdown of Notch1 inhibits invasion of human prostate cancer cells concomitant with inhibition of matrix 9metalloproteinase- and urokinase plasminogen activator. Clin Cancer Res. 2009;15:452–459. doi: 10.1158/1078-0432.CCR-08-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation. 2008;76:699–716. doi: 10.1111/j.1432-0436.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- 18.Fenner A. Prostate cancer: Unravelling AR splice variant signalling in CPRC. Nat Rev Urol. 2012 doi: 10.1038/nrurol.2012.139. [DOI] [PubMed] [Google Scholar]
- 19.Cai H, Memarzadeh S, Stoyanova T, Beharry Z, Kraft AS, Witte O. Collaboration of Kras and Androgen receptor signaling stimulates EZH2 expression and tumor propagating cells in prostate cancer. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen Y, Bi P, Liu W, Asakura A, Keller C, Kuang S. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol. 2012;32:2300–2311. doi: 10.1128/MCB.06753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang CY, Wei Q, Han I, Sato S, Ghanbari-Azarnier R, Whetstone H, Poon R, Hu J, Zheng F, Zhang P, Wang W, Wunder JS, Alman BA. Hedgehog and Notch signaling regulate selfrenewal of undifferentiated pleomorphic sarcomas. Cancer Res. 2012;72:1013–1022. doi: 10.1158/0008-5472.CAN-11-2531. [DOI] [PubMed] [Google Scholar]


