Abstract
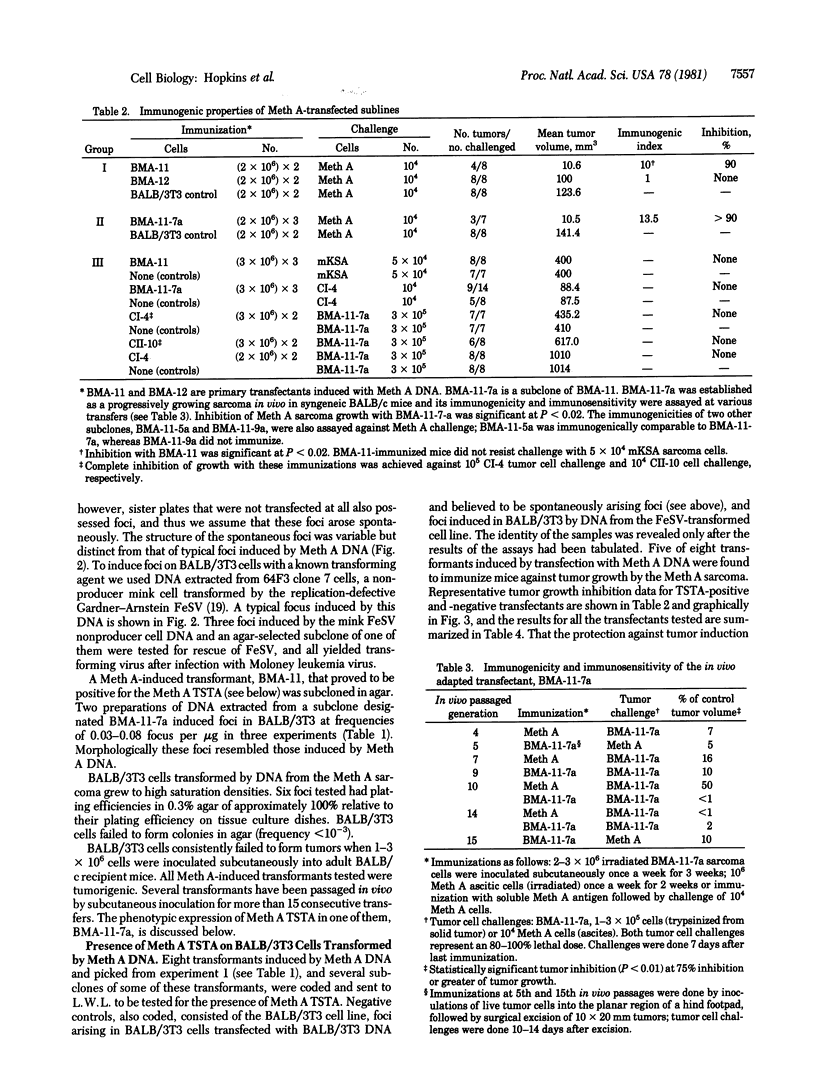
We transformed BALB/3T3 mouse cells with cellular DNA extracted from the Meth A sarcoma, a 3-methylcholanthrene-induced tumor of BALB/c mice, and asked whether foci arising in the transfection possess the previously defined Meth A tumor-specific transplantation antigen (TSTA). Five of eight foci selected from one experiment possessed Meth A TSTA. DNA extracted from one of the five TSTA-positive clones was used in secondary rounds of transfection transformation. Four out of five foci tested from the secondary transfections possessed Meth A TSTA. These results suggest that in the Meth A sarcoma a transforming gene and a genetic determinant of TSTA are intimately related: they may be identical or very closely linked; alternatively, a particular transforming gene might induce the expression of a particular TSTA. Another possible explanation for these results is that the cotransfer of certain cellular genes by DNA transfection is considerably higher than predicted from the limited studies presently available.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Andersson P., Goldfarb M. P., Weinberg R. A. A defined subgenomic fragment of in vitro synthesized Moloney sarcoma virus DNA can induce cell transformation upon transfection. Cell. 1979 Jan;16(1):63–75. doi: 10.1016/0092-8674(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Borberg H., Oettgen H. F., Choudry K., Beattie E. J., Jr Inhibition of established transplants of chemically induced sarcomas in syngeneic mice by lymphocytes from immunized donors. Int J Cancer. 1972 Nov;10(3):539–547. doi: 10.1002/ijc.2910100312. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Neiman P. E. Transforming genes of neoplasms induced by avian lymphoid leukosis viruses. Nature. 1980 Oct 16;287(5783):656–659. doi: 10.1038/287656a0. [DOI] [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Shiku H., Takahashi T., John M., Old L. J. Cell surface antigens of chemically induced sarcomas of the mouse. I. Murine leukemia virus-related antigens and alloantigens on cultured fibroblasts and sarcoma cells: description of a unique antigen on BALB/c Meth A sarcoma. J Exp Med. 1977 Sep 1;146(3):720–734. doi: 10.1084/jem.146.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois G. C., Appella E., Law L. W., DeLeo A. B., Old L. J. Immunogenic properties of soluble cytosol fractions on Meth A sarcoma cells. Cancer Res. 1980 Nov;40(11):4204–4208. [PubMed] [Google Scholar]
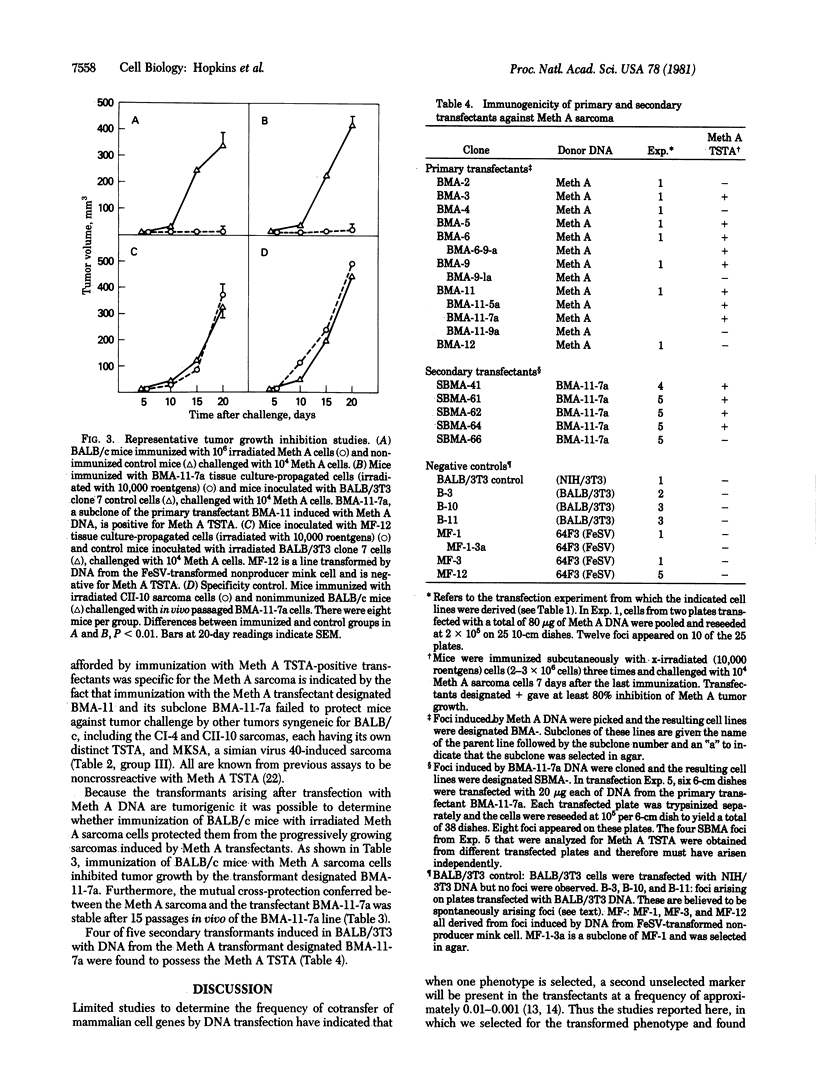
- DuBois G. C., Appella E., Law L. W., DeLeo A. B., Old L. J. The soluble antigens of BALB/c sarcoma Meth-A: a relationship between a serologically defined tumor-specific surface antigen (TSSA) and the tumor-associated transplantation antigen (TATA). Transplant Proc. 1981 Dec;13(4):1765–1773. [PubMed] [Google Scholar]
- FOLEY E. J. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res. 1953 Dec;13(12):835–837. [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN G., SJOGREN H. O., KLEIN E., HELLSTROM K. E. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960 Dec;20:1561–1572. [PubMed] [Google Scholar]
- Kit S., Kurimura T., Dubbs D. R. Transplantable mouse tumor line induced by injection of SV40-transformed mouse kidney cells. Int J Cancer. 1969 Jul 15;4(4):384–392. doi: 10.1002/ijc.2910040403. [DOI] [PubMed] [Google Scholar]
- Law L. W. Changes in tumor-specific antigen expression during passage in vitro and in vivo of newly derived methylcholanthrene-induced sarcomas of BALB/C mice. Int J Cancer. 1980 Feb 15;25(2):251–259. doi: 10.1002/ijc.2910250213. [DOI] [PubMed] [Google Scholar]
- Natori T., Law L. W., Appella E. Biological and biochemical properties of Nonidet P40-solubilized and partially purified tumor-specific antigens of the transplantation type from plasma membranes of a methylcholanthrene-induced sarcoma. Cancer Res. 1977 Sep;37(9):3406–3413. [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Peterson J. L., McBride O. W. Cotransfer of linked eukaryotic genes and efficient transfer of hypoxanthine phosphoribosyltransferase by DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1583–1587. doi: 10.1073/pnas.77.3.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Siminovitch L. Transfer of anchorage independence by isolated metaphase chromosomes in hamster cells. Cell. 1977 Nov;12(3):675–682. doi: 10.1016/0092-8674(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Steffen D., Bird S., Rowe W. P., Weinberg R. A. Identification of DNA fragments carrying ecotropic proviruses of AKR mice. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4554–4558. doi: 10.1073/pnas.76.9.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]