Abstract
The acute administration of ethanol to intestinal epithelial cells causes increased intestinal permeability and the translocation of endotoxins. The changes caused by ethanol in intestinal cells may be related to oxidative stress and DNA damage. However, DNA damage and repair-related molecules which act against stresses, including ethanol, have not been fully investigated in intestinal cells. Heat shock proteins (Hsps) are involved in the recovery and protection from cell damage and may be associated with DNA repair. Therefore, the aim of our study was to investigate cytotoxicity, DNA damage and the expression of DNA repair-related molecules, antioxidant proteins and Hsps in intestinal cells exposed to ethanol. Human intestinal Caco-2 cells were incubated with 1–8% ethanol for 1 h. Cell viability and DNA damage were determined using the MTT and comet assays, respectively. We measured DNA repair-related molecules, including DNA polymerase β, apurinic/apyrimidinic endonuclease/redox factor-1 (APE/Ref-1), growth arrest and DNA damage 45α (GADD45α) and proliferating cell nuclear antigen (PCNA), in Caco-2 cells using western blot analysis. We also measured glutathione peroxidase-1 (GPx-1), peroxiredoxin-1 (PRX-1), superoxide dismutase-2 (SOD-2), Hsp10, Hsp27, Hsp60, heat shock cognate (Hsc)70, Hsp70 and Hsp90. The viability of the Caco-2 cells exposed to ethanol decreased at concentrations ≥7% (P<0.05). The Olive tail moment, indicating DNA damage, increased dose dependently in ≥3% ethanol (P<0.05). Among the DNA repair proteins, the expression of PCNA and APE/Ref-1 increased significantly at 1% ethanol. Antioxidant enzymes, including GPx-1, PRX-1 and SOD-2, had an increased expression at 1% ethanol. Hsp10, Hsp27 and Hsp70 expression also increased significantly at 1% ethanol. In conclusion, the expression of DNA repair molecules, antioxidants and Hsps increased in intestinal Caco-2 cells exposed to low concentrations of ethanol. In particular, PCNA, APE/Ref-1, Hsp10, Hsp27 and Hsp70 were sensitive to low ethanol concentrations, indicating that they may be useful in evaluating the DNA repair and cytoprotective effects of the drug against stress in intestinal cells.
Keywords: Caco-2 cells, DNA repair molecules, heat shock proteins
Introduction
The acute administration of ethanol to intestinal epithelial cells causes increased intestinal permeability and the translocation of endotoxin, which is a component of the outer wall of Gram-negative bacteria and is normally excluded by the intestinal barrier. Despite several studies, the pathogenesis of the effects of ethanol on the small intestine is not yet clear (1–3). One study indicated that structural and functional abnormalities caused by ethanol are associated with oxidative stress and this was suggested as the mechanism by which ethanol increases intestinal permeability (4). This model has recently been demonstrated in lung cells (5). Increases in reactive oxygen species (ROS) by oxidative stress increase lipid, protein and DNA peroxidation. ROS from xenobiotics, including ethanol, cause oxidative DNA damage through single-strand breaks (6). According to Bradford et al (7), when ethanol is administered to the liver of rats, the expression of DNA repair enzymes increases and may be used as a sensitive DNA damage marker. Interest in the small intestine has been on the increase due to the development of capsule endoscopy and enteroscopy (8). However, only a few studies have been conducted on DNA damage and repair associated with oxidative stress caused by ethanol in the small intestine.
Base excision repair (BER) is the main DNA repair mechanism, which maintains the stability of the genome in response to DNA damage caused by reactive chemicals that are constantly created (9). A variety of repair-related enzymes and proteins, including DNA polymerase β, apurinic/apyrimidinic endonuclease/redox factor-1 (APE/Ref-1), proliferating cell nuclear antigen (PCNA) and growth arrest and DNA damage 45α (GADD45α) are used in BER. However, DNA damage and repair-related enzymes in intestinal cells which act against stresses including ethanol have not been fully investigated.
Heat shock proteins (Hsps) are cell proteins that remove or repair denatured proteins and prevent protein aggregation. Hsps are involved in recovery and protection from cell damage. Hsps are associated with DNA repair enzymes and related proteins during cellular oxidative damage (10,11).
Therefore, the aim of our study was to investigate cytotoxicity, DNA damage and the expression of DNA repair-related molecules, antioxidant proteins and Hsps in intestinal cells exposed to ethanol for a short time.
Materials and methods
Chemicals
Urea, thiourea, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate, dithiothreitol, acrylamide, NN′-methylene-bisacrylamide, tris and sodium dodecyl sulfate (SDS) were purchased from Sigma Chemical (St. Louis, MO, USA). Ethanol was purchased from Merck (Merck Co., Darmstadt, Germany).
Cell culture
Caco-2 cells, a human colorectal adenocarcinoma cell line, were obtained from American Type Culture Collection (ATCC-HTB-37TM; Manassas, VA, USA). These cells form polarized monolayers that morphologically and functionally resemble adult human small intestinal mucosal cells subsequent to reaching confluence. Cells were maintained in MEM containing 20% fetal bovine serum albumin (ATCC), penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37˚C in a 5% CO2 in air atmosphere. Cells cultured for 1 h with 1, 2, 3, 4, 5, 6, 7 and 8% ethanol were used for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and comet assays, as well as in western blot analysis.
MTT assay
Caco-2 cells (1×104) were incubated with different concentrations (1, 2, 3, 4, 5, 6, 7 and 8%) of ethanol in 96-well plates for 1 h and cell viability was determined using the MTT assay (Calbiochem, San Diego, CA, USA) (12). Briefly, 20 μl of 5 mg/ml MTT in phosphate-buffered saline (PBS; Gibco BRL, Paisley, Scotland, UK) was added to each well and incubated for 3 h at 37˚C. The media were then removed and formazan crystals in the cells were dissolved in the presence of 200 μl lysis buffer (5% w/v of SDS in 0.01 N HCl). The plates were read at a 590 nm wavelength using an ELISA reader (Molecular Devices Co., Sunnyvale, CA, USA). The percentage of cell proliferation and cytotoxicity was determined by comparing the optical densities of the cells treated with different concentrations of ethanol with that of the control. Each experiment was repeated seven times.
Comet assay
DNA damage was determined using the comet assay. This assay was performed according to Singh et al with minor modifications (13). Briefly, normal and low melting point agarose (NMA and LMA, respectively; Ameresco, Solon, OH, USA) was added to fully frosted slides that were precoated with 50 μl 1% NMA for firm attachment and the slides were then allowed to solidify with cover slips in the refrigerator for 5 min. Following solidification of the gel, the cover slips were removed and 50 μl cells mixed with 50 μl of 1% LMA were added. The cover slips were added on to the layer and the slides were allowed to solidify in the refrigerator for 5 min. Following the removal of the cover slips, 100 μl of 0.5% LMA was added as a third layer and the slides were again placed with cover slips in the refrigerator for 5 min. The slides were submersed in a lysing solution (2.5 M NaCl, 100 mM EDTA-2Na, 10 mM Tris-HCl, pH 10; 1% Triton X-100 and 10% DMSO, pH 10 added fresh) for 1 h. The slides were then placed in an unwinding buffer (1 mM EDTA and 300 mM NaOH, pH 13) for 20 min and electrophoresis was performed using the same solution for 20 min at 25 V and 300 mA (0.8 v/cm). Following electrophoresis, the slides were neutralized by washing three times with neutralization buffer (400 mM Tris-HCl, pH 7.4) for 5 min each and were then stained with 50 μl of 10 μg/ml ethidium bromide. The slides were examined using a Komet 4.0 image analysis system (Kinetic Imaging, Liverpool, UK) fitted with an Olympus BX50 fluorescence microscope (Olympus, Tokyo, Japan) and equipped with a 515–560 nm excitation filter and a 590 nm barrier filter. Two slides were prepared for each treatment group and 50 randomly selected cells (total 100 cells) were scored manually. The parameter Olive tail moment [=(Tail.mean-Head.mean) × Tail%DNA/100] was automatically calculated using the Komet 4.0 image analysis system, which was used for global comet description. Each experiment was repeated three times.
Western blot assay
Caco-2 cells were solubilized in a lysis buffer (pH 7.4) on ice using a homogenizer. The lysates were then clarified by centrifugation at 12,000 rpm for 15 min at 4˚C and the protein concentration of the total lysate was determined using a Bradford protein assay (Bio-Rad Laboratories, Richmond, CA, USA). An equal amount of protein per lane was separated by electrophoresis on 8 and 15% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA, USA) at 400 mA for 160 min using a transfer buffer (pH 8.3). The membranes were blocked with blocking buffer (PBS containing 5% skimmed milk) for 1 h at room temperature, followed by incubation with primary antibodies overnight at 4˚C. Subsequent to the membranes being washed three times with PBS-T for 20 min, they were further incubated with horseradish peroxidase-conjugated secondary antibodies [anti-rabbit IgG and anti-mouse IgG (1:2,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA)] for 1 h at room temperature and washed three times for 20 min with PBS-T. Following extensive washing, the immune complexes were then detected using the enhanced chemiluminescence (ECL) and ECL Plus systems (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Primary antibodies against DNA polymerase β (1:1,000; Abcam, Cambridge, MA, USA), APE/Ref-1 (1:4,000; Santa Cruz Biotechnology), PCNA (1:100; Santa Cruz Biotechnology), GADD45α (1:500; Santa Cruz Biotechnology), glutathione peroxidase-1 (GPx-1; 1:1,000; Abcam), peroxiredoxin-1 (PRX-1; 1:1,000; Santa Cruz Biotechnology), superoxide dismutase-2 (SOD-2; 1:10,000; Abcam), Hsp10 (1:20,000; Abcam), Hsp27 (1:1,000; Cell Signaling Technology, Inc., Danvers/Beverly, MA, USA), Hsp60 (1:1,000; Santa Cruz Biotechnology), Hsp70 (1:1,000; Biosciences, San Diego, CA, USA), heat shock cognate (Hsc)70 (1:2,000; Santa Cruz Biotechnology) and Hsp90 (1:4,000; BD Transduction Laboratories) were applied at the optimal concentrations. Bands were visualized by ECL and scanned using a flat-bed scanner. The digitalized images were analyzed using Scion image analysis software (Scion Co., Frederick, MD, USA).
Statistical analysis
Variables were presented as the mean ± SD and the statistical analysis was performed using an analysis of variance followed by Dunnett's test. P<0.05 was considered to indicate statistically significant results. Data were analyzed using statistical software (SPSS for Windows version 12.0; SPSS Inc., Chicago, IL, USA).
Results
Cytotoxicity
The exposure of Caco-2 cells to 1–8% ethanol for 1 h resulted in a dose-dependent decrease in viability as measured by the MTT assay. The effect was statistically significant at ethanol concentrations ≥7% (P<0.05; Fig. 1).
Figure 1.
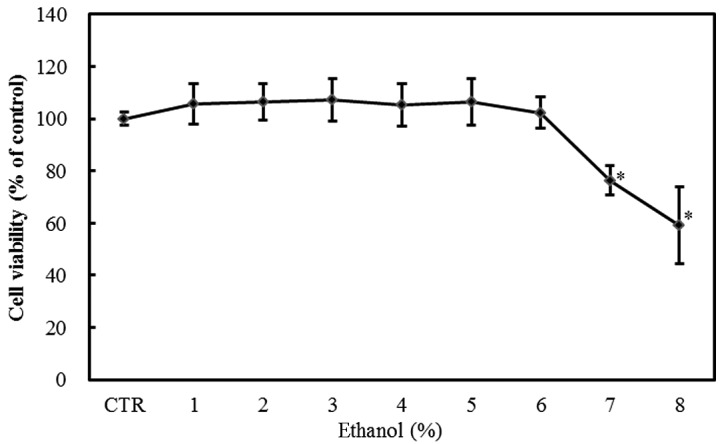
Effect of graded ethanol concentrations (1–8%) on the viability of Caco-2 cells as assessed by the MTT assay. Values are mean ± SD. *P<0.05 versus control.
DNA damage
The exposure of Caco-2 cells to 1–8% ethanol for 1 h caused a dose-dependent increase in the Olive tail moment, which is a DNA damage parameter in the comet assay. The effect was statistically significant at ethanol concentrations ≥3% (P<0.05; Fig. 2).
Figure 2.
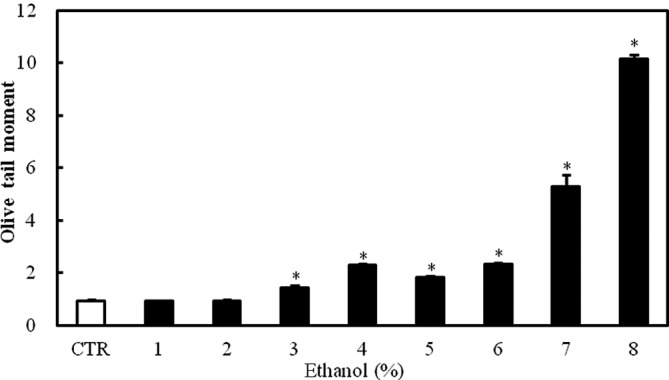
Effect of graded ethanol concentrations (1–8%) on DNA damage in Caco-2 cells as assessed by the comet assay. The figure shows the distribution of Olive tail moments, which indicate DNA damage, in Caco-2 cells exposed to ethanol. Values are mean ± SD. *P<0.05 versus control.
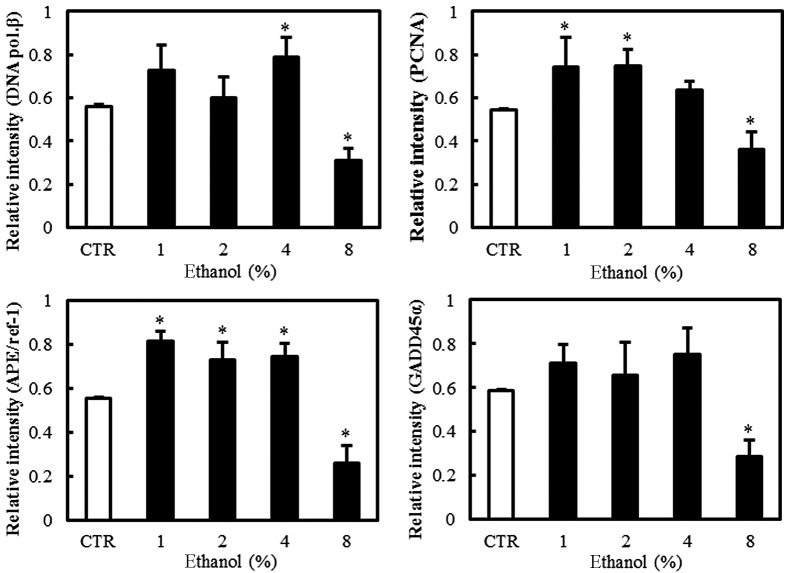
Effect of ethanol on DNA repair-related molecules
Western blot analyses were performed to evaluate the expression of the BER pathway DNA repair-related molecules, including DNA polymerase β, PCNA, APE/Ref-1 and GADD45α, in Caco-2 cells exposed to graded ethanol concentrations (1, 2, 4 and 8%) for 1 h.
The relative intensities revealed that PCNA and APE/Ref-1 expression increased significantly in Caco-2 cells exposed to 1% ethanol, the level of DNA polymerase β expression increased in 4% ethanol and GADD45α expression did not increase significantly in low concentrations of ethanol. The expression of PCNA, APE/Ref-1, DNA polymerase β and GADD45α all decreased at 8% ethanol (Fig. 3).
Figure 3.
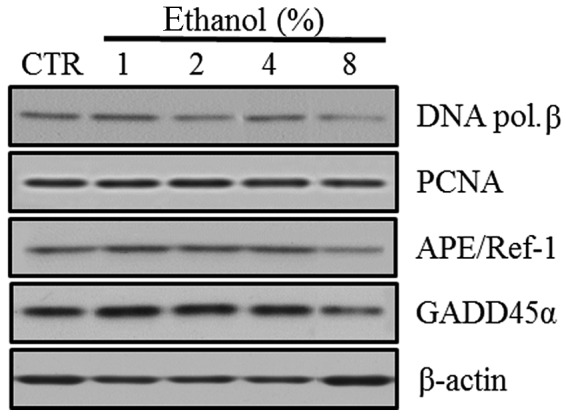
Effect of ethanol on DNA repair enzymes and molecules in Caco-2 cells. (A) Western blot analysis of DNA polymerase β, PCNA, APE/Ref-1 and GADD45α. (B) Quantitation of the relative expression intensities of DNA polymerase β, PCNA, APE/Ref-1 and GADD45α. Values are mean ± SD. *P<0.05 versus control (CTR). PCNA, proliferating cell nuclear antigen; APE/Ref-1, apurinic/apyrimidinic endonuclease/redox factor-1; GADD45α, growth arrest and DNA damage 45α; DNA pol. β, DNA polymerase β.
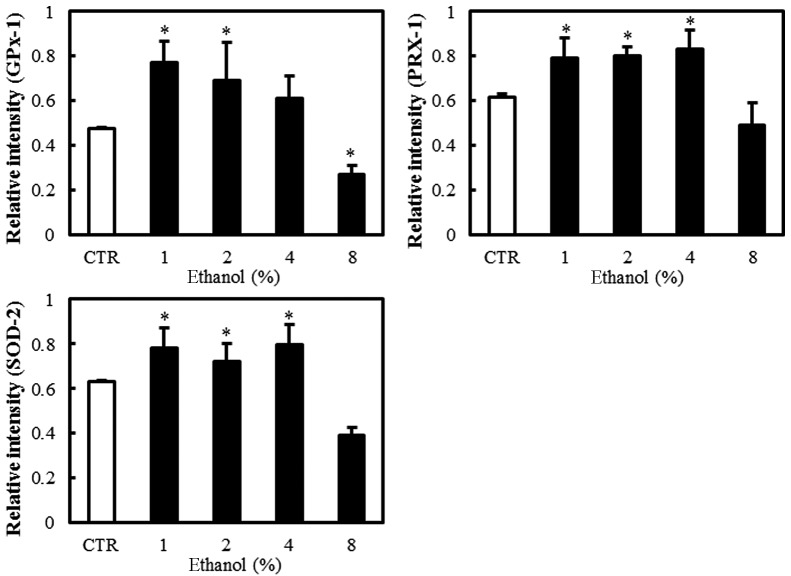
Effect of ethanol on antioxidant enzymes
Western blot analyses were performed to evaluate the expression of the antioxidant enzymes GPx-1, PRX-1 and SOD-2 in Caco-2 cells exposed to graded ethanol concentrations (1, 2, 4 and 8%) for 1 h.
The relative intensities revealed that GPx-1, PRX-1 and SOD-2 expression increased significantly in Caco-2 cells exposed to 1% ethanol; however, their expression decreased with the 8% ethanol exposure (Fig. 4).
Figure 4.
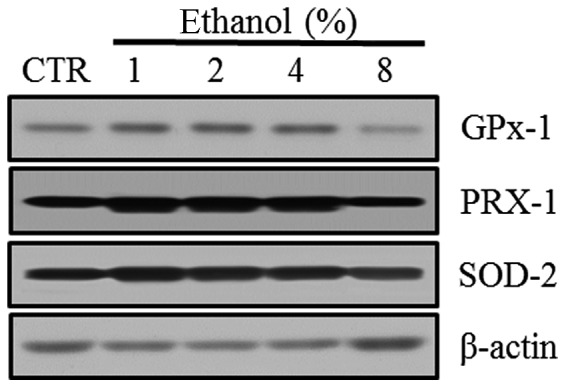
Effect of ethanol on antioxidant enzymes in Caco-2 cells. (A) Western blot analysis of GPx-1, PRX-1 and SOD-2. (B) Quantitation of the relative expression intensity of GPx-1, PRX-1 and SOD-2. Values are mean ± SD. *P<0.05 versus control (CTR). GPx-1, glutathione peroxidase-1; PRX-1, peroxiredoxin-1; SOD-2, superoxide dismutase-2.
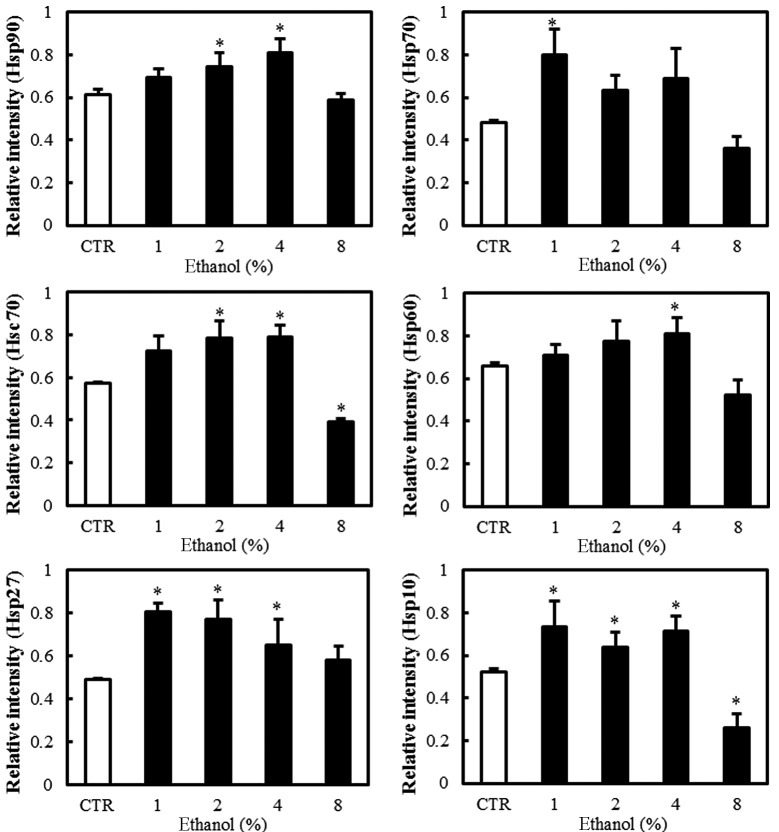
Effect of ethanol on Hsps
Western blot analyses revealed the level of Hsp expression in Caco-2 cells exposed to graded ethanol concentrations (1, 2, 4 and 8%) for 1 h. The relative intensities of Hsp10, Hsp27 and Hsp70 expression increased significantly at 1% ethanol. Hsp10 decreased significantly at 8% ethanol. Hsp27 and Hsp70 also decreased gradually with further increases in ethanol concentration (Fig. 5).
Figure 5.
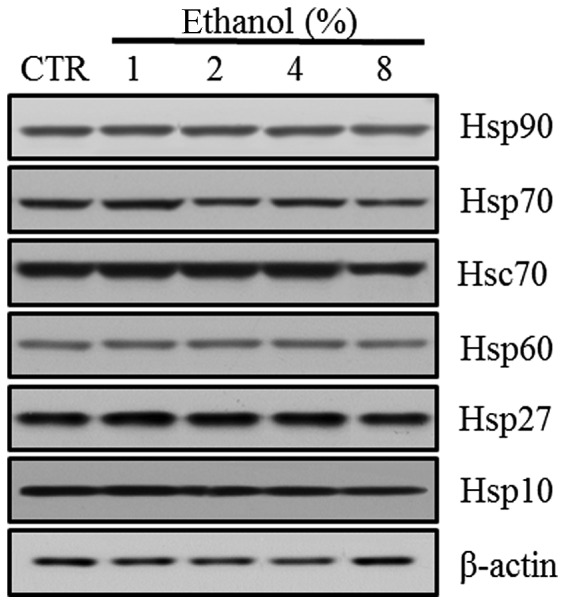
Effect of ethanol on Hsps in Caco-2 cells. (A) Western blot analysis of Hsp10, Hsp27, Hsp60, Hsc70, Hsp70 and Hsp90. (B) Quantitation of the relative expression intensity of Hsp10, Hsp27, Hsp60, Hsc70, Hsp70 and Hsp90. Values are mean ± SD. *P<0.05 versus control (CTR). Hsp, heat shock protein; Hsc, heat shock cognate.
However, Hsp60 expression increased significantly at a concentration of 4% ethanol. Hsc70 and Hsp90 expression increased with 2% ethanol exposure. Hsc70 expression decreased significantly with 8% ethanol exposure (Fig. 4).
Discussion
This study examined the cytotoxicity, DNA damage and the expression of DNA repair-related molecules, antioxidants and Hsps in intestinal epithelial cells exposed to low concentrations of ethanol for 1 h.
Acute and chronic exposure of the small intestine to ethanol causes various structural and functional abnormalities. The acute administration of ethanol increases intestinal permeability within 30 min and causes the transfer of endotoxin into the blood, leading to endotoxemia. Intermittent endotoxemia then stimulates Kupffer cells in the liver and facilitates the formation of ROS and inflammatory mediators, resulting in liver damage.
In our study, 3 and 7% concentrations of ethanol induced statistically significant DNA damage and cytotoxicity, respectively. Therefore, low ethanol concentrations (<10%) may cause intestinal epithelial cell death, as reported in previous studies (14,15). Additionally, genotoxicity may be associated with cytotoxicity. Based on these results, the ethanol concentrations required to observe the expression of proteins and enzymes involved in DNA repair in intestinal epithelial cells were determined. Accordingly, Caco-2 cells were exposed to 1, 2, 4 and 8% graded ethanol concentrations for 1 h.
The mechanism of BER is as follows (13): DNA glycosylase removes damaged DNA bases by cutting the N-glycosyl bond. The apurinic or apyrimidinic site lacking the DNA base is known as the abasic or the AP site. AP endonuclease (another name for APE/Ref-1) cleaves the phosphodiester backbone near the AP site. DNA polymerase then removes the damaged part of the strand and synthesizes a new strand. Unlike short-patch repair, in which DNA polymerase β inserts a single nucleotide, if the AP site is oxidized or reduced, it is resistant to DNA polymerase β and long-patch repair occurs. During long-patch repair, DNA polymerases δ and ɛ act with PCNA, which is a repair-related protein. APE/Ref-1 is a significant indicator of DNA repair enzymes and an essential element in cancer research. GADD45α is the transcriptional target of p53 and delays carcinogenesis and decreases mutation frequency. A recent study suggested that GADD45α improves the BER response by binding to DNA and influencing the interaction between cellular APE/Ref-1 and PCNA (16). More research concerning the role of these proteins in DNA damage-repair mechanisms is needed.
In this study, the level of DNA repair-related molecules increased in intestinal epithelial cells exposed to low concentrations of ethanol for a short time. First, PCNA and APE/Ref-1 increased in the 1% ethanol-exposed cells. DNA polymerase β increased at higher concentrations than PCNA and APE/Ref-1. GADD45α did not show a significant increase. Therefore, PCNA and APE/Ref-1 may be useful in evaluating the level of DNA damage in intestinal epithelial cells. In a previous study, which evaluated DNA damage in human lymphocytes exposed to hydrogen peroxide (H2O2) and methyl methane sulfonate (MMS) for a short time, GADD45α was more sensitive to DNA damage than other repair-related molecules, including DNA polymerase β, PCNA and APE/Ref-1 (17). However, different expression patterns of repair-related molecules in Caco-2 cells compared with lymphocytes were observed in this study. Therefore, although the toxicants used were different, the response to genotoxic compounds may vary according to cell type.
All antioxidant enzymes, including GPx-1, PRX-1 and SOD-2, increased in 1% ethanol-exposed cells, indicating that oxidative stress is involved in the intestinal cell damage caused by ethanol. Oxidative stress may induce the synthesis of Hsps. Hsps function as molecular chaperones by facilitating protein synthesis, folding, transport, translocation and processing. Hsps are therefore essential for cell survival (5). Hsps are classified into subfamilies depending on their molecular weight, including Hsp60, Hsp70, Hsp90 and the small Hsp family (18).
Among the Hsps, the expression of Hsp10, Hsp27 and Hsp70 increased significantly at 1% ethanol. Hsc70 and Hsp90 also had significant changes in expression at higher concentrations than those of Hsp10, Hsp27 and Hsp70. Hsp60 also had significant changes in the levels of expression. Different Hsps are induced in different organs. In intestinal epithelial cells, the Hsp70 family and Hsp25 are induced (19,20). Hsp60 has no protective role in the intestine and Hsp10 is a molecular chaperone that functions in protein stabilization and folding with Hsp60 (21). The overexpression of Hsp10 has been reported at a higher rate than Hsp70 in cancer cells, including colorectal cancer (22). It is speculated that Hsp10 affects cell life and death unlike Hsp60, which does not affect cell life and death. In other words, Hsp10 is considered an active factor in the cell signaling pathway and affects the cell cycle, nucleocytoplasmic transport and metabolism (23).
DNA repair-related molecules and Hsps had similar expression patterns based on the ethanol concentration. Their expression intially increased with the increasing ethanol concentration. Expression of most of these molecules significantly changed at an ethanol concentration <3%, at which point significant DNA damage occurred. However, the expression of all the repair-related molecules decreased at an 8% ethanol concentration, suggesting that intestinal epithelial cells lose their repair capacity due to cell death, which occurred at a 7% ethanol concentration in the MTT assay.
It could be inferred that Hsps are associated with DNA repair. Mendez et al (11) revealed that Hsp70 stimulates the BER enzyme, DNA polymerase β. Kenny et al (10) stated that Hsp70 binds to human AP endonuclease and stimulates endonuclease activity at abasic sites. These studies suggest that a single-strand DNA binding protein may be needed for BER and that Hsps may play a role as BER accessory proteins. If DNA repair-related molecules could be measured according to the induction or suppression of Hsps, they may be useful for clarifying the association between DNA repair and Hsps.
The limitation of our study was the use of a tumor cell line that was not suited to showing physiological phenomena. Although Caco-2 cells originated from human colorectal adenocarcinoma cells, they have similar characteristics to small intestinal epithelial cells. In addition, normal intestinal cell lines, including FHs74Int, are difficult to culture. Therefore, Caco-2 cells were used to investigate the heat shock response and gene expression in this study.
In conclusion, the results revealed that acute and low (<10%) concentrations of ethanol induced DNA damage and cytotoxicity in human intestinal epithelial Caco-2 cells. In particular, PCNA, APE/Ref-1, Hsp10, Hsp27 and Hsp70 were more sensitive to ethanol than the other DNA repair molecules and Hsps. These proteins may be useful in evaluating the genotoxicity of toxicants and the DNA repair and cytoprotective effects of drugs in intestinal cells under stress.
References
- 1.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–592. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 2.Bujanda L. The effects of alcohol consumption upon the gastrointestinal tract. Am J Gastroenterol. 2000;95:3374–3382. doi: 10.1111/j.1572-0241.2000.03347.x. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa K, Adachi J, Wong MCY, Ueno Y. Protective effect of daidzein against acute ethanol-induced lipid peroxidation in rat jejunum. Kobe J Med Sci. 2006;52:141–149. [PubMed] [Google Scholar]
- 4.Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316:1–7. doi: 10.1124/jpet.105.085449. [DOI] [PubMed] [Google Scholar]
- 5.Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Mutlu-Turkoglu U, Dogru-Abbasoglu S, Aykac-Toker G, Mirsal H, Beyazyurek M, Uysal M. Increased lipid and protein oxidation and DNA damage in patients with chronic alcoholism. J Lab Clin Med. 2000;136:287–291. doi: 10.1067/mlc.2000.109097. [DOI] [PubMed] [Google Scholar]
- 7.Bradford BU, Kono H, Isayama F, et al. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 8.Tennyson CA, Semrad CE. Advances in small bowel imaging. Curr Gastroenterol Rep. 2011;13:408–417. doi: 10.1007/s11894-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 9.Christmann M, Tomicic MT, Roos WP, Kaina B. Mechanisms of human DNA repair: an update. Toxicology. 2003;193:3–34. doi: 10.1016/s0300-483x(03)00287-7. [DOI] [PubMed] [Google Scholar]
- 10.Kenny MK, Mendez F, Sandigursky M, et al. Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J Biol Chem. 2001;276:9532–9536. doi: 10.1074/jbc.M009297200. [DOI] [PubMed] [Google Scholar]
- 11.Mendez F, Kozin E, Bases R. Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell Stress Chaperones. 2003;8:153–161. doi: 10.1379/1466-1268(2003)008<0153:hspsot>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 14.Asai K, Buurman WA, Reutelingsperger CP, Schutte B, Kaminishi M. Low concentrations of ethanol induce apoptosis in human intestinal cells. Scand J Gastroenterol. 2003;38:1154–1161. doi: 10.1080/00365520310006252. [DOI] [PubMed] [Google Scholar]
- 15.Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- 16.Jung HJ, Kim EH, Mun JY, et al. Base excision DNA repair defect in Gadd45a-deficient cells. Oncogene. 2007;26:7517–7525. doi: 10.1038/sj.onc.1210557. [DOI] [PubMed] [Google Scholar]
- 17.Cho EK, Park SY, Sul DG. Comparison of DNA damage and the expression of repair related molecules, including DNA polymerase β, APE/ref-1, PCNA, and GADD45, in human T and B lymphocytes exposed to hydrogen peroxide and methyl methanesulfonate. In: Kimura H, Suzuka A, editors. New Research on DNA Damage. Nova Science Publishers, Inc; Hauppauge, NY: 2008. pp. 305–318. [Google Scholar]
- 18.Tominaga M, Ohta M, Kai S, Iwaki K, Shibata K, Kitano S. Increased heat-shock protein 90 expression contributes to impaired adaptive cytoprotection in the gastric mucosa of portal hypertensive rats. J Gastroenterol Hepatol. 2009;24:1136–1141. doi: 10.1111/j.1440-1746.2008.05763.x. [DOI] [PubMed] [Google Scholar]
- 19.Otani S, Otaka M, Jin M, et al. Effect of preinduction of heat shock proteins on acetic acid-induced colitis in rats. Dig Dis Sci. 1997;42:833–846. doi: 10.1023/a:1018832618275. [DOI] [PubMed] [Google Scholar]
- 20.Ropeleski MJ, Tang J, Walsh-Reitz MM, Musch MW, Chang EB. Interleukin-11-induced heat shock protein 25 confers intestinal epithelial-specific cytoprotection from oxidant stress. Gastroenterology. 2003;124:1358–1368. doi: 10.1016/s0016-5085(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 21.Iwabuchi A, Otaka M, Otani S, et al. Specific preinduction of 60-kDa heat shock protein (chaperonin homolog) by TRH does not protect colonic mucosa against acetic acid-induced lesion in rats. Dig Dis Sci. 2000;45:1480–1489. doi: 10.1023/a:1005597113024. [DOI] [PubMed] [Google Scholar]
- 22.Junhui C, Liming C, Shaobin W, Jiexiong H, Qiancheng Q, Liyan X. Clinical significance of heat shock protein 10 in large bowel carcinoma. Chinese-German J Clin Oncol. 2007;6:334–338. [Google Scholar]
- 23.Stribinskis V, Heyman HC, Ellis SR, Steffen MC, Martin NC. Rpm2p, a component of yeast mitochondrial RNase P, acts as a transcriptional activator in the nucleus. Mol Cell Biol. 2005;25:6546–6558. doi: 10.1128/MCB.25.15.6546-6558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]