Abstract
When pathogenic bacteria breach the epithelial lining at mucosal surfaces, rapidly available innate immune mechanisms are critical to halt the infection. In the present study, we characterized the production of antibacterial polypeptides released by epithelial cells. IFN-γ, but neither TNF nor IL-1β alone, induced release of antibacterial activity to a cell culture medium, causing a lytic appearance of killed bacteria as revealed by electron microscopy. Addition of the protein streptococcal inhibitor of complement, derived from Streptococcus pyogenes, known for its ability to neutralize antimicrobial polypeptides (AMPs), reduced the antibacterial activity of the medium. Characterization of the antibacterial incubation medium using mass spectrometric approaches and ELISAs, displayed presence of several classical AMPs, antibacterial chemokines, as well as complement factors and proteases that may interfere with bacterial killing. Many were constitutively produced, that is, being released by cells incubated in a medium alone. While a combination of IFN-γ and TNF did not increase bacterial killing, the presence of TNF boosted the amounts and detectable number of AMPs, including antibacterial chemokines. However, the methods applied in the study failed to single out certain AMPs as critical mediators, but rather demonstrate the broad range of molecules involved. Since many AMPs are higly amphiphatic in nature (i.e., cationic and hydrophobic), it is possible that difficulties in optimizing recovery present limitations in the context investigated. The findings demonstrate that epithelial cells have a constitutive production of AMPs and that IFN-γ is an important inducer of an antibacterial response in which is likely to be a critical part of the innate host defense against pathogenic bacteria at mucosal surfaces.
Introduction
When pathogens start colonizing a mucosal surface, readily available host defense mechanisms at the epithelial lining are crucial to prevent disease. Normally, pathogens are eliminated, but if the defense is insufficient, the pathogen can invade underlying tissues and the disease may evolve from a local infection into a systemic, life-threatening disease (Bisno and others 2003; Sansonetti 2004). Many individuals are colonized by potentially virulent bacteria without having clinical signs of infection (Zwart and others 2003). However, if the epithelial lining is injured, an inflammatory response is initiated through activation of immune cells residing in submucosal tissues. This response generates production of proinflammatory cytokines that orchestrate appropriate host responses against the invading bacteria. Interferon (IFN)-γ is a key regulator of Th1-polarized immune responses seen during bacterial infections (Kolls and others 2008), and can affect epithelial cells of the mucosal layer, resulting in an activated phenotype (Sauty and others 1999; Strieter and others 2002; Sansonetti 2004).
The activated epithelium can elaborate antimicrobial polypeptides (AMPs), among them human β-defensins (hBD) and antibacterial chemokines that play important roles during early stages of bacterial infection (Sauty and others 1999; Egesten and others 2007). Distinguished features of AMPs are that they are mostly small (molecular weights of less than 15 kDa) and cationic (pI often above 9). The latter is a feature that, together with intermingled hydrophobic amino acid residues, results in domains with an amphipathic secondary structure. This structure provides a propensity to disrupt bacterial membranes (Hancock and Diamond 2000; Boman 2003; Ganz 2004). However, there may be other targets in bacteria, for example, mechanisms resulting in bacteriostatic effects, such as depletion of iron in the case of neutrophil gelatinase-associated lipocalin (NGAL) (Borregaard and Cowland 2006). Most AMPs are multifunctional and many possess chemotactic activity and some act as growth factors (De and others 2000; Malmsten and others 2007). Some AMPs belong to the large family of chemokines that are peptides sharing glycosaminoglycan (GAG)-binding properties, and that attract and activate leukocytes during immune responses. In addition, some chemokines act as growth factors and yet some posses antibacterial activity (Baggiolini 2001; Cole and others 2001; Yang and others 2003; Egesten and others 2009). In parallel with AMPs, other arms of innate immunity include, for example, the complement system, ficolins, unstable oxygen derivatives, and cell-mediated responses executed by neutrophils and NK cells (Rada and Leto 2008; Matsushita 2010).
Pharyngitis is one of the most common mucosal infections in humans, often caused by the Gram-positive bacterium Streptococcus pyogenes (Cunningham 2000). In most cases, the infection is resolved without the use of antibiotics, indicating that robust local host defense mechanisms normally eradicate the bacteria (Zwart and others 2003). In a previous study, we used the cDNA microarray technique to analyze the differential regulation of AMP gene expression in pharyngeal epithelial cells stimulated with a combination of IFN-γ and M1 protein of S. pyogenes. The most upregulated and expressed genes were several antibacterial chemokines (Eliasson and others 2007). Previously, we have also showed that bactericidal activity is retained on the surface of pharyngeal epithelial cells stimulated by a combination of IFN-γ and tumor necrosis factor (TNF), and dependent on the chemokine CXCL9/MIG (monokine induced by IFN-γ), possibly associated with GAGs (Egesten and others 2007). We also observed a decreased bactericidal activity in a medium from IFN-γ-stimulated pharyngeal epithelial cells where CXCL9/MIG expression was subjected to siRNA knockdown. In vivo, neutralization of CXCL9/MIG, CXCL10/IP-10, and CXCL11/I-TAC using antibodies, impaired host defense in an anthrax-model of airway infection (Crawford and others 2010). Furthermore, using genetically modified mice, IFN-γ-induced responses were required to prevent local dissemination of S. pyogenes, the draining lymph nodes (Hyland and others 2009). Taken together, these findings suggest important roles for IFN-γ-dependent antibacterial chemokines in hot defense at epithelial surfaces.
In the present study, proteomic approaches were used to characterize the production of AMPs and antibacterial chemokines. We demonstrate that epithelial cells alone, after appropriate stimuli, can produce a broad range of AMPs and antibacterial chemokines, resulting in antibacterial activity. This bactericidal response is likely to be important for the outcome of mucosal infections.
Materials and Methods
Reagents
Human recombinant IFN-γ, TNF, interleukin (IL)-1β, CXCL9/MIG, CXCL10/IP-10, CXCL11/I-TAC, CXCL1/GRO-α, CXCL2/GRO-β, CXCL3/GRO-γ, CCL20/MIP-3α, CCL28/MEC, and ELISAs for the detection of hBD-1, hBD-2, and hBD-3 were from Peprotech, Rocky Hill, NJ. Recombinant secretory leukocyte protease inhibitor (SLPI) and ELISAs for the detection of CXCL9/MIG, CXCL10/IP-10, CXCL11/I-TAC, CXCL1/GRO-α, CXCL2/GRO-β, CCL20/MIP-3α, CCL28/MEC, and SLPI were purchased from R&D Systems, Abingdon, United Kingdom. Additional ELISAs were as follows: CXCL3/GRO-γ (Antigenix America, Huntington Station, NY), lactotransferrin (Kamiya Biomedical, Seattle, WA), and lysozyme (Calbiotech, Spring Valley, CA). Protein streptococcal inhibitor of complement (SIC) of the S. pyogenes strain AP1 was purified as described (Åkesson and others 1996). A cellular luminescence enhancement system for superoxide detection (Diogenes) was from National Diagnostics, Atlanta, GA. L-NAME was from Cayman Chemical, Ann Arbor, MI. Diphenyleneiodonium chloride, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, NADPH, nitrate reductase, and Griess reagent, were from Sigma-Aldrich, Stockholm, Sweden. Centrifugal filter devices (Nanosep; cutoff 100 kDa) were from PALL Life Sciences, Port Washington, NY.
Cell culture and stimulation with cytokines
The human pharyngeal epithelial cell line Detroit 562 (ATCC CCL 138; ATCC) was cultured in 25-cm2 cell culture flasks (NUNC, Slangerup, Denmark) using the Minimal Essential Medium (MEM) with Earle's salt (Invitrogen, Stockholm, Sweden) supplemented with 2 mM glutamine (Invitrogen), 10% heat-inactivated fetal bovine serum (FBS; Invitrogen), amphotericin B (0.25 μg mL−1; Invitrogen), penicillin/streptomycin (100 U mL−1 and 0.1 ng mL−1, respectively; Invitrogen), referred to as the “culture medium,” at 37°C in an atmosphere containing 5% CO2 with 100% relative humidity. To investigate conditions resulting in soluble bactericidal activity, Detroit cells were cultured to near confluence in 96-well plates, washed 3 times followed by stimulation in the “incubation medium” (MEM with Earle's salt supplemented with 2 mM glutamine) for 24 h as follows: IFN-γ (100 U mL−1), IFN-γ+TNF (10 ng mL−1), IFN-γ+IL-1β (10 ng mL−1), IFN-γ+TNF+IL-1β, TNF alone, and IL-1β alone. In addition, cells were stimulated with IFN-γ (100 U mL−1) for 1, 2, 4, 8, 16, and 24 h, respectively, to investigate a possible time-dependency with respect to bactericidal activity. The incubation medium was collected and used in a viable count assay and characterization of AMP content with ELISA and liquid chromatography–tandem mass spectrometry (LC-MS/MS) as described below.
Bacteria
The S. pyogenes strain AP1 of the M1 serotype was from the WHO Collaborating Centre for Reference and Research on Streptococci, Institute of Hygiene and Epidemiology, Prague, Czech Republic.
Bacterial killing assay (viable count)
The incubation medium, collected as described above, was used in a viable count assay. AP1 bacteria were grown at 37°C, 5% CO2, to mid-logarithmic phase (OD620=0.4) in the Todd-Hewitt broth (TH; Difco, Stockholm, Sweden), washed, and diluted in 10 mM Tris (pH 7.5) containing 5 mM glucose (Tris-glucose) to a concentration of 1% [2×106 colony-forming units (cfu) mL−1]. About 25 μL of the incubation medium was incubated with 25 μL of suspended bacteria for 1 h at 37°C (in an atmosphere containing 5% CO2 with 100% relative humidity). To quantify bactericidal activity, serial dilutions of the incubation mixtures were plated on TH agar, and the number of cfu was determined.
To determine the bactericidal activity of recombinant peptides against S. pyogenes, 50 μL of AP1 bacteria (2×106 cfu mL−1) was incubated with peptides, at various concentrations or Tris-glucose alone (control) for 1 h at 37°C. To quantify bactericidal activity, serial dilutions of the incubation mixtures were plated on TH agar, and the number of cfu was determined.
To investigate if the protein SIC of S. pyogenes interfered with the bactericidal activity, the incubation medium from IFN-γ-stimulated cells were preincubated with SIC at increasing concentrations (0, 0.01, 0.1, 0.5, 1 μM) or heparin-Sepharose (GE-Healthcare, Uppsala, Sweden) for 20 min, and then incubated with AP1 bacteria for 1 h followed by measurement of bactericidal activity using the viable count assay.
Electron microscopy
AP1 bacteria were grown to the mid-logarithmic phase followed by incubation for 1 h at 37°C in the incubation medium from nonstimulated or IFN-γ-stimulated (24 h) Detroit cells. To determine the bactericidal activity, part of the sample was plated on TH agar plates, incubated at 37°C overnight, and the cfu were counted the following day. The remaining parts of the samples were processed for electron microscopy as described (Bengtson and others 2008). In short, samples (5 μL) were absorbed for 45 s onto the grids followed by 2 washes with distilled water. The samples were stained for 3 s with 0.75% uranyl formate droplets followed by staining for an additional 15–20 s with 0.75% uranyl formate. Samples were examined using a Jeol JEM 1230 EX transmission electron microscope operated at 60 kV accelerating voltage.
Electrophoresis and LC-MS/MS
To precipitate polypeptides, the incubation medium from nonstimulated Detroit cells (control), and from cells stimulated with IFN-γ (100 U mL−1) or IFN-γ in combination with TNF (10 ng mL−1) was incubated with acetone (volume 1:5) at −20°C over night and cold centrifuged 20,000 g for 30 min the following day.
The supernatant was discarded and the precipitated polypeptides were extracted by dissolving the pellet in 6 M urea (100 μL). Large complexes (e.g., glycoproteins) were removed by centrifuging the material over a Nanosep® centrifugation device (cutoff 100 kDa, 10 min, 3000 g, room temperature). The flow through material was collected and the polypeptides were separated on a Tris-tricine gel (16.5%). The peptides and proteins of the gel were silver stained using a commercial kit (SilverQuest; Invitrogen) and visualized bands were cut out and destained. The polypeptides were reduced by DTT, alkylated using iodacetamide, and digested using porcine trypsin (Promega, Madison, WI). This was followed by extraction from the gel, drying, and resuspension in formic acid (0.1%). The samples were analyzed by LC-MS/MS on a QTOF Ultima API (ESI-MS/MS; Waters, Manchester, United Kingdom). After injection, the peptides were trapped on a C18 precolumn (300 μm×5 mm, 5 μm, 100 Å; LC-Packings/Dionex, Stockholm, Sweden) and separated on an Atlantis C18 reversed-phase analytical column (75 μm×150 mm, 3 μm, 100 Å; Waters). The mass spectrometer analysis was made by data dependent acquisition. The mass range was from 400 to 1600 for MS and from 50 to 1800 for MS/MS. Only spectra from ions with charge states 2 and 3 were acquired. The MS/MS data generated by the LC-MS experiment were analyzed using MASCOT (Matrix Science, Boston, MA) against the human section of the IPI protein database (as of 22th September 2009 with 84,300 entries; European Bioinformatics Institute). Enzyme specificity was set to trypsin with one missed cleavage using carbamidomethylcysteine as a fixed modification and oxidation of methionines as a variable modification. The tolerance of the precursor ion was set to 0.1 mass units for both parent and fragment ion matches. All hits with a P value<0.05 were manually verified and only proteins detected by at least 2 different peptides were considered positively identified.
Microcapillary reversed-phase liquid chromatography and MS/MS
Fifty microliters of the incubation medium (nonstimulated and stimulated) were mixed with 50 μL 8 M urea and 100 mM ammonium bicarbonate. The proteins were reduced with 5 mM TCEP, and alkylated with 10 mM iodoacetamide, before diluted with 100 mM fresh ammonium bicarbonate. The polypeptides were digested using porcine trypsin (Promega, Madison, WI) 1 μg over night. After digestion, the peptides were cleaned-up using spin columns from Harvard Apparatus (Holliston, MA) according to the manufacturer's instructions. The setup of the microcapillary reversed-phase liquid chromatography (μRPLC) system was as described previously (Yi and others 2003). ESI-based LC–MS/MS (LTQ Thermofinnigan, San Jose, CA) analysis was carried out using an Agilent 1100 series (Agilent Technologies, Paolo Alto, CA) on a 75-μM×10.5-cm fused silica microcapillary reversed-phase column as described previously (Malmström and others 2006a). MS/MS spectra were searched using the XTandem search tool (Craig and Beavis 2003) against the human Swissprot database version 57.4. The search was performed allowing for unconstrained enzyme search, specifying monoisotopic mass and mass tolerance of 3 Da, and with methione oxidation as a variable modification. The database search results were first processed using the PeptideProphet program (Keller and others 2002). Peptides were assembled into proteins and the probabilities at the protein level were computed by the protein inference program ProteinProphet (Nesvizhskii and others 2003) and a reversed database search strategies (Elias and Gygi 2007). The selected cutoff was at 1% false discovery rate. The spectral counting was achieved using the 2DDB software (Malmström and others 2002, 2006b).
Results
IFN-γ induces a soluble bactericidal response in epithelial cells
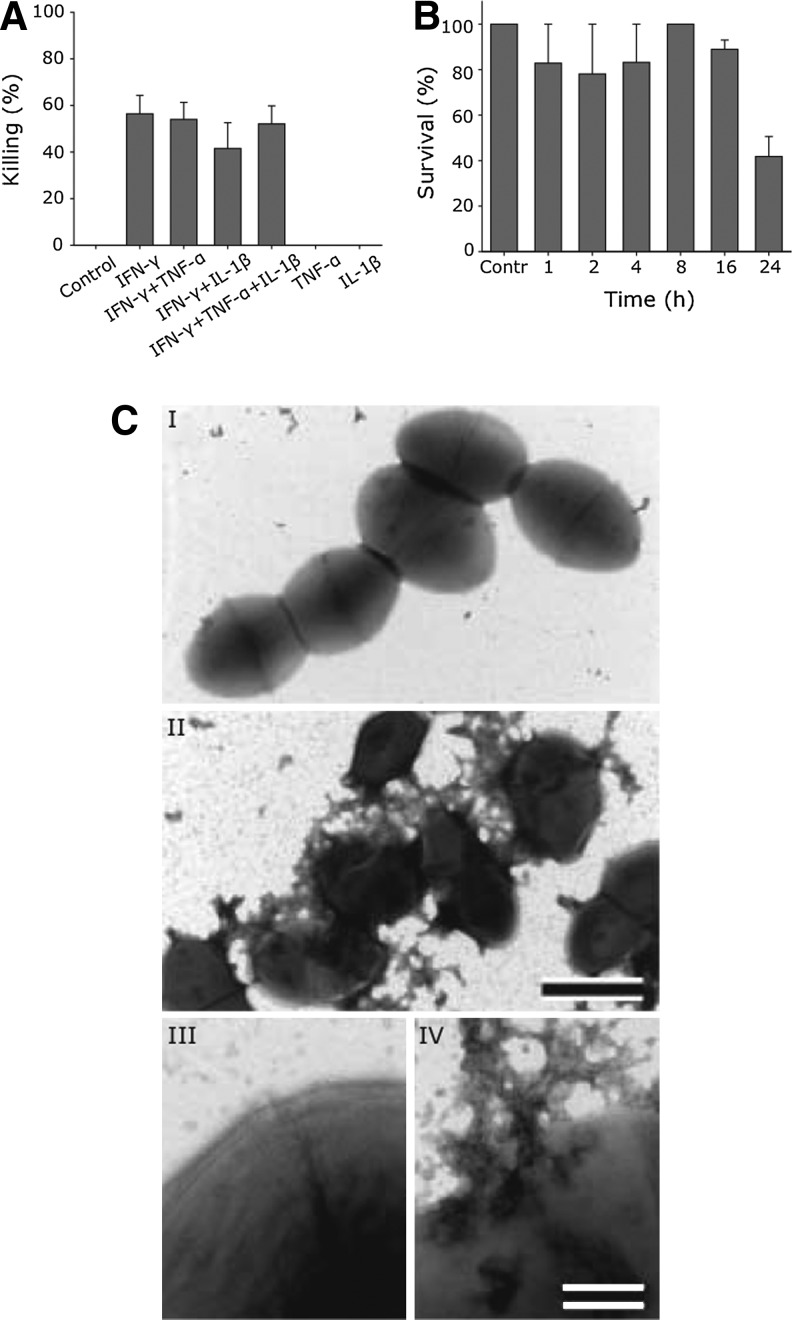
Pharyngeal epithelial cells were stimulated with cytokines, and the bactericidal activity of the incubation medium was determined using a viable count assay. Significant bactericidal activity, resulting in ∼50% killing of S. pyogenes, was seen in the presence of IFN-γ, either alone or in combination with TNF or IL-1β. Neither TNF nor IL-1β alone induced bactericidal activity in the cell incubation medium (Fig. 1A). Investigation of possible time-dependence of the response revealed that the bactericidal effect against S. pyogenes became evident with a threshold-effect after 24 h of stimulation with IFN-γ (Fig. 1B). IFN-γ itself, at the concentration used, showed no bactericidal effect (data not shown). Electron microscopy was used to investigate the morphologic appearance of S. pyogenes after exposure to the incubation medium from nonstimulated cells and IFN-γ-stimulated epithelial cells, respectively. Bacteria exposed to the incubation medium from IFN-γ-stimulated cells showed disrupted integrity and leakage of intracellular contents, whereas bacteria incubated with the incubation medium from nonstimulated cells were unaffected (Fig. 1C).
FIG. 1.
IFN-γ-dependent bactericidal activity in the incubation medium from pharyngeal epithelial cells. (A) The incubation medium from pharyngeal epithelial cells, incubated in a medium alone (control) or stimulated with IFN-γ (100 U mL−1), and/or TNF (10 ng mL−1) and/or IL-1β (10 ng mL−1) for 24 h, was incubated with S. pyogenes (AP1 strain) for 1 h. Bacterial killing was measured using a viable count assay. The number of colony-forming units (cfu) in a medium alone (control) was set to 0% killing and compared with the stimulated conditions. The data represent mean±SEM of 3 independent experiments. (B) The IFN-γ-induced bactericidal response in the incubation medium is time-dependent. The incubation medium from pharyngeal epithelial cells stimulated for 0–24 h with IFN-γ (100 U mL−1) was used in a viable counts assay against S. pyogenes. The data represent mean±SEM of 2 independent experiments. The control medium (without IFN-γ) was set to 100% survival. (C) Electron micrographs showing the morphology of S. pyogenes (AP1 strain) after exposure to the cell incubation medium from nonstimulated and IFN-γ-stimulated pharyngeal epithelial cells, respectively. Bacteria were incubated for 1 h in the cell incubation medium from nonstimulated cells (I) or a medium from cells stimulated with IFN-γ (I; 100 U ml1; 24 h). In the latter case, protrusions and leakage of intracellular contents from the bacteria are seen (micrographs II and IV), which was not the case in a bacteria-exposed medium from nonstimulated conditions (micrographs I, III). Bar in (II) is 1 μm and bar in micrograph (IV) is 100 nm. IFN, interferon; TNF, tumor necrosis factor; IL, interleukin.
Reduced bactericidal activity after addition of protein SIC or heparin
Protein SIC is a protein produced in large amounts by the most prevalent serotypes of S. pyogenes and it is released to the environment (Åkesson and others 1996). It is a strong inhibitor of several AMPs, for example, β-defensins, CXCL9/MIG, and lysozyme (Frick and others 2003; Fernie-King and others 2004; Egesten and others 2007). Addition of the protein SIC dose-dependently reduced the bactericidal effect of the incubation medium from IFN-γ-stimulated epithelial cells (Fig. 2). However, the reduction reached a plateau, demonstrating that not all bactericidal activity was sensitive to inhibition by SIC. Heparin-binding property is a common feature of many AMPs and soluble GAGs can inhibit their activity (Schmidtchen and others 2001; Andersson and others 2004). Therefore, the GAG-equivalent heparin (i.e., heparin-Sepharose) was added to the incubation medium from IFN-γ-stimulated cells, resulting in a reduction of the bactericidal activity, similar to that of the protein SIC (Fig. 2B). Taken together, a significant proportion of the bactericidal response is likely to be mediated by cationic polypeptides.
FIG. 2.
Protein streptococcal inhibitor of complement (SIC) of S. pyogenes and heparin reduce the antibacterial activity present in the IFN-γ-stimulated incubation medium from pharyngeal epithelial cells. (A)The incubation medium from IFN-γ-stimulated pharyngeal epithelial cells was preincubated with protein SIC for 20 min at the concentrations indicated before incubation with S. pyogenes for 1 h. The resulting numbers of cfu were determined using a viable count assay. The reduction of bacterial killing is shown. The data shown represent mean±SEM from 3 separate experiments. (B)The incubation medium from IFN-γ-stimulated pharyngeal epithelial cells was preincubated with heparin-Sepharose for 20 min, followed by removal of the beads and subsequent incubation with S. pyogenes for 1 h. The data shown are a representative out of 3 experiments.
Detection of AMPs by SDS-PAGE followed by LC-MS/MS
Most AMPs are highly cationic and have a size of less than 15 kDa, making it difficult to perform protein-separation using 2D-electrophoresis. Therefore, SDS-PAGE followed by silver staining was chosen to compare the patterns of produced polypeptides present in the cell incubation medium during nonstimulated and cytokine-stimulated conditions, respectively. To improve the recovery of polypeptides, it proved necessary to use acetone precipitation and an extraction procedure with urea. The separated polypeptides were further characterized and identified using LC-MS/MS. Several previously characterized AMPs, antibacterial chemokines, and additional molecules that could interfere with bacteria could be identified using this approach, including SLPI, adrenomedullin, lactotransferrin, short palate, lung, and nasal epithelium clone 1 (SPLUNC1), elafin, dermcidin, CXCL10/IP-10 (IFN-γ-induced protein 10 kDa), the lipocalin NGAL, proteins S100-A8 and S100-A9. NGAL, SLPI, and adrenomedullin were visible also in a medium from nonstimulated epithelial cells, and the intensity of the bands using SDS-PAGE was similar to that of stimulated conditions, suggesting a constitutive production of these polypeptides. However, in a particular protein S100-A8 and S100-A9 appeared after stimulation (Fig. 3). S100-A8 and S100-A9 forms a hetero tetramer in the cytoplasm of keratinocytes, and it is possible that the presence of these molecules reflects disturbed epithelial cell integrity due to necrosis or apoptosis. However, we could not see decreased cell viability as deduced by Trypan Blue exclusion or disturbed morphologic appearances during cell culture.
FIG. 3.
Detection of antimicrobial polypeptides in the incubation medium by SDS-PAGE and liquid chromatography–tandem mass spectrometry (LC-MS/MS). Polypeptides from nonstimulated pharyngeal epithelial cells (control), and from cells stimulated with either IFN-γ (100 U mL−1) or IFN-γ in combination with TNF (10 ng mL−1) were precipitated using acetone. After extraction in urea, polypeptides were separated SDS-PAGE, silver stained, and analyzed using LC-MS/MS. References characterizing antibacterial activity are shown in the column on the right. The data shown represent one representative out of 3 separate experiments.
Semiquantitative detection of AMPs using μRPLC followed by MS/MS
Using μRPLC followed by MS/MS, a sensitive and semiquantitative detection of antibacterial polypeptides was achieved. The incubation medium from nonstimulated cells and cells stimulated with either IFN-γ alone, or a combination of IFN-γ and TNF for 24 h were compared (Table 1). The semiquantitative estimation was based on the number of scans detecting the respective polypeptides. In addition, this method increased the sensitivity and additional AMPs could be detected compared with polypeptide precipitation and urea extraction followed by SDS-PAGE and LC-MS/MS. The AMPs lysozyme C, dermcidin, lactotransferrin, SLPI, and elafin, the antibacterial chemokines CXCL9/MIG and CXCL10/IP-10, midkine, protein S100-A8, and NGAL were identified. Most of them could be detected after stimulation with IFN-γ in combination with TNF. In addition, several complement factors and proteases were detected during stimulation.
Table 1.
Detection of Antimicrobial Polypeptides and Molecules That Putatively May Interact with Innate Immune Functions in the Incubation Medium Using Microcapillary Reversed-Phase Liquid Chromatography and Tandem Mass Spectrometry
| |
Distribution of scans (%) |
|||||
|---|---|---|---|---|---|---|
| Acc. No. | Control | IFN-γ | IFN-γ+TNF-α | (Total scans) | References | |
| Antimicrobial polypeptides | ||||||
| Lysozyme C | P61626 | 0 | 0 | 100 | (2) | Fleming (1922) |
| Dermcidin | P81605 | 22 | 22 | 54 | (9) | Schittek and others (2001) |
| Lactotransferrin | P02788 | 33 | 33 | 33 | (6) | Arnold and others (1977) |
| Chemokines | ||||||
| CXCL9/MIG | Q07325 | 0 | 0 | 100 | (2) | Cole and others (2001) |
| CXCL10/IP-10 | P02778 | 0 | 0 | 100 | (3) | Cole and others (2001) |
| Complement | ||||||
| Complement factor B | P00751 | 17 | 19 | 64 | (36) | |
| Complement C1s | P09871 | 0 | 0 | 100 | (4) | |
| Complement C3 | NP_000055.2 | 17 | 22 | 61 | (84) | Nordahl and others (2004) |
| Complement C4-A | P0C0L4 | 0 | 0 | 100 | (8) | Pasupuleti and others (2007) |
| Complement C1r | P00736 | 0 | 10 | 90 | (10) | |
| Complement factor H | P08603 | 0 | 20 | 80 | (15) | |
| Complement factor I | P05156 | 0 | 0 | 100 | (1) | |
| Proteases | ||||||
| Cathepsin B | P07858 | 0 | 0 | 100 | (2) | |
| Cathepsin D | P07339 | 0 | 0 | 100 | (2) | |
| Cathepsin H | Q96NY6 | 50 | 50 | 0 | (2) | |
| Cathepsin L | P07711 | 0 | 0 | 100 | (2) | |
| Cathepsin L2 | O60911 | 0 | 0 | 100 | (2) | |
| Cathepsin S | P25774 | 0 | 0 | 100 | (3) | |
| Cathepsin Z | Q9UBR20 | 0 | 0 | 100 | (1) | |
| MMP-1 | P03956 | 0 | 0 | 100 | (2) | |
| MMP-10 | P09238 | 36 | 18 | 45 | (33) | |
| Protease inhibitors | ||||||
| Calgranulin a (Protein S100-A8) | 1MR8 | 36 | 27 | 36 | (11) | Steinbakk and others (1990) |
| Cystatin C | P01034 | 33 | 0 | 67 | (3) | Björck and others (1989) |
| Elafin | P19957 | 57 | 14 | 29 | (7) | Simpson and others (1999) |
| SLPI | P03973 | 0 | 0 | 100 | (5) | Hiemstra and others (1996) |
| Other | ||||||
| Thrombospondin-1 | P07996 | 41 | 15 | 44 | (27) | Malmsten and others (2006) |
| Ceruloplasmin | P00450 | 18 | 30 | 52 | (33) | Klebanoff (1992) |
| Midkine | P21741 | 25 | 25 | 50 | (8) | Svensson and others (2010) |
| NGAL | P80188 | 31 | 31 | 38 | (45) | Goetz and others (2002) |
Polypeptides in the incubation medium were denatured using urea (8 M), reduced, and digested with trypsin. After μRPLC and obtaining MS/MS spectra, searches were performed against the human Swissprot database version 57.4. Peptides were assembled into proteins and the probabilities at the protein level were computed by a protein inference program and a reverse database search strategy. The selected cutoff was at 1% false discovery rate. References reporting antibacterial activity are indicated on the right.
IFN, interferon; μRPLC, microcapillary reversed-phase liquid chromatography; NGAL, neutrophil gelatinase-associated lipocalin; SLPI, secretory leukocyte protease inhibitor; TNF, tumor necrosis factor.
Detection of AMPs by ELISA, and determination of their bactericidal activity
Since the mass-spectrometric procedures used only provided qualitative and semiquantitative information of the polypeptide content, ELISA was used to estimate the quantitative amounts of selected AMPs and antibacterial chemokines. In addition, ELISA was used to try to detect some AMPs and antibacterial chemokines that were expected, but did not show up using the mass-spectrometric methods.
The amount of the chemokines CXCL9/MIG, CXCL10/IP-10, CXCL11/I-TAC (IFN-inducible T-cell α-chemoattractant), CXCL1/GRO-α (growth-related oncogene), CXCL2/GRO-β, CXCL3/GRO-γ, CCL20/MIP-3α (macrophage inflammatory protein 3-α), CCL28/MEC (mucosae-associated epithelial chemokine), and the polypeptide SLPI, as detected by ELISAs are shown in Table 2. Stimulated conditions showed relatively high, but not bactericidal, amounts of the IFN-γ inducible chemokines CXCL9/MIG and CXCL10/IP-10 and SLPI (Table 2). Neither the hBD-1, hBD-2, and hBD-3, nor lysozyme and lactotransferrin, could be detected by ELISA. To determine and compare the bactericidal activity of the polypeptides detected using ELISA, viable count assay with recombinant peptides and AP1 bacteria was performed. Of the polypeptides detected, CXCL9/MIG was the most efficient in killing S. pyogenes, with a MBC90 value of 0.1 μM (Table 2).
Table 2.
Detection of Antimicrobial Poylpeptides in the Incubation Medium from Pharyngeal Epithelial Cells Using ELISA
| Control | IFN-γ | IFN-γ+TNF-α | MBC90 | References | |
|---|---|---|---|---|---|
| CXCL9/MIG | N.D. | 0.5±0 | 11±1.4 | 0.1 | Cole and others (2001) |
| CXCL10/IP-10 | N.D. | 0.1±0 | 15±4 | 0.7 | Cole and others (2001) |
| CXCL11/I-TAC | N.D. | N.D. | 0.7±0.2 | 0.8 | Cole and others (2001) |
| CXCL1/GRO-α | 0.9±0.3 | 0.3±0.1 | 1.9±0.2 | 1.0 | Yount and others (2007) |
| CXCL2/GRO-β | 0.05±0 | 0.03±0 | 0.06±0.04 | 0.9 | Present study |
| CXCL3/GRO-γ | 0.2±0 | 0.2±0.06 | 0.66±0.2 | 0.9 | Present study |
| CCL20/MIP-3α | 0.01±0 | 0.01±0 | 0.4±0.2 | 0.9 | Hoover and others (2002) |
| SLPI | 9±3 | 11.9±3 | 14±2.7 | 0.8 | Hiemstra and others (1996) |
The cell culture medium from pharyngeal epithelial cells, incubated in a medium alone (control) or stimulated with IFN-γ (100 U mL−1), and/or TNF-α (10 ng mL−1) for 24 h, were analyzed using specific ELISAs for their content of antibacterial polypeptides. The values are expressed as mean (μM)±SEM from 3 separate experiments.
The bactericidal activity of the detected polypeptides against S. pyogenes was determined by incubating recombinant peptides with AP1 bacteria followed by a viable count assay. MBC90 values show the peptide concentrations (μM) at which 90% of the bacteria were killed. The values shown represent mean from at least 3 separate experiments.
N.D., not detectable.
Discussion
In the present study, we show that pharyngeal epithelial cells stimulated with IFN-γ, a key regulator of the host response during bacterial and viral infections, cause release of soluble bactericidal activity.
There are several lines of evidence, showing that IFN-γ is important during S. pyogenes infection in vivo. In an animal model, IFN-γ responsive genes were highly upregulated 24 h after infection (Hyland and others 2009). Furthermore, both neutralization of IFN-γ in vivo and IFN-γ knockout mice, showed increased susceptibility to S. pyogenes at sites of infection compared with control/wild-type mice (Raeder and others 2000). Taken together, IFN-γ seems to be a key regulator of both the Th1 response in our model in general and, in directing a local innate bactericidal response mediated by epithelial cells. Interestingly, TNF amplified the IFN-γ-induced response with respect to several AMPs, without a parallel increase in the antibacterial activity. The reason for this is unclear, and several explanations are possible. One possible explanation could be that the addition of TNF induces production of molecules that bind AMPs and interfere with their antibacterial activity, still allowing the AMPs to be detected by the methods used. One example is GAGs where the synthesis of some is increased by TNF (Nelimarkka and others 1997). GAGs can bind and inactivate AMPs (Schmidtchen and others 2001). The urea-purification step may release AMPs that are in complex with different, possibly neutralizing carriers, for example, anionic proteins, GAGs, and DNA. Another possible explanation is that the marked increase in the presence of proteases after incubation, with a combination of IFN-γ and TNF, modifies the AMPs in a manner that impair their antibacterial activity, but still allow their detection (Steffen and others 2006).
Several findings support that the bactericidal activity found in the present study is mediated by polypeptides. The time-dependent threshold effect with respect to bactericidal activity, suggests that antibacterial agents accumulate in the medium, reaching a critical concentration with time, kinetics typical of AMPs. The relatively long time necessary to reach antibacterial activity in the medium after stimulation of cells with IFN-γ is most likely due to the relatively large volume of medium used in the experimental setup. In vivo, the liquid phase on the epithelial cell surfaces is much smaller in volume and concentrations critical to achieve bactericidal activity are likely to occur at a much earlier time point. In addition, the lytic appearance of bacteria as seen by electron microscopy, indicates that the bactericidal effect is mediated by membrane disrupting AMPs. The viable count assay used in the study only reflects bactericidal activity. The bactericidal activity may be mediated both through membrane, but also through intracellular actions. In addition, several factors mediate bacteriostatic activity (e.g., lactoferrin) that is not reflected by the viable counts assay used in the present study.
Protein SIC, released from S. pyogenes, binds and inhibits the effect of many AMPs, for example, lysozyme C, CXCL9/MIG, hBD, and SLPI (Frick and others 2003; Fernie-King and others 2004; Egesten and others 2007). Similarly, the killing of S pyogenes was reduced dose-dependently after addition of the protein SIC to the incubation medium from cells stimulated with IFN-γ. However, the reduction reached a plateau, demonstrating that not all bactericidal activity was sensitive to inhibition by the protein SIC. In addition, many AMPs have heparin-binding properties and their activities are inhibited by released sulfated GAGs (Schmidtchen and others 2001; Andersson and others 2004). In line with this, addition of heparin to the incubation medium from IFN-γ-stimulated pharyngeal epithelial cells, reduced the bactericidal activity, similar to that of the protein SIC. Contribution of nonpeptide effector molecules cannot be entirely ruled out. Epithelial cells can produce reactive oxygen species and nitric oxide during inflammation. These may form antibacterial agents, especially in the presence of a combination of peroxidase activity and halides (Hampton and others 1998; Moskwa and others 2007).
Characterization of the polypeptide content in the incubation medium after cytokine stimulation demonstrated a cumulative presence of several AMPs. Lysozyme C acts synergistically with other AMPs with respect to bactericidal effect (Cole and others 1999, 2002; Singh and others 2000; Ganz 2004). Consequently, the antibacterial potential of the incubation medium may not be correctly reflected by additive effects of various AMPs, but several synergistic effects are likely to be present. Similarly to a previous report, SLPI was constitutively produced by both control cells and stimulated epithelial cells, suggesting a readiness for priming of the antibacterial effects, subsequently produced in the case of invasive infection (van Wetering and others 2000). Using a viable count assay, SLPI displayed a high antibacterial activity against S. pyogenes with a MBC90 value of 0.8 μM.
Previously, using cDNA microarray, we showed expression and upregulation of many genes coding for antibacterial chemokines when pharyngeal epithelial cells were stimulated with IFN-γ (Eliasson and others 2007). We therefore analyzed the cell incubation medium for antibacterial chemokines using ELISAs. Expression of the chemokines CXCL9/MIG, CXCL10/IP-10, and CXCL11/I-TAC is critically dependent on the presence of IFN-γ. In the present study, we found CXCL9/MIG and CXCL10/IP-10 are produced at the highest levels as detected by ELISA. Of these, CXCL9/MIG possesses the highest antibacterial activity against S. pyogenes with a MBC90 value of 0.1 μM, while CXCL10/IP-10 is somewhat less potent with a MBC90 value of 0.7 μM. In the present study, we also found that the chemokines CXCL1/GRO-α, CXCL2/GRO-β, and CXCL3/GRO-γ possesses antibacterial activity against S. pyogenes in vitro.
NGAL is a member of the lipocalin family and acts as a potent bacteriostatic agent by sequestering iron. It is less likely to play a bactericidal role in the present investigation, since its effect is bacteriostatic (Goetz and others 2002).
In vivo, the fluid lining on the epithelial cell surface presents a very small volume. Therefore, one could expect that high concentrations of AMPs are reached and, as a consequence, the bactericidal activity could be expected to be higher than in the system investigated here.
A crucial question is whether the AMPs are produced at concentrations that are bactericidal.
There are several obstacles using a proteomic approach to make a global characterization of the antibacterial content of the incubation medium. One problem is that the AMPs often are highly cationic and hydrophobic, giving them a propensity to form complexes with other molecules, such as mucins and other charged glycoproteins. In this study, precipitation followed by urea extraction was used to increase the recovery of polypeptides. Without this procedure, only a few faint bands were visible after SDS-PAGE and silver staining. In the case of ELISA, hidden epitopes and oligomerization add problems, interpreting results.
There are several examples of AMPs that we expected to find, but that could not be detected by the approaches used in this study. For example, the epithelial-produced β-defensins hBD1-3, that all are antibacterial against S. pyogenes (McCray and Bentley 1997; Singh and others 1998; Fernie-King and others 2004; Sørensen and others 2005). However, neither of these peptides could be detected by ELISA in the bactericidal cell incubation medium, nor were they found using LC-MS/MS or the highly sensitive μRPLC mass-spectrometry method. In addition, neither hBD1-3, BRAK/CXCL14, hCAP-18, nor dermcidin could be detected in resting or IFN-γ-stimulated pharyngeal epithelial cells using cDNA microarray (Eliasson and others 2007).
The finding of many molecules belonging to the complement system is intriguing. This suggests that locally produced complement can participate in both bacterial killing and in regulating the inflammatory process. It has been shown that the complement fragment C3a and C4a have strong bactericidal activity (Nordahl and others 2004). The production of several endogenous proteases may play a role in producing antibacterial polypeptides, as has been shown. Another possibility is that they play a role modulating and downregulating the inflammatory response (Malmsten and others 2007).
Taken together, the results show that stimulation of pharyngeal epithelial cells with IFN-γ, results in a bactericidal innate response against S. pyogenes, constituted of a spectrum of AMPs and antibacterial chemokines. The findings strongly emphasize an important role for the IFN-γ-stimulated epithelium in the first line of defense against pathogenic bacteria at mucosal surfaces.
Acknowledgments
The work was supported by grants from the Swedish Research Council (project # 20674), the Swedish Heart and Lung Foundation (project # 20100164), the Medical Faculty at Lund University Swedish Government Funds for Clinical Research (ALF), the Foundations of Bergh, Hierta, Kock, Marcus & Marianne Wallenberg, Österlund, and the Royal Physiographic Society in Lund.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- Åring;kesson P. Sjöholm AG. Björck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–1088. doi: 10.1074/jbc.271.2.1081. [DOI] [PubMed] [Google Scholar]
- Andersson E. Rydengård V. Sonesson A. Mörgelin M. Björck L. Schmidtchen A. Antimicrobial activities of heparin-binding peptides. Eur J Biochem. 2004;271:1219–1226. doi: 10.1111/j.1432-1033.2004.04035.x. [DOI] [PubMed] [Google Scholar]
- Arnold RR. Cole MF. McGhee JR. A bactericidal effect for human lactoferrin. Science. 1977;197:263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- Bengtson SH. Sanden C. Morgelin M. Marx PF. Olin AI. Leeb-Lundberg LM. Meijers JC. Herwald H. Activation of TAFI on the surface of Streptococcus pyogenes evokes inflammatory reactions by modulating the kallikrein/kinin system. J Innate Immun. 2008;1:18–28. doi: 10.1159/000145543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno AL. Brito MO. Collins CM. Molecular basis of group A streptococcal virulence. Lancet Infect Dis. 2003;3:191–200. doi: 10.1016/s1473-3099(03)00576-0. [DOI] [PubMed] [Google Scholar]
- Björck L. Åring;kesson P. Bohus M. Trojnar J. Abrahamson M. Olafsson I. Grubb A. Bacterial growth blocked by a synthetic peptide based on the structure of a human proteinase inhibitor. Nature. 1989;337:385–386. doi: 10.1038/337385a0. [DOI] [PubMed] [Google Scholar]
- Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- Borregaard N. Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006;19:211–215. doi: 10.1007/s10534-005-3251-7. [DOI] [PubMed] [Google Scholar]
- Cole AM. Dewan P. Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AM. Ganz T. Liese AM. Burdick MD. Liu L. Strieter RM. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- Cole AM. Liao HI. Stuchlik O. Tilan J. Pohl J. Ganz T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169:6985–6991. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- Craig R. Beavis RC. A method for reducing the time required to match protein sequences with tandem mass spectra. Rapid Commun Mass Spectrom. 2003;17:2310–2316. doi: 10.1002/rcm.1198. [DOI] [PubMed] [Google Scholar]
- Crawford MA. Burdick MD. Glomski IJ. Boyer AE. Barr JR. Mehrad B. Strieter RM. Hughes MA. Interferon-inducible CXC chemokines directly contribute to host defense against inhalational anthrax in a murine model of infection. PLoS Pathog. 2010;6:e1001199. doi: 10.1371/journal.ppat.1001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Y. Chen Q. Schmidt AP. Anderson GM. Wang JM. Wooters J. Oppenheim JJ. Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egesten A. Eliasson M. Johansson HM. Olin AI. Mörgelin M. Mueller A. Pease JE. Frick IM. Bjorck L. The CXC chemokine MIG/CXCL9 is important in innate immunity against Streptococcus pyogenes. J Infect Dis. 2007;195:684–693. doi: 10.1086/510857. [DOI] [PubMed] [Google Scholar]
- Egesten A. Olin AI. Linge HM. Yadav M. Mörgelin M. Karlsson A. Collin M. SpeB of Streptococcus pyogenes differentially modulates antibacterial and receptor activating properties of human chemokines. PLoS One. 2009;4:e4769. doi: 10.1371/journal.pone.0004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias JE. Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- Eliasson M. Frick IM. Collin M. Sørensen OE. Björck L. Egesten A. M1 protein of Streptococcus pyogenes increases production of the antibacterial CXC chemokine MIG/CXCL9 in pharyngeal epithelial cells. Microb Pathog. 2007;43:224–233. doi: 10.1016/j.micpath.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Fernie-King BA. Seilly DJ. Lachmann PJ. The interaction of streptococcal inhibitor of complement (SIC) and its proteolytic fragments with the human beta defensins. Immunology. 2004;111:444–452. doi: 10.1111/j.0019-2805.2004.01837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond B. 1922;93:306–317. [Google Scholar]
- Frick IM. Åring;kesson P. Rasmussen M. Schmidtchen A. Björck L. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J Biol Chem. 2003;278:16561–16566. doi: 10.1074/jbc.M301995200. [DOI] [PubMed] [Google Scholar]
- Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- Goetz DH. Holmes MA. Borregaard N. Bluhm ME. Raymond KN. Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Hampton MB. Kettle AJ. Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Hancock RE. Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- Hiemstra PS. Maassen RJ. Stolk J. Heinzel-Wieland R. Steffens GJ. Dijkman JH. Antibacterial activity of antileukoprotease. Infect Immun. 1996;64:4520–4524. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DM. Boulegue C. Yang D. Oppenheim JJ. Tucker K. Lu W. Lubkowski J. The structure of human macrophage inflammatory protein-3alpha/CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human beta-defensins. J Biol Chem. 2002;277:37647–37654. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- Hyland KA. Brennan R. Olmsted SB. Rojas E. Murphy E. Wang B. Cleary PP. The early interferon response of nasal-associated lymphoid tissue to Streptococcus pyogenes infection. FEMS Immunol Med Microbiol. 2009;55:422–431. doi: 10.1111/j.1574-695X.2009.00540.x. [DOI] [PubMed] [Google Scholar]
- Keller A. Nesvizhskii AI. Kolker E. Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Bactericidal effect of Fe2+, ceruloplasmin, and phosphate. Arch Biochem Biophys. 1992;295:302–308. doi: 10.1016/0003-9861(92)90522-x. [DOI] [PubMed] [Google Scholar]
- Kolls JK. McCray PB., Jr. Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–835. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsten M. Davoudi M. Schmidtchen A. Bacterial killing by heparin-binding peptides from PRELP and thrombospondin. Matrix Biol. 2006;25:294–300. doi: 10.1016/j.matbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Malmsten M. Davoudi M. Walse B. Rydengård V. Pasupuleti M. Mörgelin M. Schmidtchen A. Antimicrobial peptides derived from growth factors. Growth Factors. 2007;25:60–70. doi: 10.1080/08977190701344120. [DOI] [PubMed] [Google Scholar]
- Malmström J. Lee H. Nesvizhskii AI. Shteynberg D. Mohanty S. Brunner E. Ye M. Weber G. Eckerskorn C. Aebersold R. Optimized peptide separation and identification for mass spectrometry based proteomics via free-flow electrophoresis. J Proteome Res. 2006a;5:2241–2249. doi: 10.1021/pr0600632. [DOI] [PubMed] [Google Scholar]
- Malmström L. Malmström J. Marko-Varga G. Westergren-Thorsson G. Proteomic 2DE database for spot selection, automated annotation, and data analysis. J Proteome Res. 2002;1:135–138. doi: 10.1021/pr010004i. [DOI] [PubMed] [Google Scholar]
- Malmström L. Marko-Varga G. Westergren-Thorsson G. Laurell T. Malmström J. 2DDB - a bioinformatics solution for analysis of quantitative proteomics data. BMC Bioinform. 2006b;7:158. doi: 10.1186/1471-2105-7-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M. Ficolins: complement-activating lectins involved in innate immunity. J Innate Immun. 2010;2:24–32. doi: 10.1159/000228160. [DOI] [PubMed] [Google Scholar]
- McCray PB., Jr. Bentley L. Human airway epithelia express a beta-defensin. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- Moskwa P. Lorentzen D. Excoffon KJ. Zabner J. McCray PB., Jr. Nauseef WM. Dupuy C. Bánfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelimarkka L. Kainulainen V. Schönherr E. Moisander S. Jortikka M. Lammi M. Elenius K. Jalkanen M. Järveläinen H. Expression of small extracellular chondroitin/dermatan sulfate proteoglycans is differentially regulated in human endothelial cells. J Biol Chem. 1997;272:12730–12737. doi: 10.1074/jbc.272.19.12730. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI. Keller A. Kolker E. Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Nordahl EA. Rydengård V. Nyberg P. Nitsche DP. Mörgelin M. Malmsten M. Björck L. Schmidtchen A. Activation of the complement system generates antibacterial peptides. Proc Natl Acad Sci U S A. 2004;101:16879–16884. doi: 10.1073/pnas.0406678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti M. Walse B. Nordahl EA. Mörgelin M. Malmsten M. Schmidtchen A. Preservation of antimicrobial properties of complement peptide C3a, from invertebrates to humans. J Biol Chem. 2007;282:2520–2528. doi: 10.1074/jbc.M607848200. [DOI] [PubMed] [Google Scholar]
- Rada B. Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeder RH. Barker-Merrill L. Lester T. Boyle MD. Metzger DW. A pivotal role for interferon-gamma in protection against group A streptococcal skin infection. J Infect Dis. 2000;181:639–645. doi: 10.1086/315281. [DOI] [PubMed] [Google Scholar]
- Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- Sauty A. Dziejman M. Taha RA. Iarossi AS. Neote K. Garcia-Zepeda EA. Hamid Q. Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–3558. [PubMed] [Google Scholar]
- Schittek B. Hipfel R. Sauer B. Bauer J. Kalbacher H. Stevanovic S. Schirle M. Schroeder K. Blin N. Meier F. Rassner G. Garbe C. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- Schmidtchen A. Frick IM. Björck L. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol Microbiol. 2001;39:708–713. doi: 10.1046/j.1365-2958.2001.02251.x. [DOI] [PubMed] [Google Scholar]
- Simpson AJ. Maxwell AI. Govan JR. Haslett C. Sallenave JM. Elafin (elastase-specific inhibitor) has anti-microbial activity against gram-positive and gram-negative respiratory pathogens. FEBS Lett. 1999;452:309–313. doi: 10.1016/s0014-5793(99)00670-5. [DOI] [PubMed] [Google Scholar]
- Singh PK. Jia HP. Wiles K. Hesselberth J. Liu L. Conway BA. Greenberg EP. Valore EV. Welsh MJ. Ganz T. Tack BF. McCray PB., Jr Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK. Tack BF. McCray PB., Jr. Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- Sørensen OE. Thapa DR. Rosenthal A. Liu L. Roberts AA. Ganz T. Differential regulation of beta-defensin expression in human skin by microbial stimuli. J Immunol. 2005;174:4870–4879. doi: 10.4049/jimmunol.174.8.4870. [DOI] [PubMed] [Google Scholar]
- Steffen H. Rieg S. Wiedemann I. Kalbacher H. Deeg M. Sahl HG. Peschel A. Götz F. Garbe C. Schittek B. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother. 2006;50:2608–2620. doi: 10.1128/AAC.00181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbakk M. Naess-Andresen CF. Lingaas E. Dale I. Brandtzaeg P. Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- Strieter RM. Belperio JA. Keane MP. Cytokines in innate host defense in the lung. J Clin Invest. 2002;109:699–705. doi: 10.1172/JCI15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson SL. Pasupuleti M. Walse B. Malmsten M. Mörgelin M. Sjögren C. Collin M. Schmidtchen A. Palmer R. Egesten A. Midkine and pleiotrophin have bactericidal properties: preserved antibacterial activity in a family of heparin-binding growth factors during evolution. J Biol Chem. 2010;285:16105–16115. doi: 10.1074/jbc.M109.081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wetering S. van der Linden AC. van Sterkenburg MA. de Boer WI. Kuijpers AL. Schalkwijk J. Hiemstra PS. Regulation of SLPI and elafin release from bronchial epithelial cells by neutrophil defensins. Am J Physiol Lung Cell Mol Physiol. 2000;278:L51–L58. doi: 10.1152/ajplung.2000.278.1.L51. [DOI] [PubMed] [Google Scholar]
- Yang D. Chen Q. Hoover DM. Staley P. Tucker KD. Lubkowski J. Oppenheim JJ. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- Yi EC. Lee H. Aebersold R. Goodlett DR. A microcapillary trap cartridge-microcapillary high-performance liquid chromatography electrospray ionization emitter device capable of peptide tandem mass spectrometry at the attomole level on an ion trap mass spectrometer with automated routine operation. Rapid Commun Mass Spectrom. 2003;17:2093–2098. doi: 10.1002/rcm.1150. [DOI] [PubMed] [Google Scholar]
- Yount NY. Waring AJ. Gank KD. Welch WH. Kupferwasser D. Yeaman MR. Structural correlates of antimicrobial efficacy in IL-8 and related human kinocidins. Biochim Biophys Acta. 2007;1768:598–608. doi: 10.1016/j.bbamem.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Zwart S. Rovers MM. de Melker RA. Hoes AW. Penicillin for acute sore throat in children: randomised, double blind trial. BMJ. 2003;327:1324. doi: 10.1136/bmj.327.7427.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]