Abstract
Extinction bursts are characterized by a temporary increase in responding when drug access is withheld from rats trained to self-administer drugs of abuse. Thus far, one study has examined extinction bursts for nicotine self-administration using a 23-h access paradigm [1]. Here we examined extinction bursts using previously published and unpublished data in which rats were trained to self-administer nicotine (0.03mg/kg/infusion) or food pellets (as a comparator) in 1-h sessions under an FR5 schedule of reinforcement followed by 1-h extinction sessions. Analysis of response rates during nicotine self-administration (NSA) was indicative of a loading phase, as response rates were significantly higher at the beginning of the session, which was not observed for food self-administration. At the start of extinction for both food and nicotine, although sessional response rates did not increase, there was an increase in response rate during the first 5-min of the first extinction session relative to self-administration. This transient extinction burst following nicotine was observed in a minority of subjects and correlated with the number of nicotine infusions obtained during self-administration. This transient extinction burst following food was observed in all subjects. Nicotine and food produce more transient extinction bursts compared to other drugs of abuse and only for a minority of animals in the case of nicotine. This study supports the presence of a loading phase in rats trained to self-administer nicotine in 1-r daily sessions and the presence of a transient extinction burst.
Keywords: Nicotine, self-administration, extinction, extinction burst, drug seeking, rats
Introduction
The intravenous drug self-administration (IVSA) paradigm is utilized to study the behavioral aspects of rewarding drugs, primarily those with abuse potential. In rat models, lever pressing is commonly the behavior being reinforced by intravenous infusion of a rewarding drug. Extinction involves the rats being allowed to continue the behavior (i.e., pressing the lever) but without receiving any reinforcement (i.e., drug infusion). Though over time previously reinforced behavior will eventually decrease, the initial removal of reinforcement often results in a temporary increase in the behavior. This temporary increase is termed an “extinction burst” and has been commonly observed in rats for various drugs of abuse including alcohol [2], amphetamine [3], cocaine [4], and heroin [5].
Extinction bursts observed in animal models of drug self-administration could be relevant to the early withdrawal-induced drug craving reported to be experienced at the initiation of abstinence in drug users [6-8]. Though the initial phase of abstinence is associated with high relapse rates, a phenomenon which is commonly observed for smokers [9,10], there has been little work done using animal models to examine extinction bursts following nicotine self-administration.
In the context of rat self-administration, the extinction burst observed is characterized as a significant increase in the previously reinforced behavior at the onset of extinction compared to previous drug self-administration sessions. Apart from the work of Harris and colleagues (2007) examining extinction bursts in rats with unlimited access (23-h/day) to nicotine self-administration, very limited information is available on the presence of extinction bursts following nicotine IVSA [1]. Although not presented specifically for the presence or absence of an extinction burst, it appears that removing access to nicotine in animals trained under limited access schedules does not produce an extinction burst in rats [11-13] or in non-human primates [14]. Surprisingly, it has been reported that mice trained to self-administer nicotine displayed this extinction burst [15]. It should be noted that the failure to observe an extinction burst in rats could possibly be considered a piece of evidence suggesting that the increases in behaviour seen following nicotine administration are not indicative of reinforcement.
With regards to the work of Harris and colleagues (2007) noted above, their study demonstrated that rats trained to self-administer nicotine with unlimited access (23-h/day) display an extinction burst when mean peak 2-h infusion rates were examined. Importantly, the definition of extinction burst in that study was not limited to the 2h period at the start of the first extinction session but rather any 2-h period during the first extinction session in which the infusion rate was higher than the mean 2-h infusion rate for the baseline self-administration sessions. This definition was likely adopted due to the fact that not all animals responded immediately at the start of the 23-h self-administration sessions but rather chose particular periods during the session during which to respond. The magnitude of the burst was correlated with higher infusion rates during the first 2-h of nicotine-self administration sessions and resistance to extinction.
The current study was undertaken utilizing both unpublished and previously published data [16,17] from our laboratory in order to understand whether the findings of Harris and colleagues could be observed with the limited access paradigm (1-h/day) utilized in our own laboratory. Thus the response rates of rats during nicotine IVSA and subsequent extinction sessions (1-h) were analyzed in 5-min blocks during the sessions. We also evaluated the relation between nicotine-intake and the occurrence of an extinction burst. Response rates of rats trained to self-administer food pellets under the exact same operant conditions utilized for nicotine and that underwent extinction sessions thereafter were examined to allow for comparison to a non-drug reinforcer. The differences in the prevalence and magnitude of extinction bursts for nicotine vs. food could also help draw conclusions regarding reinforcing efficacy.
Materials and methods
Subjects
Data analyzed here are from Male Long-Evans rats (Charles River, Lachine, PQ, Canada) that were part of various nicotine (n=72) or parallel food (n=31) self-administration experiments both unpublished and published [16,17]. All data was collected in the same laboratory with similar operant chambers by three individuals in the period of 8 months with all individuals utilizing the laboratory’s standard operating procedures established for both nicotine and food self-administration. Importantly, all animals analyzed here were not subjected to any drug testing either during nicotine or food self-administration training, maintenance or during extinction. Animals were initially group housed in a humidity- and temperature-controlled (21-22°C) vivarium on a 12-h reversed light/dark cycle with access to ad libitum water and food. After a week of habituation to the colony room, animals were singly housed and diet restricted (~20g/day) throughout the experiments with food being given in the home cage after daily nicotine self-administration, food self-administration or extinction sessions. All experimental procedures were carried out in compliance with the guidelines of the Canadian Council on Animal Care.
Apparatus
All experiments were conducted in standard operant conditioning chambers located inside sound-attenuating, ventilated cubicles (Med Associates, St. Albans, VT). Each chamber was equipped with two response levers on a panel on one side of the chamber, with a 28-V white cue light above each lever, and with a white chamber light mounted on the ceiling on the opposite side of chamber. There was a recessed food receptacle between the two levers in the chambers utilized for food self-administration and for the initial food training procedure prior to self-administration. In the nicotine self-administration chambers, intravenous nicotine infusions were delivered by a syringe pump (PHM-100, Med Associates) located outside each cubicle.
Drugs
(−)Nicotine hydrogen tartrate (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in saline, and the pH of the solution was adjusted to 7.0 ± 0.2. Nicotine solution was freshly prepared and filtered through a 0.22-mm syringe filter (Fisher Scientific, Pittsburgh, PA, USA) to minimize risk of infection. All nicotine doses are described as free base concentrations. Nicotine was administered intravenously (i.v.) in a volume of 100 μl/kg/infusion.
Food self-administration
Sessions resembled exactly those of nicotine self-administration sessions as described below, with the exception of each nicotine infusion being replaced with a single 45 mg rodent purified diet pellet (BioServ, Frenchtown, NJ, USA). These pellets were delivered by a pellet dispenser which dispensed pellets into a receptacle located in between the two levers of the chamber. Presentation of a food pellet was also followed by a 1-min timeout period and a concurrent cue light being presented above the active lever during which responding on either lever had no consequence but were recorded and active lever responses were included in response rate calculations. Prior to extinction, all rats were required to have achieved a minimum of 50 pellets/session for the last 3 sessions.
Surgical procedure
Jugular vein catheterization was conducted for all animals undergoing nicotine self-administration procedures as already reported [16-23]. Surgery was performed under anesthesia induced by a xylazine/ketamine mixture (10/75 mg/kg, intraperitoneal). Incision sites were injected with bupivacaine. Animals were implanted with catheters in the right jugular vein which exited from the animal’s back between the scapulae. Buprenorphine was administered for post-operative analgesia (0.01 mg/kg, subcutaneous), and a single dose of penicillin (30,000 U, intramuscular) was administered at surgical completion. Animals were allowed to recover for a period of 1-week before drug self-administration sessions were begun.
Self-administration procedure
All animals were trained for one week to press a lever on a fixed ratio 1 (FR1) schedule in which each press resulted in the delivery of a 45-mg rodent purified diet pellet (BioServ, Frenchtown, NJ, USA) followed by a 5-sec timeout period. Animals were required to reach the criterion of obtaining 100 pellets in under 1-h by the fifth session in order to continue onto self-administration. Once trained, all animals undergoing nicotine self-administration were surgically prepared with a chronic intravenous catheter as described above.
Nicotine self-administration sessions were carried out in experimental chambers equipped with two levers. Session start was signaled by the illumination of a house-light; extinction of this light and the presentation of a nicotine-associated cue light above the active lever (1-min) indicated the time-out (TO) period during which responding on either lever had no consequence but were recorded and active lever responses were included in response rate calculations. MED-PC software (Med Associates, St. Albans, Vt., USA) along with programming developed on-site were utilized to control the chambers and record data. In all cases, rapid delivery of the self-administered drug (approximately 1- sec delivery time) was achieved with Med Associates Model PHM-104 pumps. Unit doses were 0.03mg/kg per infusion (0.1ml); volume adjustments were used to accommodate inter-animal and between-session differences in body weight. Responding on one of the levers (active lever) resulted in drug delivery when schedule requirements were met, while responding on the other lever (inactive lever) was recorded but had no programmed consequences. Self-administration sessions (1-h duration) occurred once daily Monday to Friday. An FR1 schedule of reinforcement was utilized for the first 5 days of self-administration, followed by FR2 for 3 days, and finally FR5 for 7 days.
Extinction
Once self-administration training was completed, all animals which had reached the acquisition criteria (minimum of 10 infusions/session for the last three sessions) were then exposed to daily 1-h sessions of extinction training with the houselight being on continuously. Extinction training was similar to self-administration training with the following exceptions: nicotine infusions, food pellets, and cue lights were unavailable and accordingly there were no time-out periods. Responses on either lever had no consequence but were recorded.
Data analysis
Response rates (responses/min) for each session or block were calculated by dividing the total number of active lever responses during a session/block by the total session/block length. Response rates include responses made during the 1-min timeout periods. Response rates were individually calculated for each rat and then expressed as group means (±SEM). Data was then inputted into statistical software where analysis was conducted. One-way repeated measures (RM) analysis of variance (ANOVAs) were conducted for all data examining response rates over multiple sessions for both food and nicotine with Bonferroni multiple comparison tests used for post hoc analysis. Two-way RM ANOVAs were conducted for all data examining comparing response rates for two separate groups or sessions with Bonferroni multiple comparison tests used for post hoc analysis. An extinction burst was defined as responding during the first 5-min interval of the first extinction session being greater than the first 5-min intervals of the last three self-administration sessions. A student’s t-test was conducted to compare number of infusions between bursting and non-bursting animals. All statistical analysis was performed using SPSS 15.0 for Windows (SPSS Inc.).
Results
Average response rates (responses/min) of animals over the last 3 sessions of nicotine self-administration followed by 5 extinction sessions were first analyzed (Figure 1A). A RM one-way ANOVA produced a statistically significant effect of session (F7,568 = 52.3, p < 0.0001). Bonferroni’s multiple comparison test was utilized for post hoc analysis. No significant differences were observed between the three nicotine self-administration sessions prior to the first extinction session. All 4 were found to be statistically significantly different from extinction sessions 2 to 5. However, 16.7% of animals did display an increased response rate for the first extinction session when compared to the average response rate for the last three nicotine self-administration sessions. Response rates during self-administration ranged from 0.16-7.73 (responses/min) with the data being bimodal with a large group of animals having response rate centered around 1.87 and a smaller group centered around 3.93 (responses/min). These two peaks correspond to the slightly overlapping subgroups (high vs. low intake), described further below. All of the 16.7% of animals described above to have displayed an increased response rate for the first extinction session were also in the high intake subgroup (ie. 45% of this group).
Figure 1.
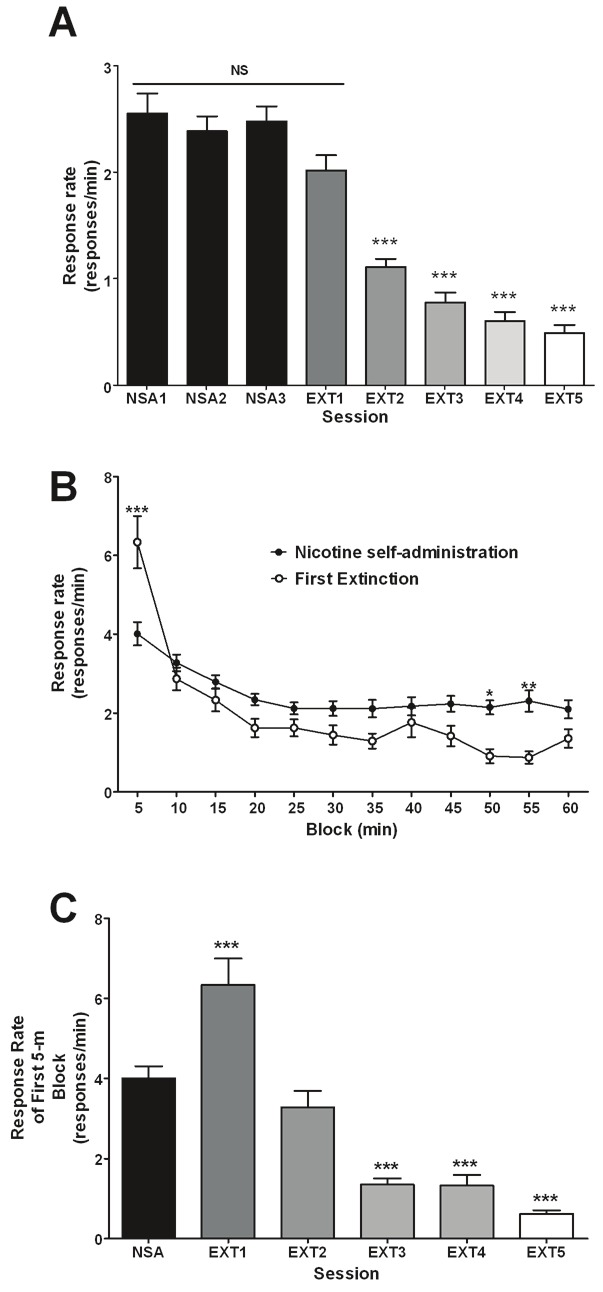
A. Response rates (responses/min) for animals trained to self-administer nicotine at a unit dose of 0.03mg/kg/infusion in 1-h/day sessions over a multiple number of 1-h nicotine-self administration (NSA1-3) and extinction (EXT1-5) sessions. *** p<0.001, Bonferroni’s multiple comparison test following significant RM one way-ANOVA, where EXT2-5 are significantly different from NSA1-3 and EXT1. B. Response rates (responses/min) for each 5-min block for the average of the last three nicotine self-administration sessions prior to extinction (nicotine self-administration) and the first extinction session. C. Response rates (responses/min) for the first 5-min interval for NSA and the EXT sessions. *p<0.05, **p<0.01, ***p<0.001 versus NSA, Bonferroni’s multiple comparison test following a significant RM two- and one- way-ANOVA (for B and C, respectively). Response rates expressed as means (±SEM) with n = 84 for all figures.
Response rates for each 5-min block of either nicotine self-administration or extinction were analyzed (Figure 1B). The last three self-administration sessions prior to extinction were combined and the average response rates are reported along with response rates from the first extinction session. A RM two-way ANOVA revealed a significant effect of session (F1,1704 = 21.03, p < 0.0001), block (F11,1704 = 30.52, p < 0.0001) and interaction (F11,1704 = 6.45, p < 0.0001). Bonferroni’s multiple comparison test was utilized for post hoc analysis and revealed significant differences between sessions for the 1st, 10th and 11th blocks. A RM one-way ANOVA conducted solely on the nicotine self-administration data revealed a significant effect of block (F11,863 = 8.023, p < 0.0001). Subsequently, Bonferroni’s multiple comparison test revealed the 5-min block to be significantly different from the 15-min to the 60-min blocks and the 10-min block to be significantly different from the 20-60min blocks.
Response rates for the first 5-min of various sessions were then analyzed (Figure 1C). The last three self-administration sessions prior to extinction were combined and the average response rate is reported. A repeated measures one-way ANOVA revealed a significant effect of session (F5,431 = 45.91, p < 0.0001) with Bonferroni multiple comparison test revealing that the first, third, fourth, and fifth extinction session were significantly different (p < 0.001) from NSA; however, the second extinction session was not. It also revealed that the last three extinction sessions were not significantly different from each other.
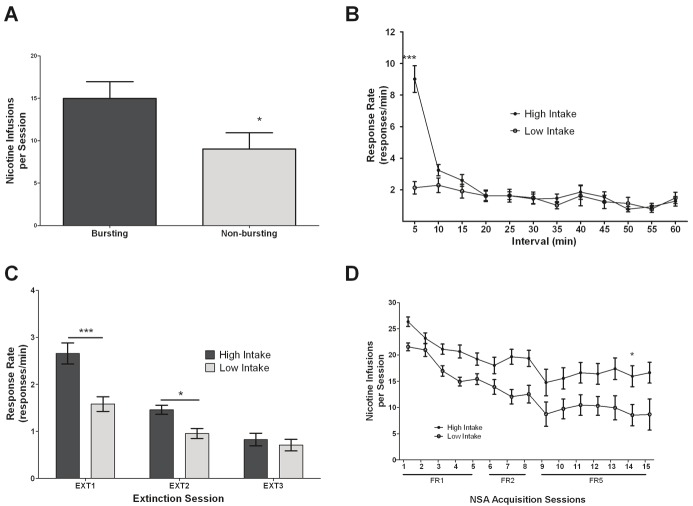
Based on the fact that some animals were observed to display an extinction burst in the first 5-min interval (responding greater than the first 5-min interval of the average of nicotine self-administration sessions) while others were not (62.5% of animals), we looked into differences in nicotine self-administration between these two groups. Upon conducting a Student’s t-test of the data, it was observed that the average (i.e., last three self-administration sessions) number of nicotine infusions earned per session of animals displaying an extinction burst was significantly greater (p < 0.05) than that of their non-bursting counterparts (Figure 2A).
Figure 2.
A. Difference in nicotine infusions per session between the extinction burst group (n = 31) and non-bursting (n = 53) extinction group of animals that were both trained to self-administer nicotine (0.03mg/kg/infusion) in 1-h/day sessions. *p<0.05, Student’s t-test. B. Response rates (responses/min) for each 5-min block from the EXT1 session for the high-intake and low-intake (> 12.75 and < 12.75 infusions during nicotine self-administration, respectively). C. Response rates (responses/min) for the high intake and the low intake animals across the EXT1-3 sessions. D. Nicotine infusions per session over the nicotine self-administration acquisition sessions with FR-1, -2 and -5 scheduling between the high intake and low intake group. *p<0.05, ***p<0.001, Bonferroni’s multiple comparison test following a significant RM two way-ANOVA (for B, C and D). All data expressed as means (±SEM).
The majority (>90%) of bursting animals were observed to have an average number of nicotine infusions per session of greater than 12.75, which led us to split all the animals into two groups: high intake (infusions > 12.75) and low intake (infusions < 12.75). The range was observed to be between 6 to 35 infusions in a single session. We then re-examined the response rate block data from the first extinction sessions looking specifically at these two groups (Figure 2B). A repeated measures two-way ANOVA revealed a significant effect of intake (F1,840 = 19.92, p < 0.0001), block (F11,840 = 19.02, p < 0.0001), and interaction (F11,840 = 11.46, p < 0.0001). Post hoc analysis using Bonferroni multiple comparison test revealed a significant difference (p < 0.001) in response rates between intake groups only for the first 5-min block of the session.
A repeated measures two-way ANOVA was conducted comparing intake groups for their sessional response rates during initial extinction sessions (Figure 2C) which revealed a significant effect of intake (F1,207 = 22.22, p < 0.0001), session (F2,207 = 43.93, p < 0.0001), and interaction (F2,207 = 5.369, p = 0.0053). Post hoc analysis with Bonferroni multiple comparison test revealed significant differences between intake groups for the first (p < 0.001) and second (p < 0.05) extinction sessions but not the third.
A repeated measures two-way ANOVA was conducted on the number of infusions acquired by each intake group for the initial nicotine self-administration acquisition sessions (Figure 2D) which revealed a significant effect of intake (F1,1035 = 73.33, p < 0.0001) and session (F14,1035 = 7.924, p < 0.0001), but no significant interaction (F14,1035 = 1.046, p > 0.05). Bonferroni multiple comparison test for post hoc analysis revealed only a significant difference (p < 0.05) between intake groups on the final session of acquisition (FR5-7).
Average response rates (responses/min) of animals over multiple sessions of food self-administration followed by extinction were analyzed (Figure 3A). A RM one-way ANOVA revealed a significant effect of session (F7,288 = 20.56, p < 0.0001). Bonferroni’s multiple comparison test was utilized for post hoc analysis. No significant differences were observed between the last three food self-administration sessions; however, all 3 were found to be significantly (p < 0.01) greater from the extinction sessions while extinction sessions were not found to be significantly different from each other. All animals displayed the same general pattern of responding during self-administration and extinction with the range of responding being between 11.3-18.6 (responses/min) during self-administration and 2.1-7.4 (responses/min) during the first extinction session.
Figure 3.
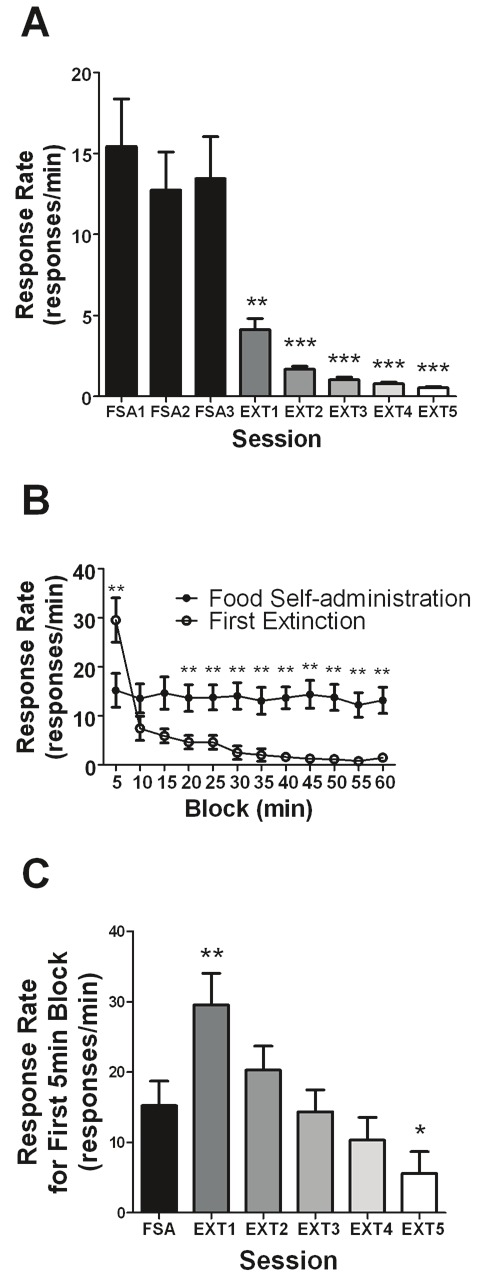
A. Response rates (responses/min) of animals trained to self-administer food pellets (45mg) in 1-h/day sessions over a multiple number of 1-h food-self administration (FSA1-3) and extinction (EXT1-5) sessions. **p<0.01, ***p<0.001, Bonferroni’s multiple comparison test following significant RM one way-ANOVA, where FSA1-3 were significantly different from all EXT sessions. B. Response rates (responses/min) for each 5-min block interval for the average of the last three food self-administration sessions prior to extinction and the first extinction session. C. Response rates (responses/min) for the first 5-min interval of the food self-administration and EXT sessions. *p<0.05, **p<0.01, Bonferroni’s multiple comparison test after significant RM two- and one- way-ANOVA (for B and C, respectively), where EXT sessions are compared to FSA. All response rates are expressed as means (±SEM) with n = 31 for all figures.
Response rates for each 5min block of either food self-administration or extinction were analyzed (Figure 3B). The last three self-administration sessions prior to extinction were combined and the average response rates are reported along with response rates from the first extinction session. A RM two-way ANOVA revealed a significant effect of session (F1,720 = 77.89, p < 0.0001), block (F11,720 = 7.572, p < 0.0001) and interaction (F11,720 = 5.989, p < 0.0001). Bonferroni’s multiple comparison test was utilized for post hoc analysis and revealed significant differences (p < 0.01) between sessions for the 1st block and blocks 20-min to 60-min. Importantly, all animals were observed to display a burst (responding during the first 5-min interval of the first extinction session greater than the first 5-min interval of the last three self-administration sessions) in response rate during the first block. A RM one-way ANOVA conducted solely on the food self-administration data revealed no significant (F11,349 = 0.62, p > 0.05) effect of block. All animals showed the same pattern of responding, with responding being stable during self-administration and being very high for the first 5-min interval of the first extinction session.
Response rates for the first 5-min of various sessions were then analyzed (Figure 3C). The last three self-administration sessions prior to extinction were combined and the average response rate is reported. A RM one-way ANOVA revealed a significant effect of session (F5,349 = 4.62, p = 0.0004) with Bonferroni multiple comparison test revealing that response rates for the first 5-min of the first and fifth extinction session were significantly different (p < 0.01) from the averaged food self-administration sessions; however, the second to fourth extinction sessions were not.
Discussion
This study has been the first to our knowledge to examine extinction bursts of nicotine-associated lever responding in a limited access (1-h sessions) model of nicotine self-administration. Our analysis confirmed the observations of prior studies [11-13], demonstrating the lack of an extinction burst in terms of the sessional response rate for the first extinction session (Figure 1A). Importantly, the sessional response rate for the first extinction session was not significantly different from the baseline (average of last three) nicotine-self administration sessions. This was not true for the food self-administration animals, where the sessional response rate for the first extinction session was significantly decreased compared to the baseline food self-administration sessions (Figure 3A).
This can be explained by examining the intra-session responding for the first extinction session of both the nicotine and food trained animals (Figure 1B and 3B, respectively). We see that though all food trained animals displayed a large burst (nearly 100% increase in responding compared to first 5-min of baseline self-administration) in the first 5-min of the extinction session, their responding quickly dropped to very low levels relative to that of baseline self-administration sessions (Figure 3B). In the case of nicotine, though the burst was not nearly as large (55% increase in responding compared to first 5-min of baseline self-administration), their responding for the remainder of the session was only slightly lower than that of baseline self-administration sessions.
The smaller magnitude of the burst is likely because the majority of animals (62.5%) trained under nicotine self-administration did not show an extinction burst in the initial 5-min of the first extinction session (i.e., an increase in response rate compared to the average response rate for the first 5-min of the last three nicotine self-administration sessions). Yet the burst magnitude (125% increase in responding compared to first 5-min of baseline self-administration) of high intake animals (Figure 2B) resulted in a burst being observed in the first 5-min of extinction when all animals were averaged (Figure 1B). In contrast, all food self-administration animals analyzed displayed an extinction burst in the first 5-min of the first extinction session, with a much larger magnitude (Figure 3B). That may be explained by the greater magnitude of responding and greater number of reinforcements earned during food self-administration sessions compared to nicotine self-administration.
Finally, the extinction burst observed in the first 5-min of the first extinction session does not appear to last longer than that one session (Figure 1C). The extinction burst seems to decrease over multiple sessions, but this decrease appears different from the extinction burst decline observed for food self-administration (Figure 3C); however, the extinction burst observed for food is also much greater in magnitude compared to that of nicotine.
The observation of greater nicotine intake in animals displaying a burst (Figure 2A) presents the possibility that there may be two subsets of animals self-administering nicotine. The evidence presented here shows small differences in acquisition of nicotine self-administration between these two groups (Figure 2D) along with slight differences in the deterioration of drug seeking behavior during extinction (Figure 2C). Further studies should examine these groups for reinstatement behavior as well which may be of greater importance. Interestingly, though there appears to be a range of different levels of intake for other psychostimulant drugs, the large majority of animals still appear to display a large extinction burst [4,5,24,25]. Future study should examine whether the parameters of the paradigm utilized in the current study could be better manipulated to produce a greater magnitude of extinction burst in a greater proportion of animals.
Though not directly related to the extinction burst, it is interesting to note the difference in within-session responding between food and nicotine during self-administration sessions (Figure 1B and 3B). The food self-administration response rates remain fairly constant throughout the session with no significant differences between the blocks. This however is not the case for nicotine as there appears to be clearly greater responding earlier in the session that stabilizes towards the end of the session. This phenomenon is compatible with the presence of a loading phase, as it has been typically reported for other psychostimulant drugs [4,5,24,25].
It should be noted that the current study lacked conditions examining response-independent reinforcement with either food or nicotine and thus a definitive conclusion regarding nicotine or food-seeking behavior cannot be stated. Our lab has previously demonstrated that light cue presentations without nicotine or food are not sufficient for producing the level of responding that is observed for nicotine or food paired with light cue presentations [20]; however, we have yet to examine nicotine or food self-administration without the light cue pairing. Thereby, there is a possibility that nicotine alone was only a reinforcer for the majority of the rats.
This should be verified in future studies. The present study demonstrates that there can be a statistically significant, but transient, extinction burst in rats trained to self-administer nicotine under a 1-h limited-access paradigm. This extinction burst was found during the first 5-min of the first extinction session in 37.5% of animals that acquired nicotine self-administration. There appears to be moderate though statistically significant differences between animals displaying the burst and those not in terms of nicotine intake and greater response rates during the initial extinction sessions. The extinction burst was also observed in animals trained to self-administer food; however, the pattern, magnitude and prevalence were quite different from that of nicotine.
The extinction burst observed in self-administration models for drugs of abuse is thought to be useful for examining the influence of early withdrawal-induced drug seeking immediately following abstinence when relapse is most likely to occur [9,10,26]. However, the majority of studies focus primarily on the reinstatement of drug-seeking as a model of relapse [10].
The data presented here suggests that the extinction burst, though significant, is only present in a minority of animals trained to self-administer nicotine under the parameters of the current study. Future studies should attempt to determine whether operant conditioning, pharmacologic, neuroanatomic or genetic manipulations can result in an increase in the magnitude and prevalence of extinction bursts for nicotine self-administration Such a model would allow for the study of extinction burst behavior which has yet to be properly examined for its relevancy to the efficacy of pharmacotherapies used in smoking cessation.
References
- 1.Harris AC, Pentel PR, Lesage MG. Prevalence, magnitude, and correlates of an extinction burst in drug-seeking behavior in rats trained to self-administer nicotine during unlimited access (23 h/day) sessions. Psychopharmacology (Berl) 2007;194:395–402. doi: 10.1007/s00213-007-0848-2. [DOI] [PubMed] [Google Scholar]
- 2.Lyness WH, Smith FL. Influence of dopaminergic and serotonergic neurons on intravenous ethanol self-administration in the rat. Pharmacol Biochem Behav. 1992;42:187–192. doi: 10.1016/0091-3057(92)90465-r. [DOI] [PubMed] [Google Scholar]
- 3.Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–549. doi: 10.1126/science.1114313. [DOI] [PubMed] [Google Scholar]
- 4.Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- 5.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert RM, Pope MA. Early effects of quitting smoking. Psychopharmacology (Berl) 1982;78:121–127. doi: 10.1007/BF00432247. [DOI] [PubMed] [Google Scholar]
- 7.Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Exp Clin Psychopharmacol. 2004;12:276–287. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- 8.al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 10.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 1997;130:396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg SR, Spealman RD, Risner ME, Henningfield JE. Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol Biochem Behav. 1983;19:1011–1020. doi: 10.1016/0091-3057(83)90408-2. [DOI] [PubMed] [Google Scholar]
- 13.Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berlin) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- 14.Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS ONE. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Garcia E, Barbano MF, Galeote L, Maldonado R. New operant model of nicotine-seeking behaviour in mice. Int J Neuropsychopharmacol. 2009;12:343–356. doi: 10.1017/S1461145708009279. [DOI] [PubMed] [Google Scholar]
- 16.Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010;35:1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, Goldberg SR, Le Foll B. Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotineseeking behavior in rats. Neuropsychopharmacology. 2012;37:685–696. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forget B, Hamon M, Thiebot MH. Involvement of alpha1-adrenoceptors in conditioned place preference supported by nicotine in rats. Psychopharmacology (Berl) 2009;205:503–515. doi: 10.1007/s00213-009-1559-7. [DOI] [PubMed] [Google Scholar]
- 19.Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biological Psychiatry. 2010;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Khaled MA, Farid Araki K, Li B, Coen KM, Marinelli PW, Varga J, Gaal J, Le Foll B. The selective dopamine D3 receptor antagonist SB 277011-A, but not the partial agonist BP 897, blocks cue-induced reinstatement of nicotine-seeking. Int J Neuropsychopharmacology. 2010;13:181–190. doi: 10.1017/S1461145709991064. [DOI] [PubMed] [Google Scholar]
- 21.Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, Goldberg SR, Le Foll B. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol. 2012;17:47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- 22.Gamaleddin I, Guranda M, Goldberg SR, Le Foll B. The selective anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking behaviour, but does not affect nicotine intake. Br J Pharmacol. 2011;164:1652–1660. doi: 10.1111/j.1476-5381.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a Selective Cannabinoid CB2 Agonist and Antagonist on Intravenous Nicotine Self Administration and Reinstatement of Nicotine Seeking. PLoS One. 2012;7:e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson MC, Hitomi M, Schuster CR. Psychomotor stimulant self administration as a function of dosage per injection in the rhesus monkey. Psychopharmacologia. 1971;22:271–281. doi: 10.1007/BF00401789. [DOI] [PubMed] [Google Scholar]
- 25.Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB Jr. Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120:10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- 26.Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]