Abstract
Pancreatic carcinoma is one of the leading causes of cancer mortality worldwide, although the molecular mechanisms of this disease are poorly understood. The aim of this study was to examine the expression of cyclin-dependent kinase inhibitors (CDKIs) and the epigenetic modifications in the promoters of these genes. We also evaluated the correlation between the methylation status of CDKI genes and smoking habit in clinical pancreatic carcinoma specimens. Western blotting and real-time PCR were performed to assess CDKI expression. Methylation-specific PCR was carried out to examine the methylation status of the promoters of CDKI genes. In this study, we revealed that reduced levels of the CDKI proteins, p15INK4b, p16INK4a, p21cip1 and p27kip1, are a prominent feature of pancreatic carcinoma patients. The DNA hypermethylation of the promoter was observed in 40% (2 of 5) of the p15INK4b genes, 60% (3 of 5) of the p16INK4a genes and 60% of the p21cip1 genes, which markedly correlated with their decreased mRNA expression. No hypermethylation was detected in the p27kip1 gene promoter in 5 pancreatic carcinoma patients with markedly decreased expression of p27kip1 mRNA, suggesting an alternative mechanism of p27kip in these patients. In this study, patients with a smoking habit displayed methylation of 2 CDKI genes in their pancreatic carcinoma specimens. We concluded that epigenetic modification via hypermethylation represents a critical mechanism for the inactivation of CDKI genes in pancreatic carcinoma.
Keywords: p15INK4b, p16INK4a, p21cip1, p27kip1, methylation, pancreatic carcinoma
Introduction
Pancreatic carcinoma is one of the leading causes of cancer mortality worldwide. Pancreatic cancer has been reported to be associated with various environmental and lifestyle risk factors. Although the molecular etiology of pancreatic carcinoma is unclear, age and cigarette smoking are the unequivocal risk factors (1).
Due to the aggressive nature of the disease and the difficulties in diagnosis, the overall 5-year survival rate of pancreatic carcinoma is less than 5% (2,3). Novel approaches for the diagnosis and treatment of pancreatic cancer are necessary to improve the survival rate.
Cancer is a genetic disease where alterations in several genes accumulate and lead to a cancer cell growth advantage. Cell-cycle progression is driven by cyclins and cyclin-dependent kinases (CDKs). The activities of cyclin-CDK complexes are modulated by two classes of CDK inhibitor (CDKI) (4). The INK4 CDKI proteins (p15INK4, p16INK4, p18INK4 and p19INK4) sequester CDKs and inhibit the formation of CDK-cyclin complexes, whereas the Cip/Kip CDKIs (p21Waf1/Cip1, p27Kip1 and p57Kip2) bind to cyclin-CDK complexes (5). p21 (also known as waf1, cip1 or sdi1) is an important cellular checkpoint molecule for the inhibition of a range of cyclin-CDK activities. In our previous study, we demonstrated that association of p21cip1 with CDK2/cyclin E blocks cell-cycle progression at multiple points (6,7). The INK4A locus encodes two unrelated proteins, p14ARF and p16INK4a. p16INK4a is a specific inhibitor of the cyclin D-dependent kinases CDK4 and CDK6 (8) and antagonizes their ability to phosphorylate the retinoblastoma (Rb) family of proteins and so prevent exit from the G1 phase of the cell cycle (9). INK4A is important in mediating the signals that constrain the cell cycle in response to hyperproliferative signals, and, furthermore, are the most frequently inactivated tumor suppressor genes in human cancer (10,11).
Promoter methylation is an alternative form of gene silencing, which relies on epigenetic factors. Previous reports have revealed that aberrant INK4a or Cip/Kip promoter methylation is a frequent event in human tumors (12). Studies have indicated that suppressed expression by aberrant promoter methylation may be an alternative mechanism for inactivation of the tumor suppressor gene in pancreatic cancer cases (13).
In the present study, we reported that reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 CDKI proteins and mRNA are prominent features of pancreatic carcinoma. We observed that promoter hypermethylation of genes was partly correlated with decreased p15INK4b, p16INK4a and p21cip1, but not p27kip1, mRNA in the tumors from clinical patients.
Materials and methods
Study subjects
Five frozen fresh tumor specimens were surgically isolated from patients with pancreatic carcinoma. All of these patients were admitted into the third General Surgery Department, Zhongshan Hospital. Ethical approval for this study from the Zhongshan hospital and agreement by all patients were obtained.
Western blot analysis
The tumor and human normal tissues were prepared in lysis buffer from the MC-CelLytics kit (Shenergy Biocolor, Shanghai, China). The protein content was determined using the Bradford calorimetric assay method (Shenergy Biocolor). The lysate was resolved by 10% polyacrylamide-sodium lauryl sulfate gel electrophoresis and Immobilon-P transfer membrane (Millipore, MA, USA). Antibodies used for detection were p15INK4b (Santa Cruz), p16INK4a (Santa Cruz), p21cip1 (Cell Signaling) and p27kip1 (Santa Cruz). Then, the blot was incubated with a secondary antibody, IRDye 800 conjugated affinity purified anti-mouse or anti-rabbit IgG (Rockland Immunochemicals, Gilbertsville, PA, USA), and detected with an Odyssey Infrared Imaging System (LI-COR Bioscienceces, Lincoln, NE, USA).
Real-time RT-PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from tumor specimens and normal tissues. RNA was reverse transcribed using a PrimeScript™ RT reagent kit (DRR037A; Takara, Dalian, China). Real-time PCR analysis was performed in a final volume of 25 μl, containing 2 μl of cDNA template, 0.5 μl of each primer (10 μM) and 12.5 μl of a SYBR-Green master mix (2X) according to the instructions of the Real-time PCR kit (Takara, Japan) to evaluate the levels of mRNA expression. The following primers were used: 5′-AAGCTGAGCCCAGGT CTCCTA-3′ (forward) and 5′-CCACCGTTGGCCGTAAACT-3′ (reverse) for p15INK4b; 5′-ACCCTTGTGCCTCGCTCAG-3′ (forward) and 5′-GGTCTGCCGCCGTTTTC-3′ (reverse) for p21cip1, and published primers for p16INK4a (14) and p27kip1 (15). The average amount of the genes was normalized to the levels of GAPDH (16), an endogenous housekeeping gene.
Methylation analysis
The tumor and normal tissue DNA was extracted by Tissue Genomic Isolation kits (Dingguo Bio-tech, Beijing, China). For methylation-specific PCR (MSP), samples were prepared according to the instructions of the CpGenome™ FAST DNA Modification kit (S7824; Chemicon International, CA, USA). Bisulfite-treated DNA was amplified using MSP primers specific for either methylated or unmethylated DNA using published primers for p15INK4b (17), p16INK4a (17), p21cip1 (18) and p27kip1 (18). PCR was carried out with Ex-Taq Hot Start DNA polymerase (Takara). The annealing temperatures used were 60˚C for p15 U/M, 65/60˚C for p16 U/M, 57˚C for p21 U/M and 55˚C for p27 U/M.
Results
Reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 proteins detected in pancreatic carcinoma
The events leading to pancreatic carcinoma development remain largely unknown. Several studies have shown that the Rb tumor-suppressive pathway is abrogated in almost all studied cases of pancreatic carcinoma, and this disruption is caused exclusively by inactivation of CDKI (19). p15INK4b, p16INK4a, p21cip1 and p27kip1 are considered the most important CDKIs. This led us to ask whether these CDKIs are involved in pancreatic tumor formation. We examined p15INK4b, p16INK4a, p21cip1 and p27kip1 expression in 5 pancreatic carcinoma specimens and controls using western blotting. As shown in Fig. 1, p15INK4b, p16INK4a, p21cip1 and p27kip1 protein levels in 5 pancreatic carcinoma specimens (PC1-5) were much lower than in normal human pancreatic tissue (NP). These data suggest that reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1, the negative regulators of cell progression, may be causative of tumor formation in pancreatic carcinoma.
Figure 1.
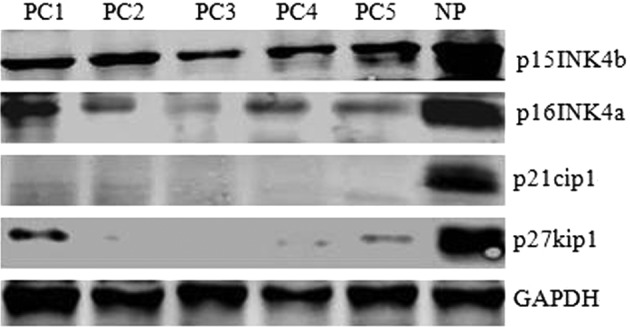
Reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 were detected in pancreatic carcinoma. Western blotting was performed to assess p15INK4b, p16INK4a, p21cip1 and p27kip1 protein levels in pancreatic carcinoma specimens (PC) and normal pancreatic tissue (NP) with GAPDH as the control.
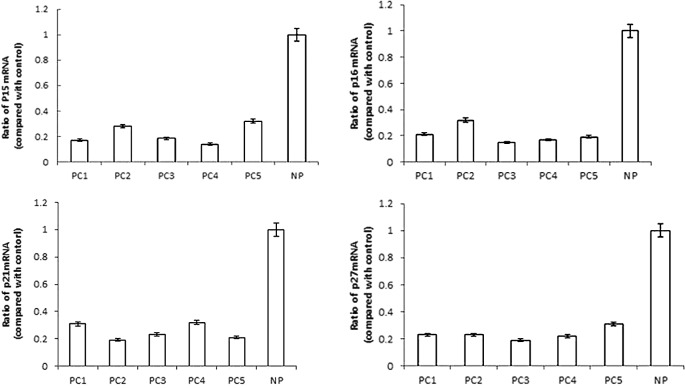
Reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 mRNA expression detected in pancreatic carcinoma
Subsequently, we were interested in whether aberrant mRNA expression levels contributed to these CDKI alterations in pancreatic carcinoma specimens from patients. We evaluated p15INK4b, p16INK4a, p21cip1 and p27kip1 mRNA by real-time RT-PCR analysis to determine whether decreased mRNA accumulation contributed to their protein levels. As shown in Fig. 2, it was preceded by a >3-fold reduction in p15INK4b mRNA expression, a >4-fold reduction in p16INK4a mRNA expression, a >3-fold reduction in p21cip1 mRNA expression and a >4-fold reduction in p27kip1 mRNA expression of PC specimens, as compared to human NP tissue. Our findings suggested that the reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 proteins were likely due to a reduction in their mRNA synthesis, stability or translation in pancreatic carcinoma.
Figure 2.
Reduced p15INK4b, p16INK4a, p21cip1 and p27kip1 mRNA expression was detected in pancreatic carcinoma. Real-time PCR was performed to assess p15INK4b, p16INK4a, p21cip1 and p27kip1 mRNA in pancreatic carcinoma specimens (PC) and normal pancreatic tissue (NP) as the control, and normalized to GAPDH expression. The error bars indicate the standard error of the mean of 3 independent experiments.
Methylation status of the p15INK4b, p16INK4a, p21cip1 and p27kip1 promoter region in pancreatic carcinoma
To explore the mechanism associated with the transcriptional silencing of the p15INK4b, p16INK4a, p21cip1 and p27kip1 genes, we examined 5 PC and human NP tissue samples to see whether there was methylation alteration in a CpG-rich region of the transcription initiation site of the these genes. The presence or absence of methylation in the promoters of the p15INK4b, p16INK4a, p21cip1 and p27kip1 genes was determined by MSP. MSP distinguishes unmethylated from methylated alleles based on sequence changes produced following bisulfite treatment of DNA, which converts unmethylated cytosine to uracil, and subsequent PCR using primers designed for either methylated or unmethylated DNA. As shown in Fig. 3, promoter hypermethylation was detected in PC2 and PC5 samples in the p15INK4b gene, PC1, 2 and 3 samples in the p16INK4a gene or PC1, 4 and 5 samples in the p21cip1 gene, while no detectable promoter hypermethylation of the p27kip1 gene was found in all 5 PC samples. No detectable promoter hypermethylation of the p15INK4b, p16INK4a, p21cip1 and p27kip1 genes was found in human NP tissue samples (Fig. 3). Thus, aberrant DNA promoter hypermethylation of the p15INK4b, p16INK4a and p21cip1 genes was thought to play a role in several cases of pancreatic carcinoma that had markedly decreased expression of p15INK4b, p16INK4a and p21cip1 mRNA, concomitant with loss of p15INK4b, p16INK4a and p21cip1 proteins, but not p27kip1 protein (Figs. 2 and 3). Thus, we suggest that DNA hypermethylation associated with transcriptional silencing of the p15INK4b, p16INK4a and p21cip1 genes may partly contribute to pancreatic carcinoma progression.
Figure 3.
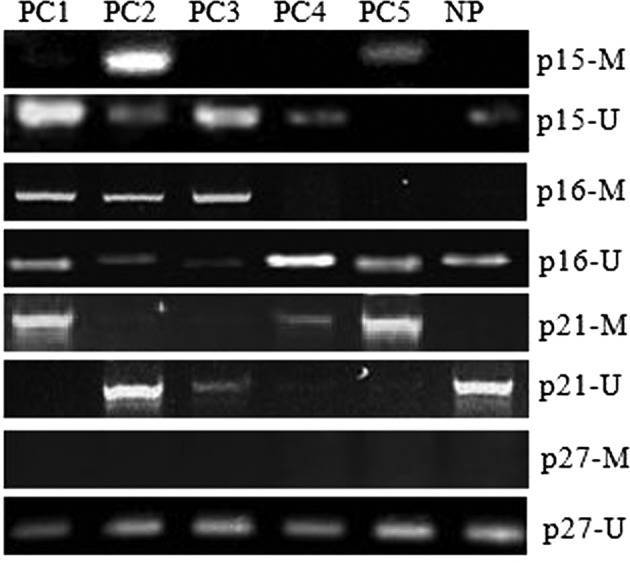
Methylation status of the p15INK4b, p16INK4a, p21cip1 and p27kip1 promoter regions in pancreatic carcinoma. Methylation-specific PCR was carried out. The presence of a PCR product in lanes M indicates the presence of methylated gene promoter and the presence of a product in lanes U indicates the presence of an unmethylated gene promoter in pancreatic carcinoma specimens (PC) and normal pancreatic tissue (NP).
Pancreatic carcinoma patients with smoking habits displayed methylation of 2 CDKI genes
According to our results, pancreatic carcinoma patients showed reduced levels of p15, p16, p21 and p27 mRNA or proteins. To investigate the correlation between smoking habit and the methylation status of the promoter region in pancreatic carcinoma, these 5 randomly selected pancreatic carcinomas removed from the patients were further studied. As shown in Table I, the patients having smoked for >15 years (PC1, 2 and 5) displayed methylation of 2 genes in their pancreatic carcinoma specimens. On the other hand, the other 2 pancreatic carcinoma specimens (PC3 and 4), with only 1 methylation region in the CDKI genes, occurred in the patients without a cigarette smoking record (Table I). Thus, we suggest that cigarette smoking may be associated with pancreatic tumorigenesis by inducing the methylation of the promoter regions of the CDKI genes.
Table I.
Association between smoking and methylation status.
| No. | Gender | Age (years) | Cigarette smoking | p15a | p16a | p21a |
|---|---|---|---|---|---|---|
| 1 | M | 42 | Yes | × | ✓ | ✓ |
| 2 | F | 62 | Yes | ✓ | ✓ | × |
| 3 | F | 49 | No | × | ✓ | × |
| 4 | F | 60 | No | × | × | ✓ |
| 5 | M | 56 | Yes | ✓ | × | ✓ |
Methylation status of the gene promoters.
Discussion
In this study, we revealed that the reduced levels of two classes of CDKI protein, including p15INK4b, p16INK4a, p21cip1 and p27kip1, are a prominent feature of pancreatic carcinomas. Moreover, a reduced amount of p15INK4b, p16INK4a, p21cip1 and p27kip1 mRNA expression was found in all of the pancreatic carcinoma samples. We observed that hypermethylation of the p15INK4b, p16INK4a and p21cip1 promoters, but not the p27kip1 promoter, was partly correlated with markedly decreased mRNA expression. Thus, we suggest that hypermethylation associated with transcriptional silencing of the p15INK4b, p16INK4a and p21cip1 gene may contribute to the progression of certain pancreatic carcinomas.
Due to the few treatment options for pancreatic carcinoma, understanding of the molecular pathology is a prerequisite for identifying potential molecular targets for drug therapy. CDKIs are negative regulators of cell-cycle progression at the G1-S restriction point. In this study, we showed that the reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 proteins were a prominent feature in the 5 pancreatic carcinoma specimens analyzed (Fig. 1), indicating that the reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1, the negative regulators of cell progression, are involved in the tumor formation in pancreatic carcinoma.
Moreover, a reduced amount of p15INK4b, p16INK4a, p21cip1 and p27kip1 mRNA normal expression was found in 5 pancreatic carcinoma samples (Fig. 2). We observed patients with a markedly decreased >3-fold reduction in p15INK4b mRNA, a 4-fold reduction in p16INK4a mRNA, a 3-fold reduction in p21cip1 mRNA and a 4-fold reduction in p27kip1 mRNA, concomitant with reduced levels of these CDKI proteins in sporadic pancreatic carcinoma (Figs. 1 and 2). The methylation status of the p15INK4b, p16INK4a, p21cip1 and p27kip1 promoter region was detected using MSP. The DNA hypermethylation promoter was found in 40% (2 of 5) of the p15INK4b genes, 60% (3 of 5) of the p16INK4a genes, 60% (3 of 5) of the p21cip1 genes and 0% (0 of 5) of the p27kip1 genes, and markedly correlated with decreased mRNA expression in pancreatic carcinoma patients (Figs. 2 and 3). Moreover, all 5 pancreatic carcinoma patients (100%; 5/5) displayed methylation of 1 or more genes, 60% (3/5) displayed methylation of 2 genes, while control tissue did not display any methylation. It has been shown that aberrant methylation is the most prominent feature of pancreatic carcinoma, causing alterations in the expressions of genes (13). Thus, we demonstrated that the epigenetic modification of p15INK4b, p16INK4a and p21cip1 via hypermethylation represents a critical mechanism for, at least in part, the inactivation of these genes in pancreatic carcinoma. In all 5 of our pancreatic carcinoma patients with markedly decreased expression of p27kip1 mRNA, we were unable to detect hypermethylation in the promoter region, suggesting an alternative mechanism of the p27kip1 gene in these patients.
Pancreatic carcinoma has been reported to be associated with various environmental and lifestyle risk factors, occupational exposures and medical conditions; however, the only risk factors consistently reported are age and smoking status, and the etiology of the disease remains largely unknown (1). In this study, we analyzed the correlation between smoking status and biological events in 5 randomly selected sporadic pancreatic carcinomas. Indeed, the patients smoking cigarettes for more than 15 years (PC1, 2 and 5) displayed methylation of 2 genes in their pancreatic carcinoma specimens. On the other hand, the other 2 pancreatic carcinoma specimens (PC3 and 4) with only 1 methylation region in the CDKI genes were surgically isolated from the patients without a cigarette smoking record (Table I). Thus, we suggest that cigarette smoking may be associated with pancreatic tumorigenesis by inducing the methylation of the promoter regions of the CDKI genes.
Our previous data suggested that expression of p21cip1 stops cell growth progression by inactivation of CDK activity, which in turn blocks the cell cycle at the G1 and G2 phases (20,21). Consistent with our previous finding that heightened CDK/cyclin signal transduction concomitant with loss of p27kip1 (22), the present study indicates that the reduced levels of the CDKI protein are a prominent feature of pancreatic carcinoma. These data suggest that the reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 may lead to defects in cell-cycle regulation and confer a selective growth advantage for pancreatic cancer cells. Thus, we showed that reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1, the negative regulators of cell progression, may be causative of tumor formation in pancreatic cancer cells.
Taken together, reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 are a fundamental event in tumor formation in the clinical pancreatic carcinoma patients. The CDK inhibitors may interact to mediate signals that are critical growth inhibitors. This growth regulatory circuit would be disrupted, such as in pancreatic carcinoma. Thus, our observations have significant implications for understanding the importance of p15INK4b, p16INK4a, p21cip1 and p27kip1; reduced levels of these CDKI proteins contribute to tumorigenesis in pancreatic carcinoma and developing ways to target aberrantly active parts of this growth regulatory pathway may lead to increased survival.
Acknowledgements
This study was supported by the Natural Science Foundation of China (81071740) and the Shanghai Science Foundation (10ZR1406300).
References
- 1.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27:87–93. doi: 10.1016/s0361-090x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Elledge SJ, Winston J, Harper JW. A question of balance: the role of cyclin-kinase inhibitors in development and tumorigenesis. Trends Cell Biol. 1996;6:388–392. doi: 10.1016/0962-8924(96)10030-1. [DOI] [PubMed] [Google Scholar]
- 5.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 6.Tang JJ, Shen C, Lu YJ. Requirement for pre-existing of p21 to prevent doxorubicin-induced apoptosis through inhibition of caspase-3 activation. Mol Cell Biochem. 2006;291:139–144. doi: 10.1007/s11010-006-9206-7. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Yamagishi N, Yagi T, Takebe H. Mutated p21(WAF1/CIP1/SDI1) lacking CDK-inhibitory activity fails to prevent apoptosis in human colorectal carcinoma cells. Oncogene. 1998;16:705–712. doi: 10.1038/sj.onc.1201585. [DOI] [PubMed] [Google Scholar]
- 8.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 9.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 10.Hainaut P, Soussi T, Shomer B, et al. Database of p53 gene somatic mutations in human tumors and cell lines: updated compilation and future prospects. Nucleic Acids Res. 1997;25:151–157. doi: 10.1093/nar/25.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996;68:67–108. doi: 10.1016/s0065-230x(08)60352-8. [DOI] [PubMed] [Google Scholar]
- 12.Perrone F, Tabano S, Colombo F, et al. p15INK4b, p14ARF, and p16INK4a inactivation in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. Clin Cancer Res. 2003;9:4132–4138. [PubMed] [Google Scholar]
- 13.Klump B, Hsieh CJ, Nehls O, et al. Methylation status of p14ARF and p16INK4a as detected in pancreatic secretions. Br J Cancer. 2003;88:217–222. doi: 10.1038/sj.bjc.6600734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurihara Y, Egawa K, Kunimoto S, Takeuchi T, Nose K. Induction of p16/INK4a gene expression and cellular senescence by toyocamycin. Bio Pharm Bull. 2002;25:1272–1276. doi: 10.1248/bpb.25.1272. [DOI] [PubMed] [Google Scholar]
- 15.Cen B, Li H, Weinstein IB. Histidine triad nucleotide-binding protein 1 up-regulates cellular levels of p27KIP1 by targeting ScfSKP2 ubiquitin ligase and Src. J Biol Chem. 2009;284:5265–5276. doi: 10.1074/jbc.M804531200. [DOI] [PubMed] [Google Scholar]
- 16.West AB, Kapatos G, O'Farrell C, et al. N-myc regulates parkin expression. J Biol Chem. 2004;279:28896–28902. doi: 10.1074/jbc.M400126200. [DOI] [PubMed] [Google Scholar]
- 17.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brakensiek K, Länger F, Kreipe H, Lehmann U. Absence of p21CIP1, p27KIP1 and p57KIP2 methylation in MDS and AML. Leuk Res. 2005;29:1357–1360. doi: 10.1016/j.leukres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Schneider G, Schmid RM. Genetic alterations in pancreatic carcinoma. Mol Cancer. 2003;22:15. doi: 10.1186/1476-4598-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Chen Y, Wang ZY, et al. Involvement of p21 (waf1) in merlin deficient sporadic vestibular schwannomas. Neuroscience. 2010;170:149–155. doi: 10.1016/j.neuroscience.2010.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Ma SL, Zhang YP, et al. A combined treatment TNF-α/Gefitinib alleviates the resistance to Gefitinib in PC-9 cells with acquired resistance to Gefitinib. Anticancer Drug. 2009;20:832–837. doi: 10.1097/CAD.0b013e32832f4b64. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Lu Y, Tang J, Wang H, Wu H. The phosphorylation status of merlin in sporadic vestibular Schwannomas. Mol Cell Biochem. 2009;324:201–206. doi: 10.1007/s11010-008-0014-0. [DOI] [PubMed] [Google Scholar]