Abstract
To investigate whether low doses of exogenous interferon (IFN)-γ attenuate airway inflammation, and the underlying mechanisms, in asthma. C57BL/6 mice (n=42), after intraperitoneal ovalbumin (OVA) sensitization on day 0 and day 12, were challenged with OVA aerosol for 6 consecutive days. Different doses of IFN-γ were then administered intraperitoneally 5 min before each inhalation during OVA challenge. Airway hyperresponsiveness, airway inflammatory cells, cytokine profiles, and Fas/FasL expression on CD4+ T cells were evaluated in an asthma model. The effect of various IFN-γ doses on Fas/FasL expression and CD4+ T cell apoptosis were assessed in vitro. We demonstrated that low doses of IFN-γ reduced pulmonary infiltration of inflammatory cells, Th2 cytokine production, and goblet cells hyperplasia (P<0.05), while high doses of endogenous IFN-γ had almost no effect. We also found that low doses of IFN-γ relocated Fas/FasL to the CD4+ T cell surface in the asthma model (P<0.05) and increased FasL-induced apoptosis in vitro (P<0.05). Furthermore, treatment with MFL-3, an anti-FasL antibody, partially abolished the anti- inflammatory properties of IFN-γ in the airway rather than affecting the Th1/Th2 balance. This research has revealed an alternative mechanism in asthma that involves low doses of IFN-γ, which attenuate airway inflammation through enhancing Fas/FasL-induced CD4+ T cell apoptosis.
Introduction
Asthma is a chronic airway disease that is characterized by airway inflammation, hyperreactivity, and recurrent episodes of airflow obstruction (Cohn and others, 2004). Although asthma has a multifactorial origin, it has been suggested that an alteration in cytokines, with overproduction of Th2 cytokines (interleukin [IL]-4, IL-5, and IL-13) combined with a reduction in Th1 cytokines (interferon [IFN]-γ and IL-2), is involved in causing asthma (Castro and others 2000; Lama and others 2011).
IFN-γ is a principal Th1 effector cytokine that could act as a central regulator in the pathogenic process of allergic inflammation. Previous studies have revealed that IFN-γ is responsible for promoting the differentiation of naive T cells toward the Th1 phenotype and inhibiting Th2 cell recruitment (Szabo and others 2003). IFN-γ also suppresses Th2 cytokine release, reducing the levels of IL-4 and IL-5 production (Lighvani and others 2001; Afkarian and others 2002). However, other compelling evidence showed opposite results. At high doses, IFN-γ may play a more important role as a proinflammatory molecule, in the development of asthma-like reactions (Doukas and Pober 1990; Farrar and Schreiber 1993; Wild and others 2000; Koch and others 2006). Thus, we speculated that other mechanisms may mediate the dual function of IFN-γ in the pathogenic process of allergic inflammation.
Persistent airway inflammation is considered to be a main contributor to the frequency and severity of asthma exacerbations. Fas/FasL-induced apoptosis provides a mechanism for the elimination of antigen-activated T cells and eosinophils, leading to resolution of the Th2-mediated immune response (Druilhe and others 1998; Jayaraman and others 1999; Vignola and others 1999). IFN-γ has been shown to induce mucous cell and eosinophil apoptosis through CD95/Fas-mediated mechanisms (Luttmann and others 2000; Shi and others 2002). Also, previous studies have demonstrated that IFN-γ inhibits allergen-stimulated CD4+ T cell proliferation in asthma by upregulating Fas and FasL surface expression in vitro, thereby triggering the apoptotic pathway (Refaeli and others 2002; De and others 2004). However, there is little evidence regarding the association between IFN-γ and CD4+ T cell apoptosis in allergic airway infiltrates in vivo.
In this study, we investigated the ability of low IFN-γ doses to induce Fas/FasL-mediated CD4+ T cell apoptosis. We developed a murine asthma model and injected IFN- γ intraperitoneally, then analyzed the following indices: pulmonary infiltration of inflammatory cells, local Th2 cytokines production, goblet cell hyperplasia, airway hyperresponsiveness (AHR), and the expression of Fas/FasL on CD4+ T cells in lung and paratracheal lymph nodes (PLNs). We also assessed the level of FasL on CD4+T cells stimulated in vitro with an allergen in the presence or absence of exogenous IFN-γ.
Materials and Methods
Animals
Male C57BL/6 mice, 4- to 6-weeks old (weight 20 to 22 g) and free of specific pathogens, were purchased from National Rodent Laboratory Animal Resources, Shanghai Branch (Shanghai, China). Experiments were performed according to protocols approved by the Animal Studies Committee of China.
OVA sensitization and challenge
A murine model of allergic asthma was established as described previously (Li and others 2008). C57BL/6 mice were sensitized using an intraperitoneal (i.p.) injection of 25 mg OVA (grade V, Sigma-Aldrich, St. Louis, MO) in 0.1 mL alum on days 0 and 12. The experimental group was challenged for 30 min per day between days 18 and 23 with aerosolized 1% OVA in phosphate-buffered saline (PBS), using an ultrasonic nebulizer. Control mice were subjected to the same protocol, but received PBS instead of OVA in the challenge phase.
IFN-γ treatment and antibody blockage
All OVA-sensitized and challenged mice (n=36) were equally divided into six groups: one OVA group, three IFN-γ groups (10 U/mouse, 100 U/mouse, 1000 U/mouse), one IFN-γ 100 U/mouse+MFL-3 group, and one IFN-γ 100 U/mouse+isotype group. The IFN-γ group was injected i.p. with murine IFN-γ (10 U/mouse, 100 U/mouse, or 1000 U/mouse, per day in 100 μl PBS for 6 days, 5 min before each inhalation; Sigma-Aldrich, St. Louis, MO). Anti-FasL-mAb:MFL-3 (100 μg/mouse, Hamster IgG1, κ; BD Biosciences, San Jose, CA) or isotype control (ebioscience, San Diego, CA) was administered i.p. at the second OVA stimulation (OVAII).
Airway responsiveness measurements
AHR was measured in vivo 24 h after the last aerosol exposure, as previously described18. Briefly, mice were anesthetized with an i.p. injection of phenobarbital (40 mg/kg), and placed in a whole-body plethysmography chamber. A small polyethylene catheter was placed in the jugular vein for intravenous (i.v.) administrations. Increasing methacholine doses of 0.5, 1.0, and 2.0 mg/kg were then administered i.v. at 5-min intervals. Tidal volume, airway flow velocity, and transpulmonary pressure were measured for 2 min after each dose by a Transducer-PCLAB Medlab (Nanjing MedEase Science and Technology, Nanjing, China) to calculate airway resistance (RL) and dynamic compliance (Cdyn). Results are expressed as the maximal resistance after each dose of methacholine minus the baseline value.
Analysis of bronchoalveolar lavage fluid
After AHR measurements, the right lung was lavaged six times with 1 mL D-Hank's solution. Total cells in the bronchoalveolar lavage fluid (BALF) were counted within a 0.05-mL aliquot. BALF was centrifuged (2500 rpm for 10 min at 4°C) and the supernatant was collected for cytokine analyses. Cell pellets were resuspended in the D-Hank's solution, and the total cell number was determined using a hemocytometer. Differential counts were performed on May-Grunwald/Giemsa-stained cytospun cells (Sigma-Aldrich).
Cytokine analysis
The concentration of cytokines in the BALF supernatant was measured using ELISA Kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. The minimum detectable level of cytokines are as follows: IL-4, less than 2 pg/mL; IL-5, less than 7 pg/mL; IL-13, less than 1.5 pg/mL; and IFN-γ, less than 2 pg/mL. IL-4, IL-5, IL-13, or IFN-γ ELISA kits show no cross-reactivity with any of the cytokines tested at 50 ng/mL.
Flow cytometry of Fas/FasL expression
Cells purified from mouse lungs and PLNs were resuspended and stained with FITC-conjugated anti-CD4, PE-conjugated anti- Fas/FasL, and the respective isotype control stains, according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). The fluorescent antibodies were added (1 μg/100 μL) and incubated for 30 min at 4°C. Flow cytometry acquisition was performed using a FACSCalibur (BD Biosciences, San Jose, CA), and the results were analyzed using CellQuest software (BD Biosciences).
Histology and quantitation of epithelial cells
Paraffin-embedded lung sections (4 μm) were stained with hematoxylin–eosin (HE) for general morphology and Alcian blue/periodic acid Schiff (AB/PAS) for detection of goblet cells. The AB/PAS-stained and epithelial areas of lung sections were captured using a light microscope (DP20, Olympus, Melville, NY) and quantification was performed as described previously (Wu and others 2001).
Cell culture, treatments, and apoptosis assay
Naive CD4+T cells were purified from the spleens of C57BL/6 mice using anti-CD4+ microbeads and a magnetic sorter (MACS; Miltenyi Biotec, Auburn, CA). The purity of the CD4+T cells was >90%. The CD4+ T cells were then suspended with or without IFN-γ (0.1 U/mL, 1 U/mL, or 10 U/mL) for 6, 12, 18, or 24 h. The anti-FasL monoclonal antibody (MFL-3, 10 mg/mL; Pharmingen, San Diego, CA, USA) was used in neutralization experiments at a concentration of 10 μg/mL. After treatment, CD4+ T cells were harvested, washed twice with cold PBS, resuspended in 100 μL of cold binding buffer plus 1 μL FITC-Annexin V and 2 μL 7-aminoactinomycin D (7-AAD), and incubated for 15 min at room temperature in the dark. Apoptosis was assessed by dual-color flow cytometry on a FACScan cytofluorometer (BD Bioscience) using CellQuest software (BD Biosciences).
Statistical analysis
Results are expressed as the mean±SD. Differences in the data were analyzed by analysis of variance (followed by the Tukey's honestly significant difference test) or correlation analysis (Spearman) as appropriate with SPSS 17.0 (SPSS, Chicago, IL). A P-value less than 0.05 denoted the presence of a statistically significant difference.
Results
Low doses of IFN-γ suppress inflammatory cell infiltration and upregulate Fas/FasL expression on CD4+ T cells in an asthma model
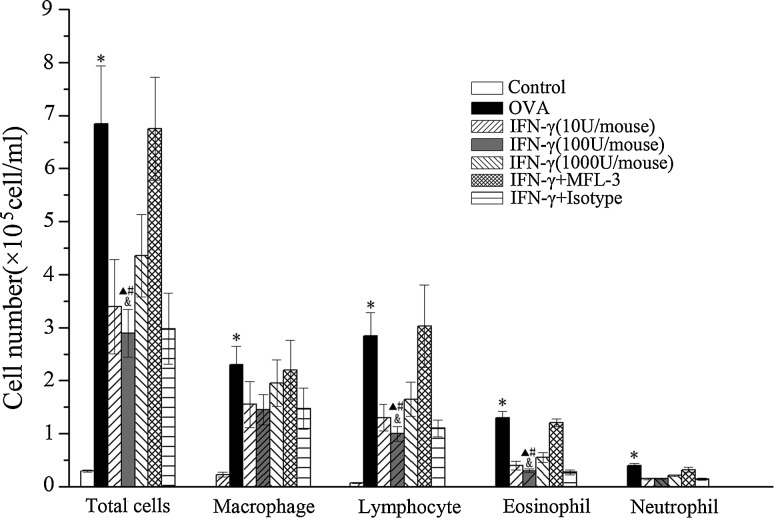
To examine the possible anti-inflammatory effect of IFN-γ on allergic airway reactivity, we used a treatment protocol, including i.p. injection of different IFN-γ doses of (10 U, 100 U, or 1000 U/mouse/day), beginning on the first day of OVA challenges. After sensitization and challenge with OVA, the total number of inflammatory cells (OVA/OVA versus control: 6.85±1.09 versus 0.31±0.02×105, P<0.01), eosinophils (OVA/OVA versus control: 1.31±0.12 versus 0×105, P<0.01), and lymphocytes (OVA/OVA versus control: 0.05±0.01 versus 2.85±0.43,×105, P<0.01) increased significantly in BALF. A low dose of IFN-γ noticeably inhibited the OVA-induced recruitment of eosinophils (100 U/mouse versus OVA/OVA: 0.31±0.09 versus 1.31±0.12,×105, P<0.01; 100 U/mouse versus 1000 U/mouse: 0.31±0.09 versus 0.55±0.07,×105, P<0.05) and lymphocyte (100 U/mouse versus OVA/OVA: 1.02±0.14 versus 2.85±0.43,×105, P<0.05; 100 U/mouse versus 1000 U/mouse: 1.02±0.14 versus 1.65±0.32,×105, P<0.05) into the BALF, whereas the low dose of IFN-γ did not affect macrophages and neutrophils (Fig. 1).
FIG. 1.
Effect of interferon (IFN)-γ on the amount of inflammatory cells in bronchoalveolar lavage fluid (BALF). The number of total cells, macrophages, lymphocytes, eosinophils, and neutrophils were determined 1 day after the final ovalbumin (OVA) challenge. Values are shown as mean±SEM of six mice per group. *P<0.01 versus control group; #P<0.01 versus OVA group; ▴P<0.05 versus MFL-3 group; &P<0.05 versus IFN-γ (1000 U/mouse) group.
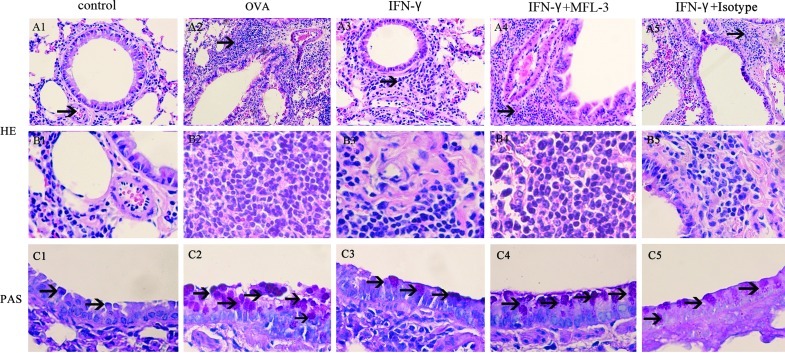
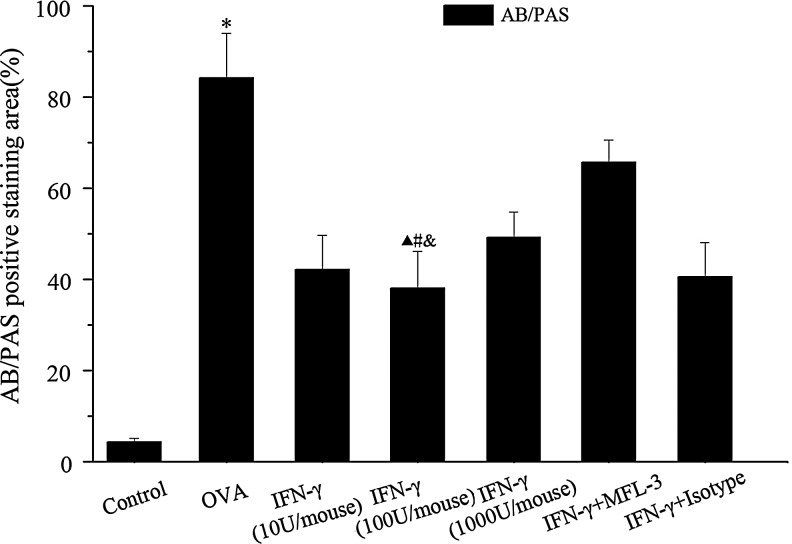
Further histological examination of H&E stained lung tissues revealed infiltration of inflammatory cells, especially lymphocytes and eosinophils after the OVA-challenge (Fig. 2). Meanwhile, OVA-challenged mice showed a strong AB/PAS-positive staining in bronchial epithelial tissue, indicating goblet cell hyperplasia. Administration of a low dose of IFN-γ during OVA challenge significantly alleviated inflammatory cell infiltration and goblet cell hyperplasia (Figs. 2 and 3).
FIG. 2.
Lung histology of C56BL/6 mice treated with IFN-γ. One day after the final challenge, lung tissues were obtained from control mice, OVA-sensitized mice and OVA-challenged mice, mice treated with IFN-γ (100 U/mouse), mice treated with IFN-γ and MFL-3 (100 μg/mouse), and IFN-γ and Isotype (100 μg/mouse). Representative photomicrographs of hematoxylin–eosin (HE)-stained (A and B) and periodic acid Schiff (PAS)-stained (C) lung sections from each group were shown. Arrows in A1–5 showed inflammatory cells; Arrows in C1–5 showed PAS-positive areas. A1–5, magnification ×100; B1–5, magnification ×400; C1–5, magnification ×400.
FIG. 3.
The percentages of positively stained epithelial areas (AB/PAS). Data are presented as the mean±SEM of six mice per group. * Significant difference (P<0.01) versus control group; #P<0.01 versus OVA group; ▴P<0.01 versus MFL-3 group; &P<0.05 versus IFN-γ (1000 U/mouse) group.
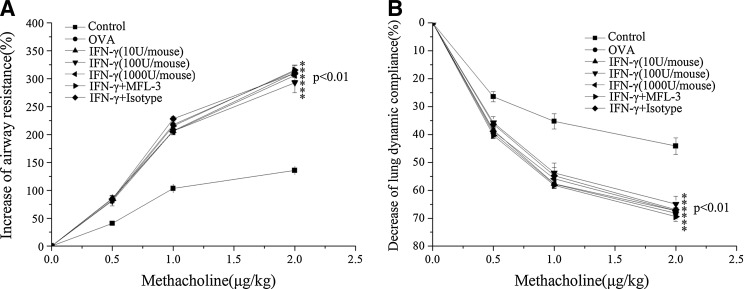
IFN-γ also inhibited AHR in the asthmatic model, and RL and Cdyn responses to methacholine were assessed in these mice. A significant increase in RL and a decrease in Cdyn were observed in OVA-challenged mice after intravenous administration of methacholine, indicating an increase in AHR compared with control mice (Fig. 4). However, low doses of IFN-γ did not inhibit the development of AHR in OVA-sensitized and OVA-challenged mice (Fig. 4).
FIG. 4.
IFN-γ has no effect on AHR. Airway responsiveness to an increased concentration of methacholine was examined 24 h after the final OVA challenge in each group. RL (A) and Cdyn (B) for each group were calculated as described in the Methods (mean±SEM, n=6). *P<0.01 versus control group.
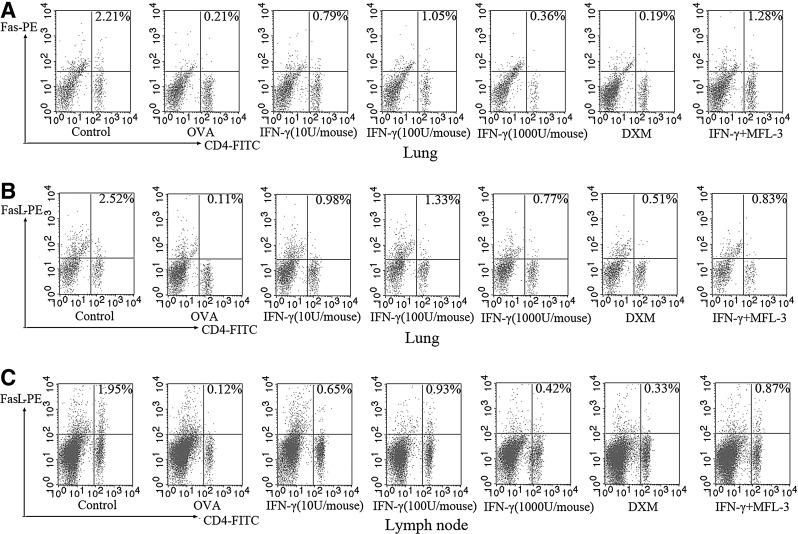
The effect of low IFN-γ doses on Fas/FasL expression on CD4+ T cells was tested in vivo using T cells derived from lung and PLNs that were analyzed by flow cytometry. After exposure to OVA, fewer CD4+/Fas+ T cells and CD4+/FasL+ T cells were observed when compared with the controls (Fig. 5). Treatment with low doses of IFN-γ significantly affected the percentages of CD4+/Fas+ T cells and CD4+/FasL+ T cells (P<0.05). Results are presented for the control group, OVA/OVA group, 10 U/mouse group, 100 U/mouse group, and 1000 U/mouse group. The ratio of CD4+ Fas+ T cells to CD4+ T cells from lung tissues were as follows: 11.3%±0.01%, 1.12%±0.01%, 3.59%±0.34%, 6.80%±0.83%, and 2.39%±0.91%, respectively. The ratio of CD4+ FasL+ T cells to CD4+ T cells from lung tissues were as follows: 15.22%±1.42%, 0.94%±0.08%, 3.07%±0.75%, 6.87%±0.85%, and 2.82%±0.31%, respectively. The ratios of CD4+FasL+T cells to CD4+T cells in PLNs were as follows: 9.39%±1.34%, 1.14%±0.76%, 3.77%±0.63%, 6.13%±1.02%, and 1.21%±0.54%, respectively). These data suggested that OVA sensitization and challenge reduce the expression of Fas/FasL on CD4+ T cells, and that a lower dose IFN-γ treatment (100 U/mouse) significantly attenuates this change, more effectively than the higher IFN-γ dose (1000 U/mouse) (P<0.05).
FIG. 5.
Effects of IFN-γ on the FAS and FASL expression on CD4+ T cells from lung tissue and peribronchial lymph nodes (PLNs). Single-cell suspensions prepared from lung tissue and PLNs were double-stained with FITC-conjugated anti-CD4, PE-conjugated anti-FAS or FASL and were analyzed by flow cytometry. (A) Percentage of CD4- and FAS-positive cells in lung tissue; (B) Percentage of CD4- and FASL-positive cells in lung tissue; (C) Percentage of CD4- and FASL-positive cells in PLNs.
Treatment with MFL-3, an anti-FasL antibody, partly abolished the anti-inflammatory property of IFN-γ in airway instead of revising the Th1/Th2 balance
To investigate whether the FasL pathway is involved in the IFN-γ-mediated anti-inflammatory mechanism in asthma, MFL-3, an anti-FasL antibody, was injected i.p. at the time of OVAII stimulation. MFL-3 partly blocked the anti-inflammatory effect of IFN-γ, as demonstrated by an increase in the numbers of total inflammatory cells, lymphocytes, and eosinophils in the MFL-3+IFN-γ group compared to those in the IFN-γ group (Fig. 1). Also, MFL-3 treatment promoted inflammatory cell infiltration and goblet cell hyperplasia in lung tissue (Figs. 2, Fig. 3), but it had no effect on AHR (Fig. 4).
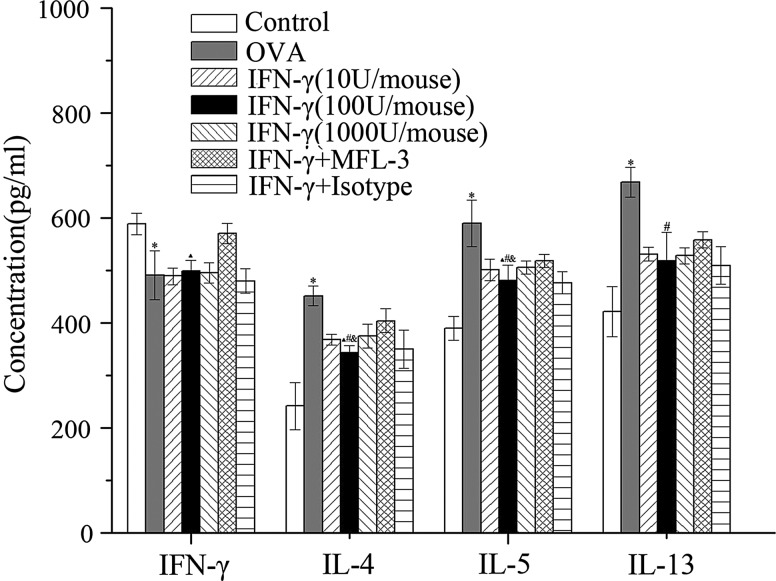
There was a significant increase in IL-4, IL-5, and IL-13 production (P<0.01) and a decrease in IFN-γ production (P<0.05) in mice sensitized and challenged with OVA compared with the control group (Fig. 6). Administration of exogenous IFN-γ during the OVA challenge significantly suppressed the OVA-induced IL-4, IL-5, and IL-13 production (P<0.05). Also, the lower dose of IFN-γ (100 U/mouse) was more effective than the higher dose (1000 U/mouse; P<0.05) at inhibiting Th2 cytokine production.
FIG. 6.
Effects of IFN-γ on cytokine production in BALF. One day after the last OVA challenge, concentrations of IFN-γ, IL-4, IL-5, and IL-13 in the BALF supernatant were assessed using ELISA. Data are presented as the mean±SEM of six mice per group. *Significant difference (P<0.01) versus control group; #P<0.05 versus OVA group; ▴P<0.05 versus MFL-3 group; &P<0.05 versus IFN-γ (1000 U/mouse) group.
MFL-3 treatment promoted Th2 cytokine production (IL-4, IL-5, IL-13) compared to mice that did not receive MFL-3 (P<0.05), and there was also an unexpectedly high level of secreted IFN-γ in the MFL-3-treated mice (Fig. 6), suggesting that MFL-3 does not influence the reversion of the Th1/Th2 imbalance induced by low doses of IFN-γ.
Low doses of IFN-γ enhance FasL-induced apoptosis in vitro
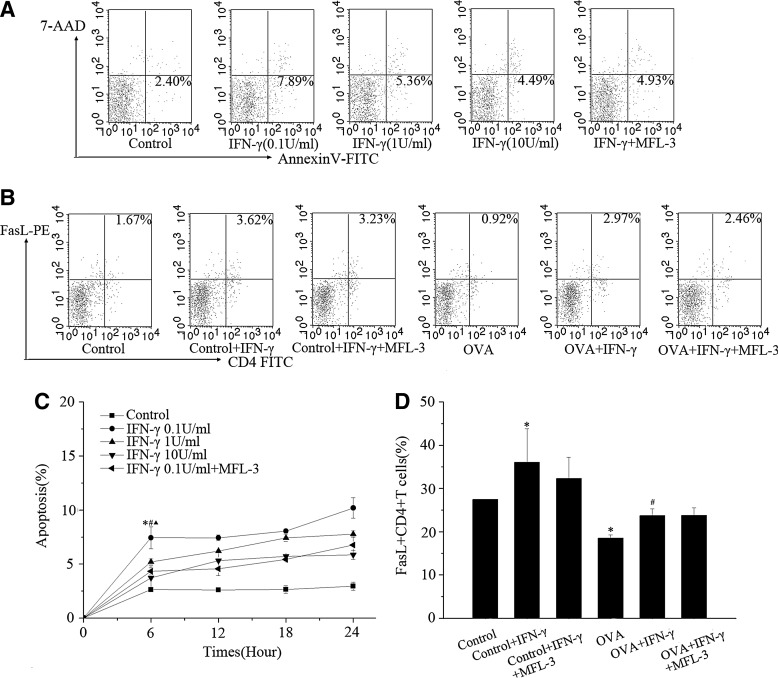
To determine whether low doses of IFN-γ enhance FasL-induced apoptosis in vitro, we isolated CD4+ T cells, which were preincubated in the presence or absence of lower (0.1 U/mL) or higher (1 U/mL or 10 U/mL) IFN-γ levels. As shown in Fig. 7, lower levels of IFN-γ-induced more CD4+ T cell apoptosis than did higher levels of IFN-γ (P<0.05) after 6, 12, 18, and 24 h. We also assessed the effect of IFN-γ on FasL and observed that lower levels of IFN-γ upregulated FasL expression.
FIG. 7.
(A, C) Effects of IFN-γ on CD4+ T cells apoptosis in vitro. After treatment with different concentrations of IFN-γ (0.1 U/mL, 1 U/mL, 10 U/mL), CD4+ T cells isolated from the murine spleen were double-stained with FITC-conjugated annexin V and 7-AAD, and were analyzed by flow cytometry at different times (6, 12, 18, 24h). *P<0.01 versus control group; #P<0.05 versus IFN-γ group (1 U/mL); ▴P<0.05 versus IFN-γ group (10 U/mL); (B, D) Effect of IFN-γ on FASL expression on CD4+ T cells in vitro. *P<0.05 versus control group; #P<0.05 versus OVA group.
MFL-3 partially inhibited IFN-γ-induced apoptosis, but did not affect the expression of FasL
The relationship between IFN-γ and Fas/FasL were also assessed in response to MFL-3 in experimental and control mice. We observed no significant increase in the levels of FasL (P>0.05) in the lung and PLN of experimental mice (Fig. 5). In vitro, the percentage of apoptotic cells, but not the percentage of FasL+ CD4+ T cells, decreased after exposure to MFL-3, compared to the IFN-γ group (P<0.05).
Discussion
Results of our study showed that i.p. administration of low doses of exogenous IFN-γ during an OVA challenge significantly reduced the numbers of different inflammatory cells in BALF, decreased Th2 cytokine production, and alleviated goblet cell hyperplasia; these results suggest that IFN-γ induced a shift from a Th2-skewed response to a more balanced Th1/Th2 response. However, high doses of endogenous IFN-γ had little effect on the airway inflammation. Also, the lower IFN-γ dose inhibited inflammatory infiltrations more effectively than did the higher dose. Our results are similar to those of other previously published studies (Flaishon and others, 2002; Lama and others 2011).
The proinflammatory effect of IFN-γ, at a relatively high level, has been shown by several researchers. Doukas and Farrar reported that IFN-γ, in conjunction with TNF-α, can expand and amplify the overall inflammatory response through enhancing expression of cell surface adhesion molecules, such as intercellular adhesion molecule-1 and endothelial leukocyte adhesion molecule-1 (Doukas and Pober 1990; Farrar and Schreiber 1993). Recently, Wild and others (2000) illustrated that IFN-γ induced IL-18 production, which increased allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. Also, Koch and his coworkers demonstrated that IFN-γ increased markers of allergic airway inflammation (IL-5, IL-13), and questioned the therapeutic value of IFN-γ in allergic asthma (Koch and others 2006). We considered that a higher level of IFN-γ may induce the release of some cytokines and inflammatory mediators, and partly abolish the anti-inflammatory function of IFN-γ.
AHR is a main pathophysiological characteristic of asthma, and the effect of IFN-γ on AHR is still controversial. IFN-γ has been previously thought to decrease AHR and inhibit airway inflammation in the asthma Th2 model (Flaishon and others 2002). However, several studies have recently revealed that IFN-γ does not suppress AHR or neutralize IFN-γ-inhibited AHR and neutrophilic accumulation in BALF and lung tissues (Kumar and others 2004; Hayashi and others, 2007; Yang and others 2009). These studies have led to the hypothesis that IFN-γ may induce the release of some chemokines, which have the capacity to recruit neutrophils in the lungs and thereby induce AHR. The data presented in this study show that low doses of IFN-γ did not affect neutrophils in BALF, which may result in failure to inhibit OVA-induced AHR.
It is well known that an alteration in cytokines, with over production of Th2 cytokines (IL-4, IL-5, and IL-13) in concert with reduction of Th1 cytokines (IFN-γ and IL-2), is predicted to play a role in causing asthma (Castro and others 2000; Lama and others 2011). However, using MFL-3, an anti-FasL monoclonal antibody, before treatment with a low dose of exogenous IFN-γ resulted in an increased level of the Th2 cytokines IL-4, IL-5, and IL-13, and the Th1 cytokine IFN-γ in BALF, and also resulted in an increase in inflammation and an increase in lymphocytes and eosinophil infiltration compared to the IFN-γ group. MFL-3 partially blocked the IFN-γ-mediated reduction in inflammation, but had no effect on a shift from Th2 to Th1 that was induced by low doses of IFN-γ. Thus, we hypothesize that a low dose of exogenous IFN-γ may attenuate airway inflammation through another mechanism that is blocked by MFL-3, but that has no effect on reversing the Th1/Th2 imbalance.
Apoptosis provides a mechanism for the removal of inflammatory and structural cells, and consequently resolves the inflammatory response. Evidence has accumulated that IFN-γ regulates the apoptosis of T lymphocytes by modulating antigen-activated T cell surface Fas and FasL expression. Kodama's study indicated a correlation between increased IFN-γ production and enhanced CD4+ T cell apoptosis in allergic airway infiltrates (Kodama and others 2003). De Rose and others (2004) have shown that exogenous IFN-γ inhibited the proliferation of allergen-stimulated CD4+ T cells from atopic, asthmatic patients by inducing the surface expression of Fas and FasL, which in turn triggered apoptosis in the cells. Our study demonstrated that OVA sensitization and challenge can reduce the number of CD4+/Fas+ T cells and CD4+/FasL+ T cells, whereas delivery of a lower IFN-γ concentration relocated Fas/FasL to the CD4+ T cell surface more effectively than did higher IFN-γ concentrations. Also, the percentages of CD4+/Fas+ T cells and CD4+/FasL+ T cells showed a negative correlation with several important indices of asthma, including the number of lung inflammatory cells, the level of IL-4 and IL-5 in BALF, and hyperplasia of airway goblet cells (data not shown). These findings suggested that low doses of IFN-γ might promote the recovery of Fas/FasL expression on CD4+ T cells during allergic airway infiltration.
Low levels of IFN-γ stimulation can also induce in vitro CD4+ T cell apoptosis. The effect of IFN-γ on CD4+ T cells was concentration dependent, with a maximum apoptosis enhancement at 0.1 U/mL. The expression of FasL on CD4+ T cells from OVA-sensitized and OVA-challenged mice were also analyzed, and we found that a low level of IFN-γ increased the number of CD4+ FasL+ T cells. Although MFL-3 did not affect FasL expression, this study revealed that MFL-3 inhibited IFN-γ-induced apoptosis in vitro, and as a result, partially blocked the anti-inflammatory function of IFN-γ in vivo. We speculated that the mechanism by which MFL-3 might suppress IFN-γ-induced apoptosis is through damage to the FasL function or destruction of the FasL structure, rather than by decreasing its expression level.
In summary, our study showed that low doses of IFN-γ indeed reversed the Th1/Th2 imbalance in asthmatic responses, but the anti-inflammatory mechanism of IFN-γ may occur through a second pathway, which decreases the asthmatic responses in a dose-dependent manner coupled with increasing the Fas/FasL expression on CD4+ T cells. This caused enhanced CD4+ T cell apoptosis, leading to attenuation of airway inflammation in asthma.
Acknowledgments
This work was supported by Grants 81000757, 81170015, and 81170038 from the National Natural Science Foundation of China, and by Grant Y2100346 from the Natural Science Foundation of Zhejiang Province, China.
Author Disclosure Statement
The authors have no competing financial interests.
References
- Afkarian M. Sedy JR. Yang J. Jacobson NG. Cereb N. Yang SY. Murphy TL. Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- Castro M. Chaplin DD. Walter MJ. Holtzman MJ. Could asthma be worsened by stimulating the T-helper type 1 immune response? Am J Respir Cell Mol Biol. 2000;22:143–146. doi: 10.1165/ajrcmb.22.2.f174. [DOI] [PubMed] [Google Scholar]
- Cohn L. Elias JA. Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- De Rose V. Cappello P. Sorbello V. Ceccarini B. Gani F. Bosticardo M. Fassio S. Novelli F. IFN-gamma inhibits the proliferation of allergen-activated T lymphocytes from atopic, asthmatic patients by inducing Fas/FasL-mediated apoptosis. J Leukoc Biol. 2004;76:423–432. doi: 10.1189/jlb.0503247. [DOI] [PubMed] [Google Scholar]
- Doukas J. Pober JS. IFN-gamma enhances endothelial activation induced by tumor necrosis factor but not IL-1. J Immunol. 1990;145:1727–1733. [PubMed] [Google Scholar]
- Druilhe A. Wallaert B. Tsicopoulos A. Lapa e Silva JR. Tillie-Leblond I. Tonnel AB. Pretolani M. Apoptosis, proliferation, and expression of bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998;19:747–757. doi: 10.1165/ajrcmb.19.5.3166. [DOI] [PubMed] [Google Scholar]
- Farrar MA. Schreiber RD. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Flaishon L. Topilski I. Shoseyov D. Hershkoviz R. Fireman E. Levo Y. Marmor S. Shachar I. Cutting edge: anti-inflammatory properties of low levels of IFN- gamma. J Immunol. 2002;168:3707–3711. doi: 10.4049/jimmunol.168.8.3707. [DOI] [PubMed] [Google Scholar]
- Hayashi N. Yoshimoto T. Izuhara K. Matsui K. Tanaka T. Nakanishi K. T helper 1 cells stimulated with ovalbumin and IL-18 induce airway hyperresponsiveness and lung fibrosis by IFN-gamma and IL-13 production. Proc Natl Acad Sci USA. 2007;104:14765–14770. doi: 10.1073/pnas.0706378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S. Castro M. O'Sullivan M. Bragdon MJ. Holtzman MJ. Resistance to Fas-mediated T cell apoptosis in asthma. J Immunol. 1999;162:1717–1722. [PubMed] [Google Scholar]
- Koch M. Witzenrath M. Reuter C. Herma M. Schütte H. Suttorp N. Collins H. Kaufmann SH. Role of local pulmonary IFN-gamma expression in murine allergic airway inflammation. Am J Respir Cell Mol Biol. 2006;35:211–219. doi: 10.1165/rcmb.2005-0293OC. [DOI] [PubMed] [Google Scholar]
- Kodama T. Kuribayashi K. Nakamura H. Fujita M. Fujita T. Takeda K. Dakhama A. Gelfand EW. Matsuyama T. Kitada O. Role of interleukin-12 in the regulation of CD4+T cell apoptosis in a mouse model of asthma. Clin Exp Immunol. 2003;131:199–205. doi: 10.1046/j.1365-2249.2003.02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RK. Herbert C. Webb DC. Li L. Foster PS. Effects of anticytokine therapy in a mouse model of chronic asthma. Am J Respir Crit Care Med. 2004;170:1043–1048. doi: 10.1164/rccm.200405-681OC. [DOI] [PubMed] [Google Scholar]
- Lama M. Chatterjee M. Nayak CR. Chaudhuri TK. Increased interleukin-4 and decreased interferon-γ levels in serum of children with asthma. Cytokine. 2011;55:335–338. doi: 10.1016/j.cyto.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Li H. Xie Q. Wang H. Shao C. Chen W. Intramuscular delivery of mIL-12 gene reduces the expression of CD44/CD49d on pulmonary leucocytes and inhibits ovalbumin-induced airway hyperreactivity. Inflam Res. 2008;57:11–17. doi: 10.1007/s00011-007-7042-1. [DOI] [PubMed] [Google Scholar]
- Lighvani AA. Frucht DM. Jankovic D. Yamane H. Aliberti J. Hissong BD. Nguyen BV. Gadina M. Sher A. Paul WE. O'Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttmann W. Dauer E. Schmidt S. Marx O. Hossfeld M. Matthys H. Virchow JC., Jr. Effects of interferon-gamma and tumor necrosis factor-alpha on CD95/Fas ligand-mediated apoptosis in human blood eosinophils. Scand J Immunol. 2000;51:54–59. doi: 10.1046/j.1365-3083.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Refaeli Y. Van Parijs L. Alexander SI. Abbas AK. Interferon γ is required for activation-induced death of T lymphocytes. J Exp Med. 2002;196:999–1005. doi: 10.1084/jem.20020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZO. Fischer MJ. De Sanctis GT. Schuyler MR. Tesfaigzi Y. IFN-γ, but not Fas, mediates reduction of allergen-induced mucous cell metaplasia by inducting apoptosis. J Immunol. 2002;168:4764–4771. doi: 10.4049/jimmunol.168.9.4764. [DOI] [PubMed] [Google Scholar]
- Szabo SJ. Sullivan BM. Peng SL. Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Vignola AM. Chanez P. Chiappara G. Siena L. Merendino A. Reina C. Gagliardo R. Profita M. Bousquet J. Bonsignore G. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–573. doi: 10.1016/s0091-6749(99)70225-3. [DOI] [PubMed] [Google Scholar]
- Wild JS. Sigounas A. Sur N. Siddiqui MS. Alam R. Kurimoto M. Sur S. IFN- gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164:2701–2710. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- Wu CA. Puddington L. Whiteley HE. Yiamouyiannis CA. Schramm CM. Mohammadu F. Thrall RS. Murine cytomegalovirus infection alters Th1/Th2 cytokine expression, decreases airway eosinophilia, and enhances mucus production in allergic airway disease. J Immunol. 2001;167:2798–2807. doi: 10.4049/jimmunol.167.5.2798. [DOI] [PubMed] [Google Scholar]
- Yang M. Kumar RK. Foster PS. Pathogenesis of steroid-resistant airway hyperresponsiveness: interaction between IFN-gamma and TLR4/MyD88 pathways. J Immunol. 2009;182:5107–5115. doi: 10.4049/jimmunol.0803468. [DOI] [PubMed] [Google Scholar]