Abstract
Aim: To investigate whether increased levels of vimentin citrullinated peptides identified by MS in articular cartilage can be measured in pathologies other than rheumatoid arthritis and be utilised for diagnostic purposes. Methods: A monoclonal antibody against the sequence RLRSSVPGV-citrulline (VICM) was developed and evaluated in a carbon tetrachloride (CCl4) (n=52 + 28 controls) rat model of liver fibrosis and two clinical cohorts of adult patients with hepatitis C (HCV) (n=92) and non-alcoholic fatty liver disease (NAFLD) (n=62), and compared to healthy controls. Results: In CCl4-treated rats, mean systemic VICM levels increased 31% at week 12 (176 ng/mL, P<0.001), 41.7% at weeks 16 (190 ng/mL, P<0.001), 49.2% at weeks 20 (200 ng/ml, P<0.001), compared to controls (134 ng/mL). VICM levels correlated with total hepatic collagen determined by Sirius red staining of rat livers (r=0.75, P<0.05). In the HCV cohort, when stratified according to the METAVIR F score, VICM levels were 63% higher in F0 (632 ng/mL ±75, p<0.05), 54% in F1 (597 ng/mL ±41.3, p<0.05) and 62% in F2 (628 ng/mL ±59, p<0.05) all compared to controls. In the NAFLD cohort, VICM levels were 20.6% higher in F0 (339 ±12 ng/mL, P<0.05), 23.8% in F1 (348 ±12 ng/mL, P<0.05) and 28.8% in F2 (362 ±25 P<0.05). Conclusion: We demonstrated increased serological levels of citrullinated and MMP degraded vimentin in an animal model of liver fibrosis and in early fibrosis associated with HCV and NAFLD patients. These data suggest that citrullinated and MMP degraded proteins are also present in liver fibrosis.
Keywords: Biomarker, citrulline, hepatitis C, NAFLD
Introduction
The extracellular matrix (ECM) consists of collagens, proteoglycans and glycoproteins, all of which play important, unique and interrelated functions in maintaining the physicochemical structure of connective tissue. The ECM is normally remodelled in a balanced way, by degradation of proteins by proteases, such as matrix metalloproteinases (MMPs), and by formation of healthy new tissue. However, in specific diseases such as liver fibrosis, the remodelling balance is disturbed in a process which involves excessive degradation of ECM, leading to excessive deposition of fibrillar ECM components, scar formation and ultimately organ failure [1-5]. Fibrosis may begin in response to various acute or chronic stimuli, including infections, autoimmune reactions, toxins, radiation and mechanical injury [6]. In the case of liver fibrosis these stimuli prompt several cell types to work in synergy, ultimately leading to liver damage [4].
MMPs are the principal family of enzymes involved in the cleavage and degradation of matrix proteins. Because of their ability to destroy normal matrices and cause tissue injury, the extracellular activity of MMPs is carefully regulated. In the healthy liver, homeostasis of ECM is sustained by a precisely regulated permanent turn-over of proteins in which MMPs are active participants. Alteration of the balance between TIMPs and MMPs contributes significantly to the progression of liver fibrosis [7]. More than 26 MMPs and 4 TIMPs have been identified so far [8] and 11 of the MMPs have been found in the liver. MMP levels are constantly changed during different stages of the progression of liver fibrosis [9,10].
ECM remodelling is a common mechanism in a wide range of diseases, which may be triggered by post-translational modifications (PTMs) [11,12]. Furthermore, PTMs and subsequent protease breakdown of proteins may cause increased antigenicity, protein interactions and subsequently an immune response due to the uncovering of cryptic epitopes or even creation of new ones. PTMs such as phosphorylation have been proven to have central regulatory roles in the functioning of vimentin [13]. The phosphorylation motifs and patterns on vimentin N and C terminal domains are known to have central role in the protein’s function. Additional PTMs have been described as of particular importance, the most prominent being auto-antibodies directed against citrullinated vimentin which have been described as highly specific for rheumatoid arthritis [14-17]. Selective citrullination of vimentin has been described in mouse peritoneal macrophages undergoing apoptosis, a process which is also known to contribute to the development of human atherosclerotic lesions [18,19].
Citrullination is the enzyme-mediated PTM of the amino acid arginine into citrulline by peptidylarginine deiminase (PAD) enzymes in the presence of calcium [20,21]. The citrulline modification irreversibly disrupts protein structure and function [22-25]. Citrullination has been shown to make proteins prone to proteolytic degradation, which may be indicative of its role in a wider variety of pathology progression [26]. Citrullination has been associated with a variety of pathologies such as rheumatoid arthritis (RA), Alzheimer’s disease, psoriasis and multiple sclerosis (MS), and PAD enzymes have been proven to play an active role in these pathologies [21,22,27-30]. Since auto antibodies against citrullinated antigens are found in RA patients as early as 15 years before clinical onset, antibodies against citrulline have been used with considerable success for the early prognosis and diagnosis of the disease [31-36]. Citrulline-containing proteins have been associated with the triggering phase of the disease and are central to immunopathogenesis in RA, thus further rising awareness for the active role of PTMs in the transition from physiology to pathology [37-39].
Vimentin is a type III intermediate filament (IF) protein [40]. Even though its exact roles are not fully elucidated, some of its functions have been linked to cell migration, adhesion and signalling, formation of active macrophage-like cells and polykaryons and, crucially, with inflammation and apoptosis [41,42,17]. Vimentin has been also shown to be expressed during Hepatic stellate cell (HSC) activation in liver fibrosis [43,44].
A number of pathologies besides autoimmune disorders feature disturbed ECM remodelling and inflammation. As inflammation is an integral part of fibrosis and the synovium could be considered as fibrotic tissue, we hypothesised that citrullination could be closely associated with fibrosis. If this was found to be true, the successful detection and targeting of citrullinated proteins for the diagnosis and prognosis of RA could be potentially used also in a range of other diseases.
The aims of this study were 1) To investigate whether a citrullinated and MMP cleaved fragment of vimentin that has been identified by MS in articular cartilage can be accurately measured and related to liver fibrosis 2) Whether the assessment of citrullinated and MMP-degraded vimentin could be used as liver fibrosis biomarker for diagnostic purposes.
Materials and methods
Mass spectrometry
Human cartilage tissue was digested by a range of proteases and the proteolyzed peptide products were identified by mass spectrometry as previously described [45]. The aim of this experiment was to identify and characterise proteolysis peptide products generated by MMP activity. The methods included proteolysis of human articular tissue with exogenous MMPs (including MMPs 2, 3, 8, 9, 12 and 13), the proteolyzed products of which were identified by LC-MS/MS. The obtained spectra were searched against both Sequest and Xtandem databases. From the peptides identified to have arginine residues in the c terminal end, a vimentin sequence was chosen to test our hypothesis due to the vimentins presence and significant role in hepatic stellate cells HSCs [46,47].
Antibody development
Reagents
All reagents used for the experiments were standard high-quality chemicals from companies such as Merck (Whitehouse Station, NJ, USA) and Sigma Aldrich (St Louis, MO, USA). The synthetic peptides used for monoclonal antibody production were purchased from the Chinese Peptide Company, Beijing, China.
Selection of peptide for immunization
The sequence used for the immunisations was selected using mass spectroscopy as described above. Several different peptides were identified by mass spectroscopy analysis. Among these ten vimentin fragments identified were selected for immunizations. The first six amino acids at the protease site were regarded as a target sequence. All relevant sequences were analysed for homology and then blasted for homology using the NPS@: network protein sequence analysis [48].
The vimentin sequence RLRSSVPGVR immunization generated the best antibody in terms of native reactivity and stability thus an antibody against this sequence was selected for assay development. The sequence located at amino acid positions 69 and 78, was identified as being generated by MMP-2 and MMP-8. An additional sequence was created in which the C-terminal arginine was substituted with a citrulline residue, i.e. RLRSSVPGV-Citrulline.
Immunization procedure
Six 4-6 week old Balb/C mice were immunized subcutaneously in the abdomen with 200μL emulsified antigen (50μg per immunization), using Freund’s incomplete adjuvant. Immunizations were performed at two-week intervals until a stable titre level was obtained. At each bleeding, the serum titre was measured and the mouse with the highest titre and best native material reactivity towards relevant biological fluids such as serum and urine was selected for fusion. The selected mice were boosted intravenously with 50μg immunogen in 100μL 0.9% sodium chloride solution three days before surgical removal of the spleen for cell fusion.
Fusion and antibody screening
The fusion procedure has been described elsewhere [49]. Briefly, the hybridoma cells were cloned using a limiting dilution method and transferred into 96-well microtitre plates for further growth. Standard limited dilution was used to promote monoclonal growth. Supernatants were screened using an indirect ELISA, while the biotinylated peptide Biotin-RLRSSVPGV-Citrulline was used as a catcher on streptavidin-coated microtitre plates.
Characterization of clones
Native reactivity and peptide binding of the monoclonal antibodies was evaluated for human and rat serum, plasma and urine, in a preliminary ELISA using 10 ng/mL biotinylated peptide coater on a streptavidin coated microtitre plate and supernatant from the growing monoclonal hybridoma. Clone specificity was tested against the free peptide RLRSSVPGV-Citrulline, RLRSSVPGV without citrulline and a non-sense peptide. Isotyping of monoclonal antibodies was performed using the clonotyping System-HRP kit, cat.5300-05 (Southern Biotech, Birmingham, AL, USA).
Assay development
The selected clones were purified using Protein G columns according to the manufacturer’s instructions and dialysed (GE Healthcare Life Science, Little Chalfont, Buckinghamshire, UK).
The selected monoclonal antibodies were labelled with horseradish peroxidase (HRP) using the Lightning-Link Horseradish Peroxidase (HRP) antibody labelling kit according to the manufacturer’s instructions (Innova Bioscience, Babraham, Cambridge, UK). A 96-well streptavidin plate (Roche Diagnostics, Basel, Switzerland) was coated with 2.5ng of the biotinylated synthetic peptide, Biotin-RLRSSVPGV-Citrulline, dissolved in assay buffer (50mM Tris, 1% BSA, 0.1% Tween-20, 0.36% Bronidox, adjusted to pH 7.4 at 20°C) and incubated for 30 minutes at 20°C. Twenty μL of the peptide calibrator or sample was added to appropriate wells, followed by 100 μL of 4 ng/ml HPR labelled monoclonal antibody and incubated for 1 hour at 20°C. Finally, 100 μL tetramethyl benzinidine (TMB) (Kem-En-Tec cat.438OH, Taastrup, Denmark) was added, and the plate was incubated for 15 minutes at 20°C in the dark. All the above incubation steps included shaking at 300 rpm. After each incubation step the plate was washed five times in washing buffer (20mM Tris, 50mM NaCl, pH 7.2). The TMB reaction was stopped by adding 100 μL of stopping solution (1% HCl) and measured at 450 nm with 650 nm as the reference. A calibration curve was plotted using a 4-parametric mathematical fit model with a starting concentration of 2000ng for the standard peptide following a 2-fold dilution.
Rat CCl4 liver fibrosis model
Liver fibrosis was induced in 52 male Wistar rats (Charles-River, Saint Aubin les Elseuf, France) as previously described. Briefly, CCl4 was inhaled twice a week and phenobarbital (0.3 g/l) was added to the drinking water. Another 28 male Wistar rats received phenobarbital only and served as controls. Animals receiving CCl4 were stratified into groups treated for 8, 12, 16 or 20 weeks (n=13 for each group). Seven control animals were terminated at the end of each of the four treatment durations. Blood was collected at termination and was allowed to stand at room temperature for 20 minutes to clot, before centrifugation at 2500 rpm for 10 min. Samples were stored at - 80°C. Liver sections 4 μm thick were stained with 0.1% Sirius red (F3B) in saturated picric acid (Sigma-Aldrich, St Louis, MO, USA). From each animal, the amount of fibrosis expressed as a percentage of total collagen in the total liver area was measured by digital quantitative histology (VisioMorph, Visiopharm, Hørsholm, Denmark) using 3 adjacent histology slides from each animal.
This animal study was performed according to the criteria of the Investigation and Ethics Committee of the Hospital Clinic Universitari (Barcelona, Spain) (reference number (B-NNP-233-09).
Liver fibrosis clinical samples
Hepatitis C patients
Ninety-two patients with hepatitis C virus (HCV), not previously submitted to antiviral treatment, non-obese (defined as a body mass index (BMI) below 30), non-diabetic, age between 18 and 60 years, from Hospital de Clínicas, Universidade Federal do Rio Grande do Sul, Brazil, were included and compared with healthy controls (blood donors in the same hospital), none of which were diagnosed with liver fibrotic-related pathology. Patients were positive for HCV antibody (detected by ELISA-3) and detectable HCV RNA. Identification of type 2 diabetes, hypertension and dyslipidaemia in patients was performed according to recommendations of the American Diabetes Association.
Patients with hepatitis B infection, hemochromatosis, recipients of a solid organ transplant, clinical evidence of cirrhosis as determined by the presence of portal hypertension defined by oesophageal or gastric varices at endoscopy, ascites, splenomegaly, or evidence of synthetic dysfunction as evaluated by laboratory measurements were excluded. Patients with prior diagnosis of cardiovascular diseases, systemic blood hypertension, chronic renal failure, cancer, alcohol abuse, pregnancy or chronic use of lipid-lowering drugs or immunosuppressants were excluded. Age, BMI, fasting glucose and lipid levels were determined among other indicators.
All patients had a liver biopsy. The liver tissue was fixed in 4% formaldehyde and stained with haematoxylin-eosin and Masson trichrome for histological analysis. Histological analyses were performed blinded by a pathologist, unaware of the HCV genotype as well as the patient’s clinical characteristics. Stages of fibrosis and grades of inflammation were scored according to METAVIR fibrosis score, which consists of F0 (no fibrosis), F1 (portal fibrosis without septa), F2 (portal fibrosis with few septa), F3 (numerous septa without cirrhosis), F4 (cirrhosis).
Non-alcoholic fatty liver disease (NAFLD) patients
Fifty two patients diagnosed by biopsy with Non-Alcoholic Fatty Liver Disease (NAFLD) and referred to the Clinic Hospital of the University of São Paulo, School of Medicine, São Paulo, Brazil, were stratified by their stage of fibrosis (F0-F4) on liver biopsy. Twelve subjects not diagnosed with any liver fibrotic-related pathology served as controls. These male or female patients aged 18-75 years had been previously screened for NAFLD. Identification of metabolic syndrome components followed the recommendations of the Adult Treatment Panel III Report: fasting glucose ≥110 mg/dL, triglycerides ≥150 mg/dL, high density lipoprotein (HDL) <40 mg/dL in men or <50 mg/dL in women; and ≥130 mmHg systolic or ≥85 mmHg diastolic blood pressure; abdominal obesity, defined as a waist circumference > 102 cm in men and > 88 cm in women. Criteria for exclusion were any other acute or chronic liver or biliary disease, substance abuse, especially alcohol intake of >100 g/week, and previous use of steatogenic medications. All the liver specimens were scored by a single liver pathologist with expertise in NAFLD, according to macro- and microvacuolar fatty change, zonal distribution, foci of necrosis, portal and perivenular fibrosis, and inflammatory and fibrotic infiltrate with zonal distribution. The slides were blindly classified according to activity score devised by the Pathology Committee of the Clinical Research Network. Fatty infiltration, which did not fulfil NAFLD criteria, was classified as steatosis only. Clinical and biochemical investigation included liver function tests which were conducted at the time of liver biopsy. Retrospective information on co-morbidities was collected as well.
In the both the HCV and NAFLD cohort, blood samples were obtained after 12h overnight fasting. Serum and plasma was stored at -80°C until use. All patients gave written informed consent. The study was approved by the institutional Internal Review Board of São Paulo and Porto Allegre Universities from which the samples were obtained (Reference number 0064/10).
Statistical analyses
Comparison of groups was performed using an ANOVA test with Dunnett correction. Correlations were performed using the Spearman correlation. Differences were considered statistically significant if p<0.05. The diagnostic value of the assay was calculated by ROC curve plots. GRAPH PAD PRISM 5 (Graph Pad Software, La Jolla, CA, USA) was used for the calculations.
Results
Mass spectroscopy analysis
Mass spectroscopy analysis of human articular cartilage revealed 1883 unique peptide sequences [45]. Increasing number of peptides with an arginine at the C-terminal end was noted. By counting the amino acids found at the C-terminal end, a map of all identified peptides was created. Such data revealed that 47% of the identified sequences contained a lysine amino acid residue, known to be associated with ubiquitination, and 37% of the remaining sequences were found to have an arginine (Table 1). No specific pattern was predominant at the N-terminal end of the identified sequences. As discussed above, from the various sequences that have been identified, vimentin was chosen to test our hypothesis.
Table 1.
Abundance of amino acids found at the C terminal end of identified peptides
| Amino acid | Abundance % |
|---|---|
| Lysine | 47 |
| Arginine | 37 |
| Glycine | 4 |
| Alanine | 3 |
| Rest amino acids | 9 |
Clone characterization & technical evaluation
The clone selected for ELISA development was the IgG1 subtype. The lower limit of detection (LLD) for the assay was found to be 21,376 ng/ mL. Dilution recovery was found to be within 100±15%. The inter- and intra-assay variation was found to be 10.9% and 4.05% respectively. Specificity of the antibody was tested using an elongated peptide as well as a non-citrullinated arginine-rich sequence in the same set up. No signal inhibition was observed, implying that the antibody was only reacting to the specific sequence of interest (Figure 1).
Figure 1.
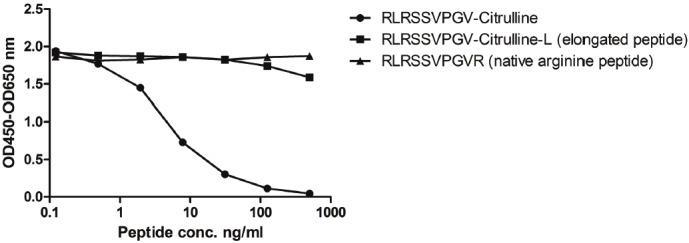
Representative standard curve for the target sequence peptide, native arginine peptide and elongated peptide. Signal inhibition is only seen towards the selected peptide sequence, and no reactivity (signal inhibition) towards either the elongated peptide or native arginine peptide implying specificity for the citrulline-rich peptide. The signal was assessed as the optical density at 450 nm, subtracting the background at 650 nm, as a function of peptide concentration. Measurement is the mean of a double determination measurement.
Rat CCl4 liver fibrosis model
CCl4-treated rats were stratified into 4 quartiles according to the total collagen increase as measured by Visiopharm quantitative histology software. The mean citrullinated vimentin level for control rats was 134 ng/ml. Compared with controls, the increase in marker levels in the first quartile of CCl4-treated rats was not statistically significant (P>0.05). However, the increase vs. controls was significant in the second 159.6 ng/ml (p<0.02), third 173.8 ng/ml (p<0.002) and fourth quartile 234.7 ng/ml (<0.001) (Figure 2A). The groups were then stratified according to the CCl4 treatment durations and a statistically significant (P<0.05) increase in citrullinated vimentin compared with controls was observed in animals treated for 12 weeks 176.7 ng/ml (p<0.001), 16 weeks 190 ng/ml (p<0.001) and 20 weeks 200 ng/ml (p<0.002) (Figure 2B). Detected levels of citrullinated vimentin were correlated with the total collagen increase as measured by quantitative histology (r=0.75) (Figure 2C). We have estimated a cut off range in rat serum of 151 ng/ml in which sensitivity was found to be 74% and specificity 65.9%.
Figure 2.
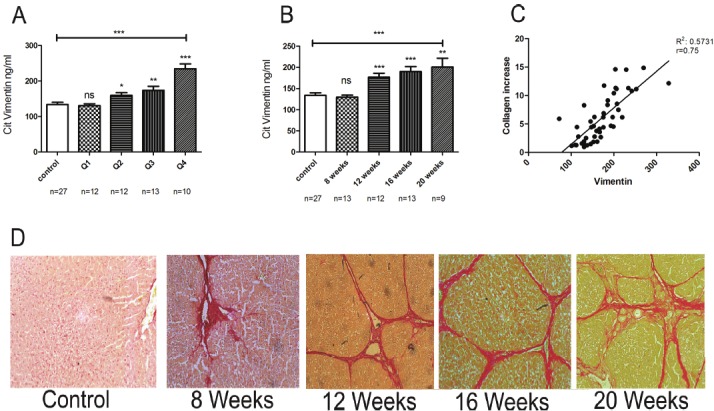
Citrullinated vimentin levels in serum from CCl4-treated rats, stratified in quartiles according to total collagen in the liver as measured by quantitative histology (Q1 P>0.5, Q2 P<0.5, Q3 P<0.05, Q4 P<0.0001) (A), treatment duration (8 weeks P>0.05, 12 weeks P>0.001, 16 weeks P>0.005 and 20 weeks P>0.005) (B) and correlation of the citrullinated vimentin marker with total collagen increase as measured by quantitative histology (C). Comparisons are between controls of each group vs. treatment period and collagen quartile. Serius red histology images from different treatment points, showing progressive fibrosis development during CCl4 treatment (D).
VICM is elevated in hepatitis C patients versus controls
Due to the low number of patients in groups F3 these two groups were not included in the analysis. Demographic and biochemical measurement data for the HCV cohort are seen in Table 2. The mean values of the citrullinated vimentin marker in the groups were: controls (386 ng/ml ±79), F0 (632 ±75.74, p<0.05), F1 (597 ±41.35, p<0.05), F2 (628 ±59.27, p=0.2) (Figure 3).
Table 2.
Characteristics of patients with Hepatitis C virus stratified according to their METAVIR fibrosis score (F). Numbers represent mean values. F3 and F4 groups were not included in the statistical calculations due to insufficient numbers for meaningful statistical analysis.
| F0 | F1 | F2 | |
|---|---|---|---|
|
|
|||
| n=11 | n=44 | n=27 | |
| Age | 46.4 ±7.2 | 44.9 ±7.9 | 45.8 ±7.9 |
| BMI | 24.9 ±3.9 | 25.5 ±3.5 | 24.7 ±3.3 |
| AST (IU/L) | 57.8 ±24.2 | 58.6 ±20.1 | 73.6 ±37.6 |
| ALT (IU/L) | 57.8 ±31.5 | 58.6 ±28 | 73.6 ±74.9 |
| Glucose (mg/dL) | 94.4 ±9.9 | 95.9 ±12.5 | 97.8 ±10.4 |
| Insulin (mcU/mL) | 9.24 ±4.7 | 11.05 ±7.3 | 12 ±5.9 |
Figure 3.
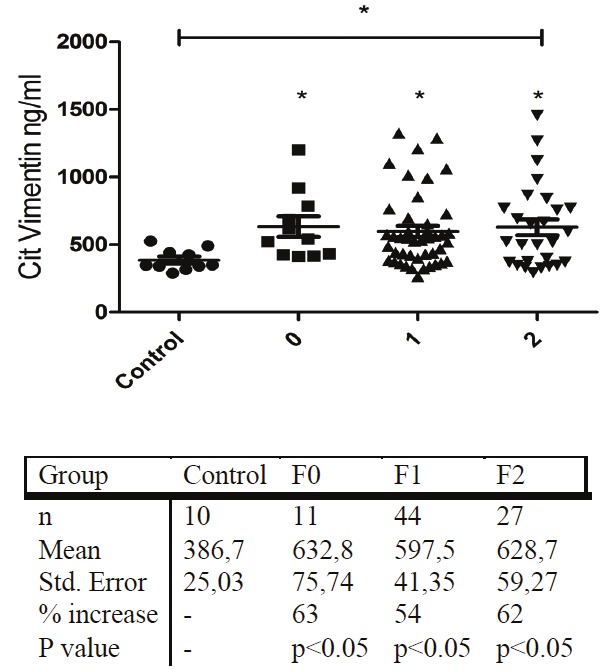
Serum levels of citrullinated vimentin measured in control and hepatitis C patient groups. Control group is comprised of 10 randomly selected individuals with BMI<25 not diagnosed with any liver fibrosis-related pathology.
Demographic and biochemical measurements table for the NAFLD cohort are seen in Table 3. The mean level of the marker in the control group was found to be 281 ng/ml ±55. When compared with the control group, a statistically significant difference (p<0.05) in the biomarker levels was observed in all groups stratified according to Metavir fibrosis score, as follows: F0 group 339 ±53 (p<0.05), F1 348 ±59 (p<0.05) and F2 362 ±80 (p<0.05) (Figure 4).
Table 3.
Demographic table for the control and NAFLD cohort stratified according to fibrosis (F) score. Numbers represent mean values. F3 and F4 groups were not included in the statistical analysis due to inadequate available numbers.
| F0 | F1 | F2 | |
|---|---|---|---|
| n=19 | n=23 | n=10 | |
| Age | 55.4 ±12 | 53.2 ±8.3 | 58 ±6.5 |
| BMI | 30.4 ±5.4 | 31 ±3.45 | 31.8 ±7.2 |
| AST (IU/L) | 36.1 ±28 | 33.9 ±14 | 60.9 ±42 |
| ALT (IU/L) | 50.5 ±44 | 53.2 ±36 | 75.2 ±49 |
| Glucose (mg/dL) | 119 ±59 | 116±40 | 124 ±29 |
| Insulin (mcU/mL) | 16.2 ±10.9 | 17.3 ±7.5 | 18.3 ±13.7 |
| HDL (mg/dL) | 50 ±11 | 51 ±12 | 47.9 ±16 |
| LDL (mg/dL) | 124.5 ±45 | 112.5 ±38 | 108.1 ±34 |
Figure 4.
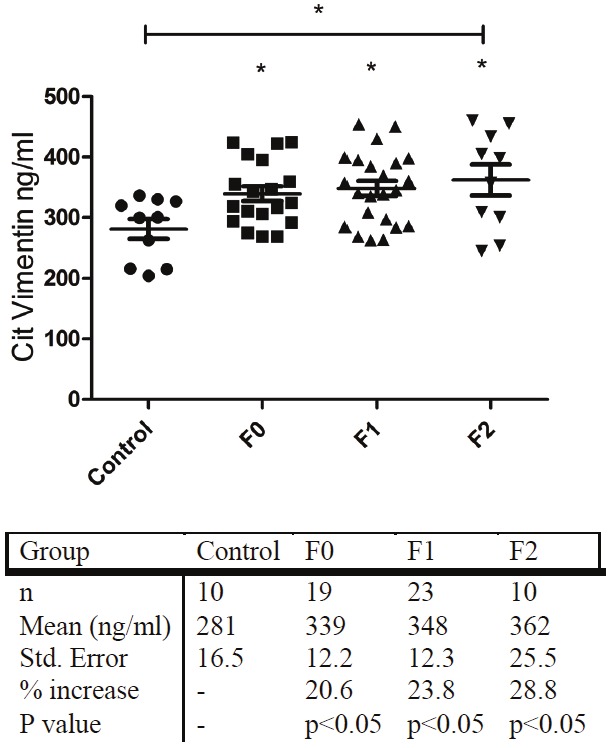
Plasma levels of citrullinated vimentin measured in control and NAFLD patient groups. Control group is comprised of 10 randomly selected individuals with BMI<25, not diagnosed with any liver fibrosis related pathology.
ROC values
The assay was found to have high ROC values for both patient groups. The ROC value for the NAFLD group was found to be higher (ROC=0.77) than the HCV (0.72). The ROC value was highly significant for the HCV groups F0-F2, and NAFLD cohorts (Table 4).
Table 4.
ROC values of citrullinated vimentin in the Hepatitis C and NAFLD clinical cohorts
| Comparison | Area | Std. Error | P Value | |
|---|---|---|---|---|
| Hepatitis C | ||||
| ROC Value | Control Vs. combined groups F0-F2 | 0.72 | 0.07 | <0.05 |
| Control Vs. F0 | 0.80 | 0.09 | <0.05 | |
| Control Vs. F1 | 0.74 | 0.07 | <0.05 | |
| Control Vs. F2 | 0.76 | 0.07 | <0.05 | |
| NAFLD | ||||
| ROC Value | Control Vs. combined groups F0-F2 | 0.77 | 0.07 | <0.01 |
| Control Vs. F0 | 0.74 | 0.09 | <0.05 | |
| Control Vs. F1 | 0.79 | 0.07 | <0.01 | |
| Control Vs. F2 | 0.77 | 0.10 | <0.05 |
Discussion
Citrullinated peptides have been successfully and widely utilised for diagnostic purposes in rheumatic diseases during the last years. Inflammation is a common denominator in both auto-immune disorders and fibrotic processes. Since a variety of ECM-derived peptides been shown to have chemotactic action against inflammatory cells and induce immune responses [50], we hypothesized that citrullinated peptides may also be present in liver fibrosis.
Well-described auto-immune protein targets such as vimentin are also contained in hepatic stellate cells which are key participants in liver fibrosis events [46]. The consideration that citrullination may be an active player in the protein degradation process in a variety of pathologies other than rheumatoid arthritis, which involve high levels of high ECM remodelling formed the basis of our hypothesis.
The first aim of our study was to investigate whether a citrullinated and MMP cleaved fragment of vimentin that has been identified by MS in articular cartilage could be accurately measured in liver fibrosis. A cleavage-site specific ELISA assay was developed and successfully evaluated in a rat model of CCl4 induced fibrosis. The marker was found to be highly statistically elevated in treatment groups when stratified according to both treatment duration and total collagen in the liver (Figure 2A and 2B). Additionally the marker was also found to be correlated with the total collagen increase as measured by quantitative histology (Figure 2C). The above results indicate that the marker can be measured and is elevated during liver fibrosis progression. The correlation between the marker and disease progression as measured by total collagen increase is an indicator of the markers relationship with the pathologic staging of the disease.
The second aim of the study was to assess whether the citrullinated and MMP-degraded vimentin marker could be used as liver fibrosis biomarker for diagnostic purposes. For this reason the marker was measured in two clinical cohorts of adult patients with HCV and NASH. A statistically significant increase of the marker was observed in both clinical cohorts in early disease onset indicating that the marker is also present not only in preclinical settings but also in two different clinical fibrosis cohorts (Figures 3 and 4). The markers ROC value of 0.71 and 0.77 for HCV and NAFLD respectively indicate a potential diagnostic value for the marker.
The marker showed a different pattern of up regulation between the pre-clinical and clinical cohort. While in the pre-clinical study the marker has shown to be slowly increasing with time and CCl4 administration, both clinical cohorts have revealed a statistically significant increase of the marker early on disease onset. The above indicates that the VICM marker is correlated to severity in the animal model but not in the clinical samples. The pre-clinical model used is an acute model of liver fibrosis induction, while fibrosis development in humans is a chronic process which cannot be mimicked by our pre-clinical study.
In both clinical cohorts, VICM was significantly increased in the early fibrosis groups (F0-F2), compared with healthy controls. Even though the results of this cross-sectional study are not adequate to determine the marker’s prognostic role, the high values obtained from ROC analysis for the F0-F2 groups indicate a potential increased value of the marker in early stages of HCV and NAFLD patients. However, a longitudinal study is required to further investigate this finding.
Our results from the two clinical cohorts indicate that increased levels of the citrullinated vimentin fragment are elevated in patients with HCV or NAFLD compared to healthy patients. We did not observe any relevance to severity in these patients. The observation that levels of the marker are also found in healthy controls suggests that citrullinated vimentin peptides may play a part in normal physiological processes.
The mass spectrometry results obtained (Table 1), showed the prevalence of lysine (47%) at the C terminal end of recognised peptides, while the second most abundant aminoacid was found to be arginine (37%). Lysine is an aminoacid residue associated with ubiquitination which is known to act as an intracellular signal for protein destruction. The high prevalence of arginine residues on the obtained mass spectrometry raises the question regarding citrullination as a signal for an extracellular signal for protein destruction which needs to be further investigated.
Our findings, together with a recent study showing that a commercially available anti-modified citrullinated vimentin antibody used in rheumatoid arthritis had also detected increased levels of citrullinated vimentin in HCV patients, suggest the following key conclusions: 1) The citrullinated and MMP cleaved fragment of vimentin RLRSSVPGV-citrulline that has been identified by MS in articular cartilage can be accurately measured in liver fibrosis in both rat CCl4 live fibrosis preclinical model and in the NAFLD and HCV clinical cohorts. 2) The marker is correlated with pathology progression in the rat CCl4 liver fibrosis model. 3) Citrullination may be implicated in a variety of pathologies other than RA. In conclusion, MMP degrade and citrullinated vimentin assessed by the VICM assay may be related to the pathology of liver fibrosis and its role should be further investigated. We believe that it is a biomarker that may be providing additional diagnostic information. Utilisation of biomarkers reflecting changes in the intensity of these processes, may assist in patient monitoring in early disease stages and could even assist in the discovery and development of novel treatments for various fibrotic diseases in which excessive citrullination occurs. Additionally, since both vimentin and citrulline have been previously linked to inflammatory events in other diseases, the elevated marker levels may reflect early inflammatory processes and should be investigated further in future.
Study limitations
The main limitations of the present study are found in the low numbers of patients especially with a fibrosis score of F3 or F4 to fully describe the relevance of the marker across the full spectrum of fibrosis development. Another limitation of the study is the relatively small size of both clinical cohorts and thus they may not accurately represent the actual marker levels during disease progression. Thus additional measurements in larger cohorts are needed in order to fully describe the marker levels and further validate its presence during fibrosis progression. A further limitation is that even though MS data shows that the described fragment is derived from MMP-2 and -8 activity, other proteases or a combination of proteases could potentially generate this fragment in vivo. The study lacks immunohistochemistry and western blot data due to the lack of tissue from the enrolled patients. Even though the marker can be accurately measured in serum/plasma, it may be also present in other body fluids such as urine and saliva. These have not been assessed in this study due to lack of relevant material, and thus prevented an assessment of the influence of renal function on marker levels. Lastly the marker should be evaluated in a well characterised rheumatoid arthritis cohort in order to validate its utilisation in rheumatic diseases.
Acknowledgement
We acknowledge the Danish Ministry of Science, Technology and Innovation and the Den Danske Forskningsfond, Centre for Clinical and Basic Research. We would also like to acknowledge the Brazilian Ministry of Education (CAPES foundation) and FIPE-HCPA and Peter Mitchell from Elli Lilly and Company.
Author contribution
Efstathios Vassiliadis: manuscript draft, study concept and design, data acquisition analysis and interpretation, statistical analysis. Claudia P Oliveira: study concept and design, data acquisition analysis and interpretation. Mario R Alvares-da-Silva: study concept and design, collection of clinical samples, data acquisition analysis and interpretation. Chen Zhang: Data acquisition, collection of clinical samples assay development and analysis, statistical analysis. Flair J Carrilho: Collection of clinical samples, data acquisition and analysis, collection of clinical samples. Jose T Stefano: Collection of clinical samples, data acquisition and analysis, statistical analysis. Fabiola Rabelo: Collection of clinical samples, data acquisition and analysis, statistical analysis. Leila Pereira: Data acquisition and analysis, statistical analysis (not this but collection of clinical samples!?). Camila R Kappel: Data acquisition and analysis, collection of clinical samples, statistical analysis. Kim Henriksen: manuscript draft. Sanne Skovgård Veidal: manuscript draft. Ben Vainer: manuscript draft. Kevin L Duffin: Data acquisition. Claus Christiansen: critical revision of the manuscript for important intellectual content. Diana J Leeming: assay development, manuscript draft, study concept and design, analysis and interpretation. Morten Karsdal: manuscript draft, study concept and design, analysis and interpretation. All authors have read and approved the manuscript
References
- 1.Charles Streuli. Extracellular matrix remodelling and cellular differentiation. Current Opinion in Cell Biology. 1999;11:634–640. doi: 10.1016/s0955-0674(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 2.Shigeki Tsukadaa, Christopher JP, Richard AR. Mechanisms of liver fibrosis. Clinica Chimica Acta. 2006;364:33–60. doi: 10.1016/j.cca.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 4.Copple BL, Allen K, Welch TP. Mechanisms of Liver Fibrosis. Comprehensive Toxicology. 2010;9:263–274. [Google Scholar]
- 5.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 6.Veidal SS, Vassiliadis E, Bay-Jensen AC, Tougas G, Vainer B, Karsdal MA. Procollagen type I N-terminal propeptide (PINP) is a marker for fibrogenesis in bile duct ligation-induced fibrosis in rats. Fibrogenesis Tissue Repair. 2010;3:5. doi: 10.1186/1755-1536-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benyon RC, Arthur MJ. Extracellular Matrix Degradation and the Role of Hepatic Stellate Cells. Semin Liver Dis. 2011;21:373–384. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- 8.Amălinei C, Căruntu ID, Giuşcă SE, Bălan RA. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51:215–228. [PubMed] [Google Scholar]
- 9.Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis –a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46:955–975. doi: 10.1016/j.jhep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:245–249. doi: 10.1152/ajpgi.2000.279.2.G245. [DOI] [PubMed] [Google Scholar]
- 11.Meri S, Baumann M. Proteomic: Post translational modifications immune responces and current analytical tools. Biomolecular Engineering. 2001;18:213–220. doi: 10.1016/s1389-0344(01)00106-x. [DOI] [PubMed] [Google Scholar]
- 12.Karsdal MA, Henriksen K, Leeming DJ, Woodworth T, Vassiliadis E, Bay Jensen AC. Novel combinations of post transaltional modification (PTM) neo-epitopes provide tissuespecific biochemical markers-are they the cause or the consequence of the disease? Clinical Biochemistry. 2010;43:793–804. doi: 10.1016/j.clinbiochem.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Erikcsson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004;117:919–932. doi: 10.1242/jcs.00906. [DOI] [PubMed] [Google Scholar]
- 14.Nijenhuis S, Zendman AJ, Vossenaar ER, Pruijn GJ, vanVenrooij WJ. Autoantibodies to citrullinated proteins in rheumatoid arthritis: clinical performance and biochemical aspects of an RA-specific marker. Clin Chim Acta. 2004;350:17–34. doi: 10.1016/j.cccn.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 15.Sghiri R, Khalifa O, Bouajina E, Ben Haj Slama F, Baccouche K, Ben Fredj H, Gaha R, Boukadida J. Presence and diagnostic performances of IgA class antibodies to mutated citrullinated vimentin in rheumatoid arthritis. Joint Bone Spine. 2010;77:279–280. doi: 10.1016/j.jbspin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Goëb V, Jouen F, Gilbert D, Le Loët X, Tron F, Vittecoq O. Diagnostic and prognostic usefulness of antibodies to citrullinated peptides. Joint Bone Spine. 2009;76:343–349. doi: 10.1016/j.jbspin.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion migration, and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 18.Asaga H, Yamada M, Senshu T. Selective Deimination of Vimentin in Calcium Ionophore-Induced Apoptosis of Mouse Peritoneal Macrophages. Biochem Biophys Res Commun. 1998;243:641–646. doi: 10.1006/bbrc.1998.8148. [DOI] [PubMed] [Google Scholar]
- 19.Müller K, Dulku S, Hardwick SJ, Skepper JN, Mitchinson MJ. Changes in vimentin in human macrophages during apoptosis induced by oxidised low density lipoprotein. Atherosclerosis. 2001;156:133–144. doi: 10.1016/s0021-9150(00)00641-9. [DOI] [PubMed] [Google Scholar]
- 20.van Gaalen F, Ioan-Facsinay A, Huizinga TW, Toes RE. The Devil in the Details: The Emerging Role of Anticitrulline Autoimmunity in Rheumatoid Arthritis. J Immunol. 2005;175:5575–5580. doi: 10.4049/jimmunol.175.9.5575. [DOI] [PubMed] [Google Scholar]
- 21.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioassays. 2003;25:1106–1118. doi: 10.1002/bies.10357. [DOI] [PubMed] [Google Scholar]
- 22.György B, Tóth E, Tarcsa E, Falus A, Buzás EI. Citrullination: A posttranslational modification in health and disease. Int J Biochem Cell Biol. 2006;38:1662–1677. doi: 10.1016/j.biocel.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Tarcsa E, Marekov LN, Mei G, Melino G, Lee SC, Steinert PM. Protein unfolding by peptidylarginine deiminase. Substrate specificity and structural relationships of the natural substrates trichohyalin and filaggrin. J Biol Chem. 1996;271:30709–30716. doi: 10.1074/jbc.271.48.30709. [DOI] [PubMed] [Google Scholar]
- 24.Bannister AJ, Kouzarides T. Reversing histone methylation. Nature. 2005;436:1103–1106. doi: 10.1038/nature04048. [DOI] [PubMed] [Google Scholar]
- 25.Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 26.Harauz G, Ishiyama N, Hill CM, Bates IR, Libich DS, Farès C. Myelin basic protein – diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron. 2004;35:503–542. doi: 10.1016/j.micron.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 27.McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immunol. 1999;67:3248–3256. doi: 10.1128/iai.67.7.3248-3256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishida-Yamamoto A, Senshu T, Takahashi H, Akiyama K, Nomura K, Iizuka H. Decreased deiminated keratin K1 in psoriatic hyperproliferative epidermis. J Invest Dermatol. 2000;114:701–705. doi: 10.1046/j.1523-1747.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- 29.Ehrlich A, Booher S, Becerra Y, Borris DL, Figg WD, Turner ML, Blauvelt A. Micellar paclitaxel improves severe psoriasis in a prospective phase II pilot study. J Am Acad Dermatol. 2004;50:533–540. doi: 10.1016/j.jaad.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Mastronardi FG, Mak B, Ackerley CA, Roots BI, Moscarello MA. Modifications of basic protein in DM20 transgenic mice are similar to those in myelin basic protein from multiple sclerosis. J Clin Invest. 1996;97:349–358. doi: 10.1172/JCI118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nienhuis RL, Mandema E. A new serum factor in patients with rheumatoid arthritis; the anti-perinuclear factor. Ann Rheum Dis. 1964;23:302–305. doi: 10.1136/ard.23.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girbal-Neuhauser E, Durieux JJ, Arnaud M, Dalbon P, Sebbag M, Vincent C, Simon M, Senshu T, Masson-Bessière C, Jolivet-Reynaud C, Jolivet M, Serre G. The epitopes targeted by the rheumatoid arthritis-associated antifilaggrin autoantibodies are posttranslationally generated on various sites of (pro) filaggrin by deimination of arginine residues. J Immunol. 1999;162:585–594. [PubMed] [Google Scholar]
- 34.Lutteri L, Malaise M, Chapelle JP. Comparison of second and third generation anti cyclic citrullinated peptide antibodies assays for detecting rheumatoid arthritis. Clinica Chimica Acta. 2007;386:76–81. doi: 10.1016/j.cca.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Vossenaar ER, van Venrooij WJ. Anti-CCP antibodies, a highly specific marker for (early) rheumatoid arthritis. Clinical and Applied Immunology Reviews. 2004;4:239–262. [Google Scholar]
- 36.Marcelletti JF, Nakamura RM. Assesment of serological markers associated with rheumatoid arthritis: Diagnostic autoantibodies and conventional disease activity markers. Clinical and Applied Immunology Reviews. 2003;4:109–123. [Google Scholar]
- 37.Goldbach-Mansky R, Lee JM, Hoxworth JM, Smith D 2nd, Duray P, Schumacher RH Jr, Yarboro CH, Klippel J, Kleiner D, El-Gabalawy HS. Active synovial matrix metalloproteinase-2 is associated with radiographic erosions in patients with early synovitis. Arthritis Res. 2000;2:145–153. doi: 10.1186/ar79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldbach-Mansky R, Lee J, McCoy A, Hoxworth J, Yarboro C, Smolen JS, Steiner G, Rosen A, Zhang C, Ménard HA, Zhou ZJ, Palosuo T, Van Venrooij WJ, Wilder RL, Klippel JH, Schumacher HR Jr, El-Gabalawy HS. Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res. 2000;2:236–243. doi: 10.1186/ar93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holm A, Rise F, Sessler N, Sollid LM, Undheim K, Fleckenstein B. Specific modification of peptide-bound citrulline residues. Analytical Biochemistry. 2006;352:68–76. doi: 10.1016/j.ab.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Evans RM. Intermediate filaments and lipoprotein cholesterol. Trends Cell Biol. 1994;4:149–151. doi: 10.1016/0962-8924(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 41.McInroy L, Määttä A. Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem Biophys Res Commun. 2007;360:109–114. doi: 10.1016/j.bbrc.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 42.Benes P, Macecková V, Zdráhal Z, Konecná H, Zahradnícková E, Muzík J, Smarda J. Role of vimentin in regulation of monocyte/macrophage differentiation. Differentiation. 2006;74:265–276. doi: 10.1111/j.1432-0436.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 43.Geerts A, Eliasson C, Niki T, Wielant A, Vaeyens F, Pekny M. Formation of normal desmin intermediate filaments in mouse hepatic stellate cells requires vimentin. Hepatology. 2001;33:177–188. doi: 10.1053/jhep.2001.21045. [DOI] [PubMed] [Google Scholar]
- 44.Miyata E, Masuya M, Yoshida S, Nakamura S, Kato K, Sugimoto Y, Shibasaki T, Yamamura K, Ohishi K, Nishii K, Ishikawa F, Shiku H, Katayama N. Hematopoietic origin of hepatic stellate cells in the adult liver. Blood. 2008;111:2427–2435. doi: 10.1182/blood-2007-07-101261. [DOI] [PubMed] [Google Scholar]
- 45.Zhen EY, Brittain IJ, Laska DA, Mitchell PG, Sumer EU, Karsdal MA, Duffin KL. Characterization of metalloprotease cleavage products of human articular cartilage. Arthritis Rheum. 2008;58:2420–2431. doi: 10.1002/art.23654. [DOI] [PubMed] [Google Scholar]
- 46.Abheen S, Olusi SO, George S. Serum anti-modified citrullinated vimentin antibody concentration is associated with liver fibrosis in patients with chronic hepatitis. Hepatic Medicine: Evidence and Research. 2011;3:13–18. doi: 10.2147/HMER.S17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Burt AD. The diffuse stellate cell system. J Mol Histol. 2007;38:53–64. doi: 10.1007/s10735-007-9078-5. [DOI] [PubMed] [Google Scholar]
- 48.Combet C, Blanchet C, Geourjon C, Deleage G. NPS@: network protein sequence analysis. Trends Biochem Sci. 2000;25:147–150. doi: 10.1016/s0968-0004(99)01540-6. [DOI] [PubMed] [Google Scholar]
- 49.Gefter ML, Margulies DH, Scharff MD. A simple method for polyethylene glycolpromoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977;3:231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- 50.ir-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


