Abstract
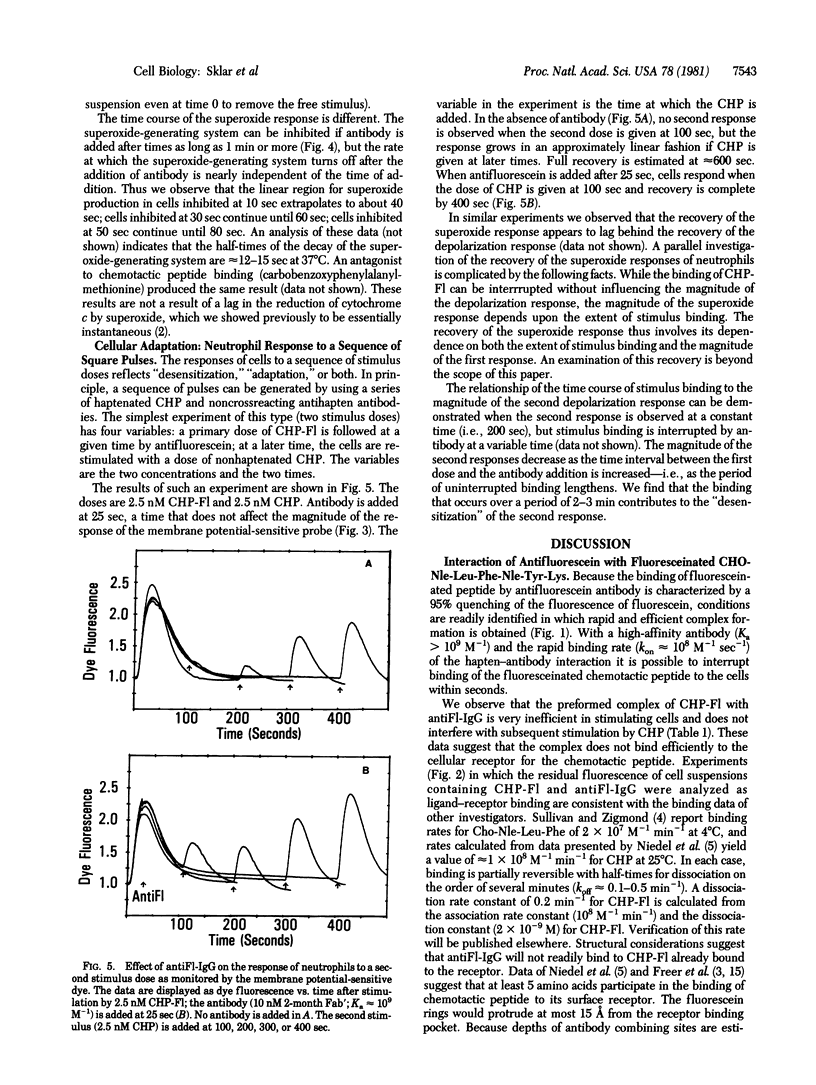
Antifluorescein antibody molecules were used to interrupt the stimulation of neutrophils by a fluoresceinated chemotactic peptide. From the results we construct a semiquantitative relationship among ligand-receptor interaction, the time course of cell triggering and response, and aspects of cellular adaptation. The interaction of the antibody with the free fluoresceinated peptide is complete within a few seconds and the peptide-antibody complex neither stimulates the cells nor inhibits subsequent stimulation by unlabeled peptide. When antibody is added to a cell suspension that has been stimulated with the fluoresceinated peptide, we observe that: (i) the apparent membrane depolarization response monitored by a fluorescent dye can be inhibited only if antibody is added within 30 sec of stimulation; (ii) the superoxide response can be inhibited even if antibody is added more than 1 min after stimulation and decays with an intrinsic half-life of about 12 sec; (iii) responses to a second dose of nonfluoresceinated peptide are enhanced if the antibody is added within 2 min of stimulation by the fluoresceinated peptide. These results suggest that different neutrophil responses depend in individual ways on the time course and extent of ligand binding to its receptor. A comparison of these data with the time course of binding permits an estimate of the number of receptors involved in these responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freer R. J., Day A. R., Radding J. A., Schiffmann E., Aswanikumar S., Showell H. J., Becker E. L. Further studies on the structural requirements for synthetic peptide chemoattractants. Biochemistry. 1980 May 27;19(11):2404–2410. doi: 10.1021/bi00552a019. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison S. A., Hicks A. N., Portmann A. J., Dandliker W. B. Fluorescence polarization and intensity kinetic studies of antifluorescein antibody obtained at different stages of the immune response. Biochemistry. 1975 Aug 26;14(17):3778–3786. doi: 10.1021/bi00688a009. [DOI] [PubMed] [Google Scholar]
- Mercola D. A., Morris J. W., Arquilla E. R. Use of resonance interaction in the study of the chain folding of insulin in solution. Biochemistry. 1972 Oct 10;11(21):3860–3874. doi: 10.1021/bi00771a005. [DOI] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Cuatrecasas P. Formyl peptide chemotactic receptors of leukocytes and macrophages. Curr Top Cell Regul. 1980;17:137–170. doi: 10.1016/b978-0-12-152817-1.50009-2. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kahane I., Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979 Sep 28;205(4413):1412–1414. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- Portmann A. J., Levison S. A., Dandliker W. B. Equilibrium binding studies on antifluorescein antibody during various stages of the immune response. Immunochemistry. 1975 Jul;12(6-7):461–466. doi: 10.1016/0019-2791(75)90066-x. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J Biol Chem. 1981 Oct 10;256(19):9909–9914. [PubMed] [Google Scholar]
- Sullivan S. J., Zigmond S. H. Chemotactic peptide receptor modulation in polymorphonuclear leukocytes. J Cell Biol. 1980 Jun;85(3):703–711. doi: 10.1083/jcb.85.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Green G., Schumaker V. N. Bivalent hapten-antibody interactions--11. Bivalent haptens as probes of combining site depth. Immunochemistry. 1975 Jan;12(1):49–54. doi: 10.1016/0019-2791(75)90048-8. [DOI] [PubMed] [Google Scholar]



