Abstract
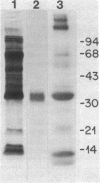
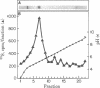
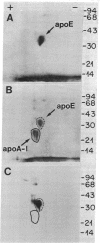
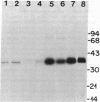
Monolayers of mouse peritoneal macrophages were shown to synthesize and secrete a protein that resembles apoprotein E (apoE), a normal constituent of plasma lipoproteins. Synthesis and secretion were studied by incubation of macrophages with L-[35S]methionine and analysis of the 35S-labeled proteins secreted into the culture medium. The 35S-labeled protein resembling apoE showed the following properties: (i) it floated in the ultracentrifuge at a density less than 1.215 g/ml, indicating that it was associated with lipid; (ii) by NaDodSO4/polyacrylamide gel electrophoresis, its Mr of 35,000 was identical to that of authentic apoE obtained from mouse plasma very low density lipoprotein; (iii) its isoelectric point of 5.4 was the same as that of authentic mouse apoE; (iv) it comigrated with authentic mouse apoE after two-dimensional isoelectric focusing/NaDodSO4/polyacrylamide gel electrophoresis; and (v) it was quantitatively precipitated by a monospecific antibody directed against rat apoE. Synthesis and secretion of the apoE-like protein was stimulated 3- to 8-fold when the macrophages were loaded with cholesterol by incubation with either acetylated low density lipoprotein (acetyl-LDL) or beta-migrating very low density lipoprotein from cholesterol-fed rabbits. When the cells were incubated with acetyl-LDL, the apoE-like protein composed approximately 2% of the total 35S-labeled protein synthesized by the cells and approximately 10% of the total 35S-labeled protein secreted into the medium. The current findings suggest a role for apoE in the plasma transport of cholesterol excreted from cholesterol-loaded macrophages.
Full text
PDF
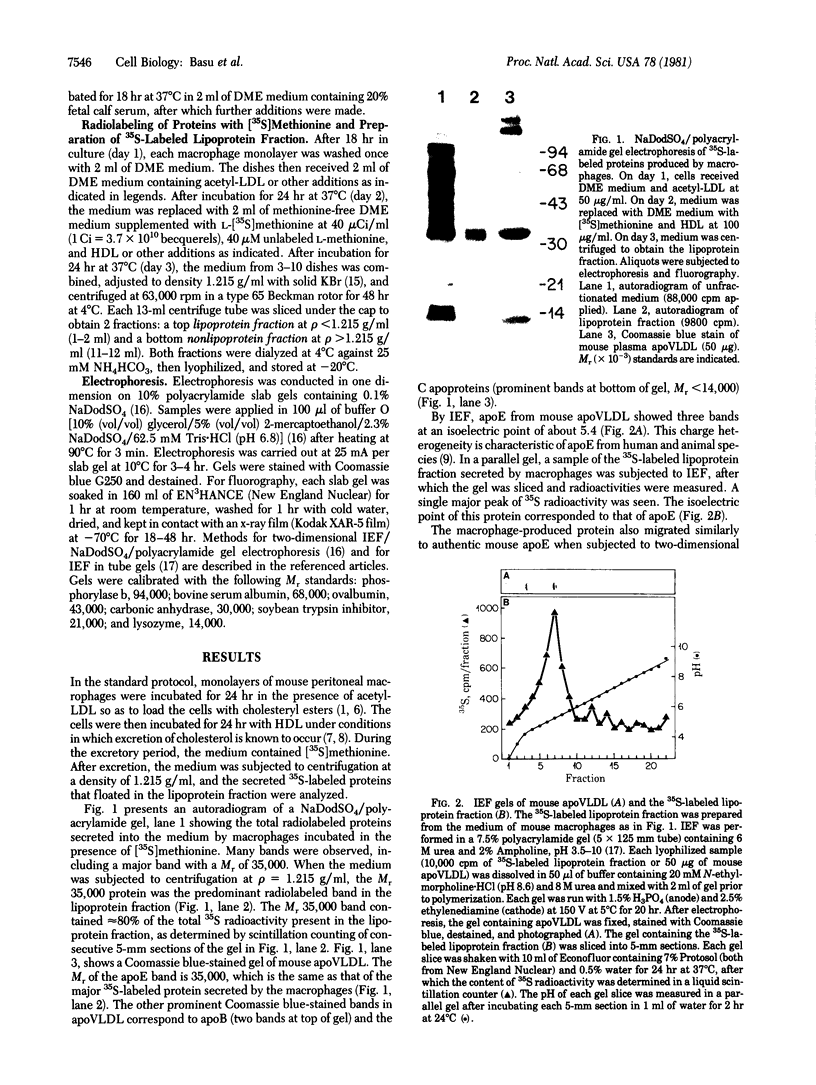
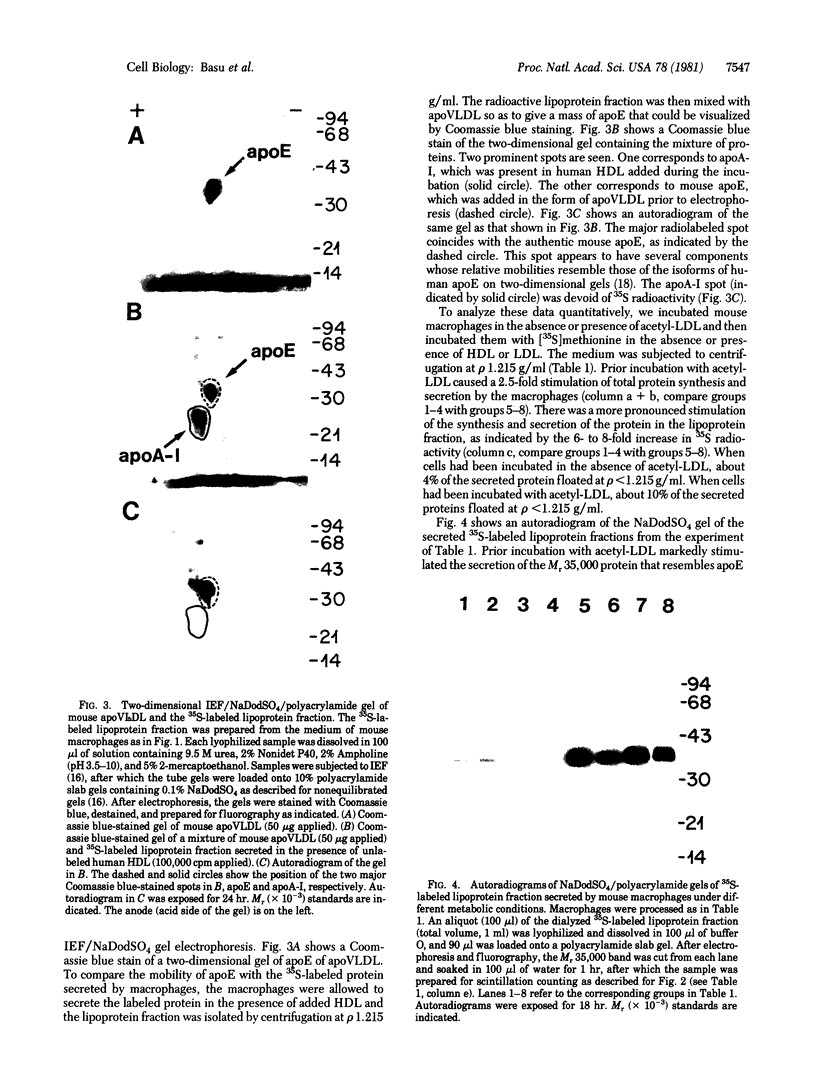
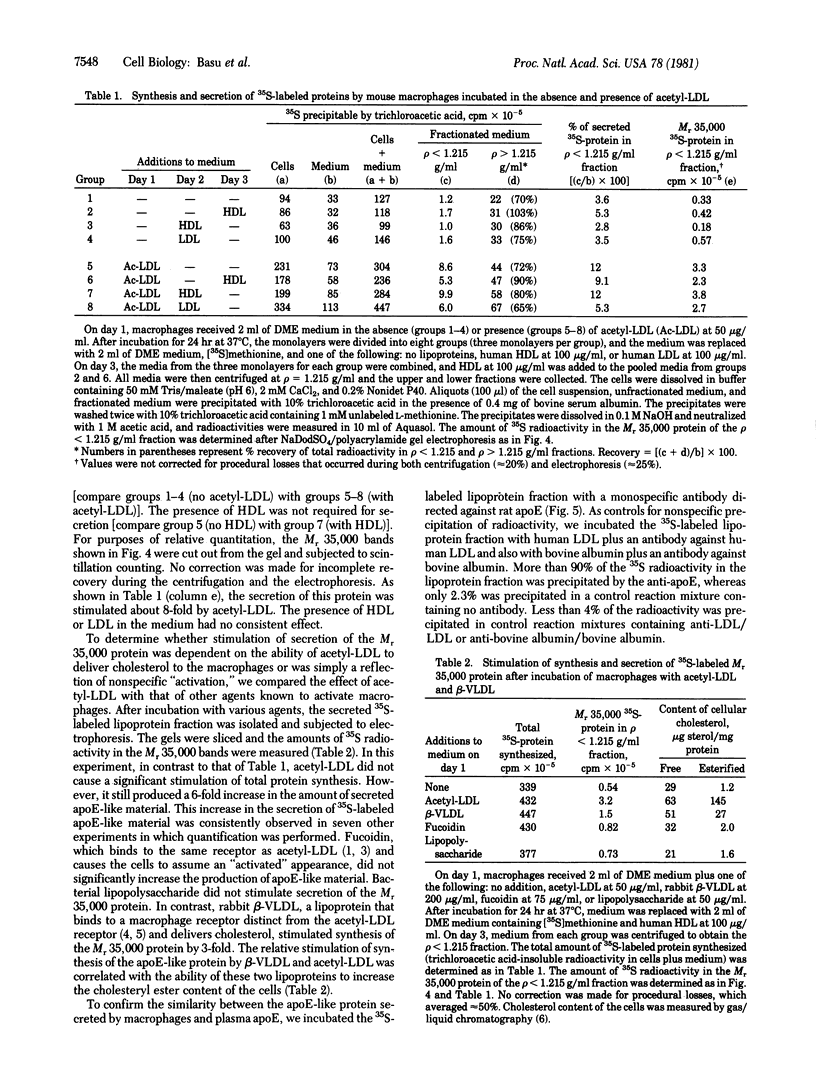
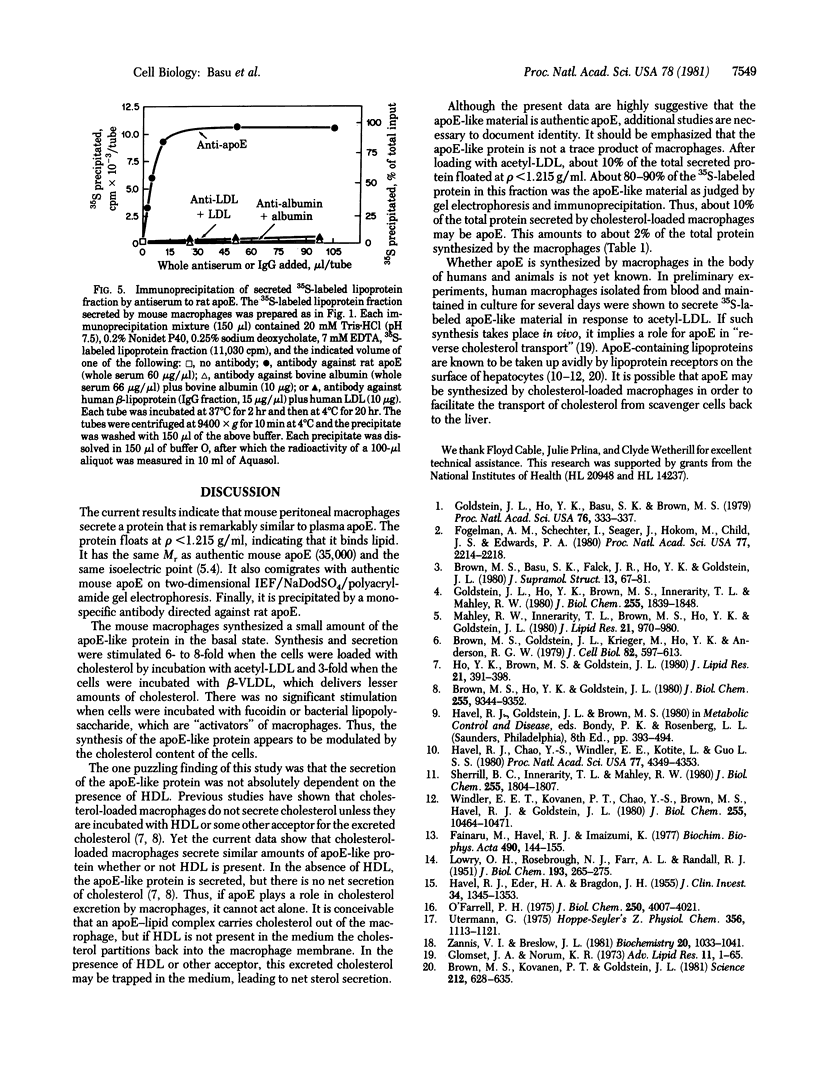
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979 Sep;82(3):597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Fainaru M., Havel R. J., Imaizumi K. Radioimmunoassay of arginine-rich apolipoprotein of rat serum. Biochim Biophys Acta. 1977 Jan 25;490(1):144–155. doi: 10.1016/0005-2795(77)90114-3. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Brown M. S., Innerarity T. L., Mahley R. W. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J Biol Chem. 1980 Mar 10;255(5):1839–1848. [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Chao Y., Windler E. E., Kotite L., Guo L. S. Isoprotein specificity in the hepatic uptake of apolipoprotein E and the pathogenesis of familial dysbetalipoproteinemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4349–4353. doi: 10.1073/pnas.77.7.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Goldstein J. L. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J Lipid Res. 1980 May;21(4):391–398. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Utermann G. Isolation and partial characterization of an arginine-rich apolipoprotein from human plasma very-low-density lipoproteins: apolipoprotein E. Hoppe Seylers Z Physiol Chem. 1975 Jul;356(7):1113–1121. doi: 10.1515/bchm2.1975.356.2.1113. [DOI] [PubMed] [Google Scholar]
- Windler E. E., Kovanen P. T., Chao Y. S., Brown M. S., Havel R. J., Goldstein J. L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980 Nov 10;255(21):10464–10471. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L. Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry. 1981 Feb 17;20(4):1033–1041. doi: 10.1021/bi00507a059. [DOI] [PubMed] [Google Scholar]