Abstract
Monocyte chemoattractant protein-1 (MCP-1) is an essential cytokine for the migration of monocytes into vessels, and is also involved in the pathogenesis of atherosclerosis. In this study, we investigated the importance of janus kinase 2 (JAK2) and the function of the Akt and glycogen synthase kinase-3β (GSK3β) pathway in toll-like receptor (TLR2)-mediated MCP-1 expression. The TLR2 agonist, Pam3CSK4, induced MCP-1 expression in the Raw264.7 cell line. The induction of MCP-1 was seen in the bone marrow-derived macrophages of wild-type mice but not in TLR2 knockout mice. The TLR2-mediated MCP-1 induction was myeloid differentiation primary response gene 88 (MyD88)-independent. By contrast, the inactivation of JAK2 attenuated TLR2-mediated MCP-1 expression. The JAK inhibitor suppressed the phosphorylation of GSK3β as well as Akt by Pam3CSK4 stimulation. While the inactivation of Akt by LY294002 suppressed TLR2-mediated MCP-1 induction, the inactivation of GSK3β by LiCl potentiated TLR2-mediated MCP-1 induction. Furthermore, Akt inhibitor suppressed TLR2-mediated phosphorylation of GSK3β. Taken together, these results suggest that a MyD88-independent pathway exists in TLR2 signaling; the JAK2-Akt-GSK3β pathway is a novel MyD88-independent pathway for MCP-1 induction.
Keywords: toll-like receptor, monocyte chemoattractant protein-1, janus kinase 2, Akt, glycogen synthase kinase-3 β
Introduction
Toll-like receptors (TLRs) are the most well-known pattern recognition receptors, due to their role in the detection of a variety of pathogen-associated molecular patterns (1). To date, 13 TLRs have been identified, and each TLR has a specific ligand (2–4). In particular, TLR2 heterodimerizes with TLR1 or TLR6, and recognizes lipopeptides or lipoproteins (5).
TLR has been suggested to play a role in atherosclerosis progression (6,7); TLR2 is the most known receptor for the development of atherosclesosis. The direct stimulation of TLR2 by the synthetic ligand, Pam3CSK4, accelerates lesion formation and, in TLR2 knockout (KO) mice, this phenomenon is inhibited (8). The TLR2-associated progression of atherosclerosis has been confirmed in various cells, including endothelial cells, smooth muscle cells and macrophages. For example, high expression of TLR2 in monocytes serves as an important risk factor for arteriosclerotic disease (9,10). In addition, increased expression of TLR2 in endothelial cells causes interruption of blood flow, thus leading to more severe early atherogenic events (11). Apolipoprotein CIII regulates atherosclerosis-related gene expression in adipocytes (12), and even Chlamydia pneumonia-induced macrophage-form cell formation is mediated by TLR2 (13). Therefore, the verification of regulatory proteins in atherosclerosis induced by TLR2 may aid in the prevention of the disease and could provide possibilities of novel target medication.
Multiple regulators contribute towards the progression of atherosclerosis. However, the recruitment of monocytes on the injured sites of blood vessels is essential for the onset of atherosclerosis, and the most essentially effective chemokine is monocyte chemoattractant protein-1 (MCP-1) (14,15). Monocytes activated by MCP-1 differentiate into macrophages and are then converted into foam cells; however, each of the cells produce more MCP-1, thus exasperating atherosclerosis progression. Although MCP-1 has been shown to be associated with the formation of foam cells and the progression of atherosclerosis, many questions remain unanswered regarding the expression mechanism during TLR2 activation. There is very limited information on studies involved in the TLR2-mediated MCP-1 production. At present, the function of ERK and JNK has been suggested in MCP-1 production by TLR4 stimulation (16,17) and, recently, the existence of multiple pathways, such as PKC, Akt and mitogen-activated protein kinase (MAPK), have also been suggested (16,18,19). We have previously reported the regulation of MCP-1 production via TLR9 by cytosolic PLA2 and JNK pathway (20). However, not much information is available regarding the MCP-1 production mechanism by TLR2 signaling. Although MAPK (ERK, p38, JNK) pathway is a well-known mechanism with the involvement of the myeloid differentiation primary response gene 88 (MyD88) complex in the TLR-mediated MCP-1 production, the participation of other types of kinases remains obscure. Therefore, in our present study, we investigated the participation of new signaling pathways, which regulate MCP-1 production by TLR2 stimulation.
Materials and methods
Cells lines and reagents
Raw264.7 cell lines were purchased from ATCC (Manassas, VA, USA). Primary bone marrow-derived monocytes were differentiated into bone marrow-derived macrophages (BMDM) for 5–7 days in DMEM supplemented with 10% L929 cell-conditioned medium [as a source of macrophage-colony stimulating factor (M-CSF)]. Cell culture reagents, including fetal bovine serum (FBS), were obtained from Life Technologies (Grand Island, NY, USA). α-Akt, α-phospho Akt (S473), α-phospho glycogen synthase kinase-3β (GSK3β) (S9) antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). α-β-actin, LiCl were purchased from Sigma-Aldrich (St. Louis, MO, USA). Reverse transcription-polymerase chain reaction (RT-PCR) kits were from Takara Bio (Kyoto, Japan). TLR2, MyD88, TIR domain-containing adapter inducing IFN-β (TRIF) siRNAs and TRIzol were purchased from Invitrogen (Carlsbad, CA, USA). Janus kinase (JAK)1, JAK2, JAK3 and TYK2 siRNA were purchased from Bioneer (Daejeon, Korea). α-GSK3β was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). LY294002 and AG-490 were purchased from Biomol (Plymouth Meeting, PA, USA). JAK inhibitor I was purchased from Merck/Calbiochem (Darmstadt, Germany). Pam3CSK4 was purchased from InvivoGen (San Diego, CA, USA).
siRNAs and transfection
Stealth control and gene-specific siRNAs against the following target genes were designed using the Block-IT Stealth RNAi designer: i) TLR2, 5′-UUA AAG GGC GGG UCA GAG UUC UCC A-3′; ii) MyD88, 5′-ACC ACC AUG CGG CGA CAC CUU UUC U-3′; iii) TRIF, 5′-UGU UGG UGG AGU GUA ACG UAU GUC C-3′; iv) JAK1, 5′-UCA CCG GGA CUU AGC AGC AAG AAA U-3′; v) JAK2, 5′-CGG GUC GGC GCA ACC UAA GAU UAA U-3′; vi) JAK3, 5′-CAC AUG ACU CGG AAG UCC UCC UGA A-3′; vii) TYK2, 5′-GCG AGG AGG AGA UCC ACC ACU UUA A-3′. For transfection, Raw264.7 cells were plated in 35-mm plates and transfected with siRNA at a final concentration of 150–200 pM using nucleoporation reagents from Lonza (Allendale, NJ, USA). Cells were nucleoporated according to the manufacturer's protocol and incubated for 18–24 h before Pam3CSK4 stimulation.
RT-PCR
Total RNA was extracted from cells using TRIzol. First-strand of cDNA was synthesized from 1 μg total RNA in the presence of random primers, oligo(dT) and reverse transcriptase (Takara Bio). Cycling conditions for PCR were 95˚C for 5 min, followed by 26–33 cycles at 95˚C for 1 min, 60–63˚C for 1 min and 72˚C for 1 min. For semi-quantitative PCR, target genes were normalized for β-actin transcription. The sequences of PCR primers used in the present study were as follows: i) MCP-1 forward, 5′-AGA GAG CCA GAC GGG AGG AA-3′ and reverse, 5′-GTC ACA CTG GTC ACT CCT AC-3′; ii) TLR2 forward, 5′-CGC CCT TTA AGC TGT GTC TC-3′ and reverse, 5′-TTA TCT TGC GCA GTT TGC AG-3′; iii) MyD88 forward, 5′-CCA GTA TCC TGC GGT TCA TCA-3′ and reverse, 5′-GCT CCG CAT CAG TCT CAT CTT-3′; iv) TRIF forward, 5′-GTA TGG GCC CTC TGA CTG AT-3′ and reverse, 5′-ATA GGT GTG GTC TTC CCT GC-3′; v) JAK1 forward, 5′-ATG GAA GAC GGA GGC AAT GGT-3′ and reverse, 5′-GGA ACT TTA GAG GCA GAA TAC-3′; vi) JAK2 forward, 5′-AAG AGC AAC GGA AGA TTG C-3′ and reverse, 5′-CGT CAC AGT TTC TTC TGC CT-3′; vii) JAK3 forward, 5′-CAC ACC TGG CAT CCC GAA TC-3′ and reverse, 5′-AGC AGT AGG CGG TCG GTG TG-3′; viii) TYK2 forward, 5′-CCT GGC CAT GAC CTG AAC AG-3′ and reverse, 5′-TGT GCC CTT CAC TGA CGG AG-3′; and ix) β-actin forward, 5′-TCC TTC GTT GCC GGT CCA CA-3′ and reverse, 5′-CGT CTC CGG AGT CCA TCA CA-3′.
Western blot analysis
Macrophages were cultured in 35-mm Petri dishes and treated with Pam3CSK4 in the presence or absence of inhibitor. Cell pellets were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.0; 5 mM EDTA; 150 mM NaCl; 0.5% Nonidet P-40; 1 mM phenylmethylsulfonyl fluoride; and protease inhibitor cocktail). Proteins were separated on an 8% reducing SDS-PAGE gel and transferred onto nitrocellulose membranes in 20% methanol, 25 mM Tris and 192 mM glycine. Membranes were then blocked with 5% non-fat dry milk and incubated overnight with primary antibody at 4˚C before washing, followed by 1 h of incubation with horseradish peroxidase-conjugated secondary antibody. Further washing steps were followed with subsequent development with an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, UK).
Results and Discussion
Pam3CSK4 stimulates MCP-1 mRNA expression through TLR2-dependent, but MyD88-independent pathway
Previously, we as well as others have reported on the formation of foam cells by the TLR2 ligand; the foam cell is a hallmark of early atherosclerosis lesions (13,20). We studied the nature of molecules involved in TLR2-induced foam cell formation and found that MCP-1 plays a major role. RT-PCR showed maximal induction of MCP-1 mRNA expression by Pam3CSK4 in both cell lines, Raw264.7 (Fig. 1A) and BMDM (Fig. 1B). Subsequently, we investigated whether the induction was TLR2-dependent using TLR2 KO mice. While MCP-1 expression was induced by Pam3CSK4 in BMDM from wild-type mice, its expression was not affected in BMDM from mice with deletions in TLR2 (TLR2 KO; Fig. 1B). Therefore, we postulated that MCP-1 expression by Pam3CSK4 is dependent upon TLR2. Many types of TLR ligands (LPS, flagellin, FSL1 and CpG-ODN) other than the TLR2 ligand have also been found to induce the expression of MCP-1 (data not shown). These results suggest that MCP-1 induction by TLR ligands is a general characteristic. MCP-1 was found at higher expression levels within atherosclerotic lesions and, thus, considered a key player in the recruitment of monocytes into the arterial wall (21). Therefore, our data suggest that the Pam3CSK4-induced MCP-1 expression is associated with atherosclerosis.
Figure 1.
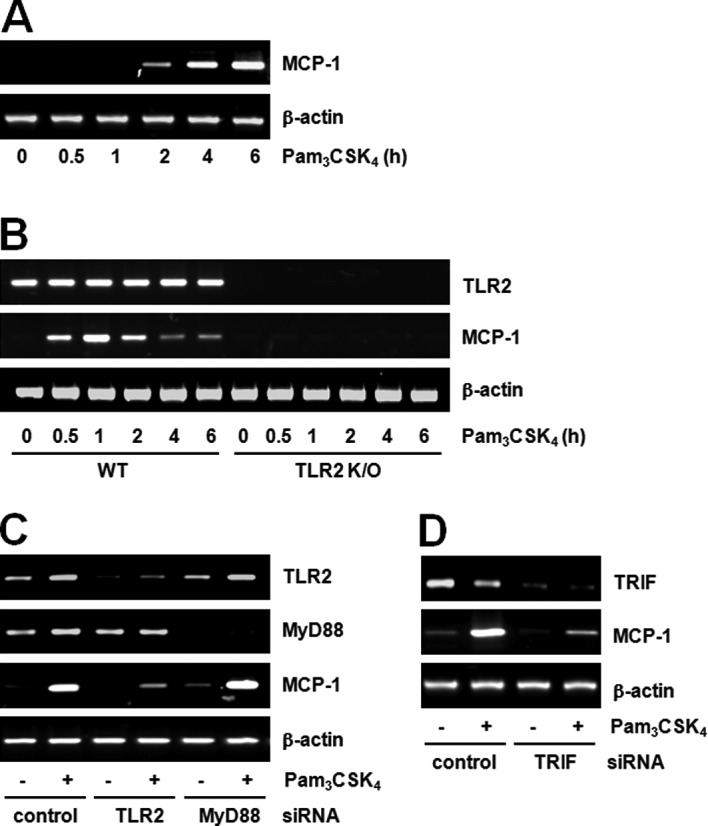
Pam3CSK4 induces MCP-1 expression by a TLR2-dependent, but MyD88-independent pathway. (A) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) for the indicated duration of times. MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control. (B) BMDMs were isolated from wild-type or TLR2 knockout (KO) mouse bone marrow and treated with Pam3CSK4 (100 ng/ml) for the indicated duration of times. MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control. (C) Raw264.7 cells were transfected with either control or TLR2 or MyD88 siRNA (200 pM) for 24 h. Cells were stimulated with vehicle or Pam3CSK4 (100 ng/ml) for 6 h. TLR2, MyD88 and MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control. (D) Raw264.7 cells were transfected with control or TRIF siRNA (200 pM) for 24 h. Cells were stimulated with vehicle of Pam3CSK4 (100 ng/ml) for 6 h. TRIF and MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control.
We further investigated the involvement of MyD88 by TLR2 stimulation in Raw264.7 cells. TLR2 or MyD88 siRNA transfected into cells was capable of successfully downregulating their respective target genes. While MCP-1 expression induced by Pam3CSK4 decreased in TLR2 siRNA cells, there were no changes in MCP-1 expression in MyD88 siRNA cells (Fig. 1C). These results suggest that MCP-1 expression induced by Pam3CSK4 is TLR2-dependent, but MyD88-independent. Currently, MyD88 is the most known adaptor molecule in the TLR2 signaling pathway (22). However, there are currently very few studies on the MyD88-independent pathway. The TLR2 signaling pathway is a well-known mechanism through which MyD88 activates the NF-κB transcription factor and induces various genes, including cytokines, chemokines and inflammatory mediators (23). However, as our results demonstrate, the fact that MCP-1 production by TLR2 stimulation is MyD88-independent is very unique.
The MyD88-independent pathway has mainly been studied in association with the TLR3 or TLR4 signaling pathway, and the functions of TRIF-related adaptor molecule (TRAM) and TRIF have mainly been focused upon. Studies on the MyD88-independent pathway in TLR2 signaling are very limited. Chen et al (24) reported the TRIF-dependent pathway in Chlamydia pneumonia, TLR2 ligand-induced foam cell formation. Our preliminary data also demonstrate the participation of TRIF in TLR2 signaling. In other words, TRIF siRNA cells exhibited decreased MCP-1 expression by TLR2 stimulation (Fig. 1D). These results suggest the existence of the MyD88-independent pathway in TLR2 signaling and the feasibility of the TRIF-dependent pathway.
JAK2 is involved in the TLR2-mediated MCP-1 expression
We tried searching for the downstream molecules of TLR2 in MCP-1 expression and found that JAK plays a role in MCP-1 induction. For example, pre-treatment with the JAK inhibitor blocked MCP-1 expression induced by Pam3CSK4 (Fig. 2A). AG490, the JAK2 inhibitor, also inhibited MCP-1 expression induced by Pam3CSK4 (Fig. 2B). To confirm the role of JAK2, the effects of Pam3CSK4 were compared after decreasing JAK expression using the siRNA method. The expression of JAK mRNA was decreased to 60–80% in each siRNA-transfected cell. While TLR2-mediated MCP-1 expression had no effects in JAK1, JAK3 or TYK2 siRNA cells, the expression of MCP-1 was decreased in the JAK2 siRNA cells (Fig. 2C).
Figure 2.
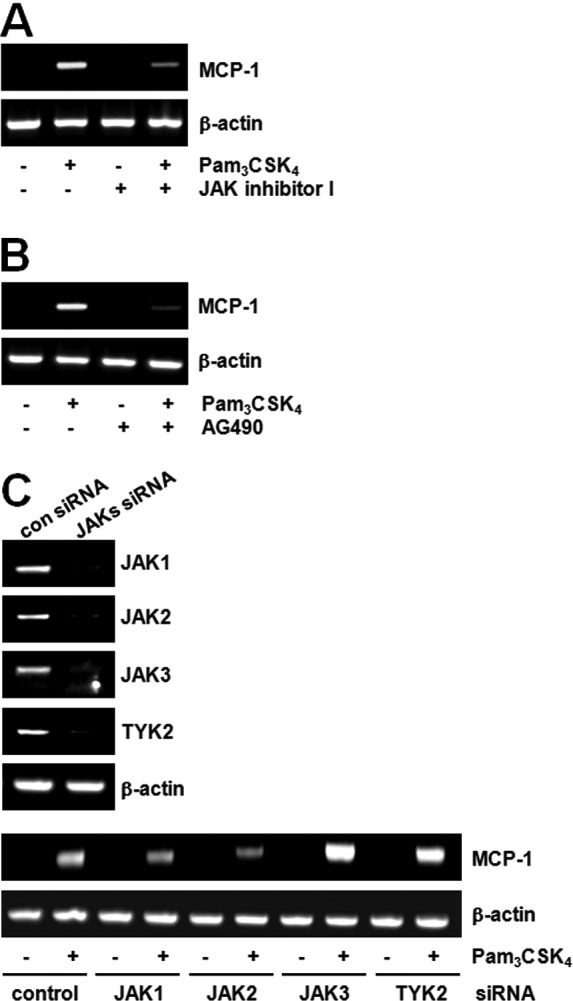
JAK2 mediates TLR2-stimulated MCP-1 expression. (A and B) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) for 6 h in the presence or absence of JAK inhibitor I (10 μM) or JAK2 inhibitor AG490 (10 μM). MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control. (C) Raw264.7 cells were transfected with JAK1, JAK2, JAK3 or TYK2 siRNA (200 pM) for 24 h. Cells were stimulated with vehicle or Pam3CSK4 for 6 h, and MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control.
Four types of JAKs have been identified and they play an essential role in tyrosine kinase receptor signaling. Although the participation of JAK2 in TLR2 signaling is of interest, systematic study has not yet been performed. While not much data is available on the correlation between TLR and JAK, the function of JAK2 in TLR4-induced macrophage activation (25) and the function of TYK2 in TLR4-induced endotoxin shock have been suggested (26). Currently, there are no reports on the direct correlation between TLR2 and JAK2. In our data, the role of JAK2 in TLR2-mediated MCP-1 production was highly feasible through the MyD88-independent (TRIF) pathway. However, we still do not know how TRIF regulates JAK2 activity for the MCP-1 expression.
Akt-GSK3β pathway is downstream of JAK2 for MCP-1 expression
We searched for the identity of the signaling proteins regulated by JAK2 and found the involvement of the Akt-GSK3β pathway. It is well-known that GSK3β is downstream kinase of Akt in many signaling pathways. We tested the phosphorylation of two proteins using the JAK inhibitor to confirm the correlation between JAK and the Akt-GSK3β pathway. The JAK inhibitor dramatically decreased Akt and GSK3β phosphorylation induced by Pam3CSK4 (Fig. 3A and B). These results suggest that JAK regulates the Akt-GSK3β pathway. Furthermore, LY294002 pre-treatment significantly inhibited both Akt phosphorylation and MCP-1 expression induced by Pam3CSK4 (Fig. 4A and B). These results show that Akt phosphorylation inhibited by JAK regulates MCP-1 expression induced by TLR2. Previously, the possibility of PI3K-Akt pathway activation by JAK in TLR2 signaling was assumed according to the role played by IL-6 (27,28). The importance of the PI3K-Akt pathway has also been suggested in TLR signaling. A number of studies have demonstrated that the activation of the PI3K pathway by a TLR2 agonist exerts effects on the production of inflammatory mediators (29). The TLR4 agonist, LPS, has been shown to induce Akt phosphorylation on both T308 and S473, in which the blockade of PI3K attenuated phosphorylation (30). However, the correlation between the JAK and PI3K pathway has not been sufficiently studied to date. Hirata et al (31) suggested that IL-10 production by LPS plus imiquimod in dendritic cells can be regulated by the JAK-PI3K axis. Our results also showed the significant inhibition of TLR2-mediated Akt phosphorylation by the JAK inhibitor, and therefore we believe that the JAK-PI3K-Akt pathway is an essential step in MCP-1 regulation.
Figure 3.
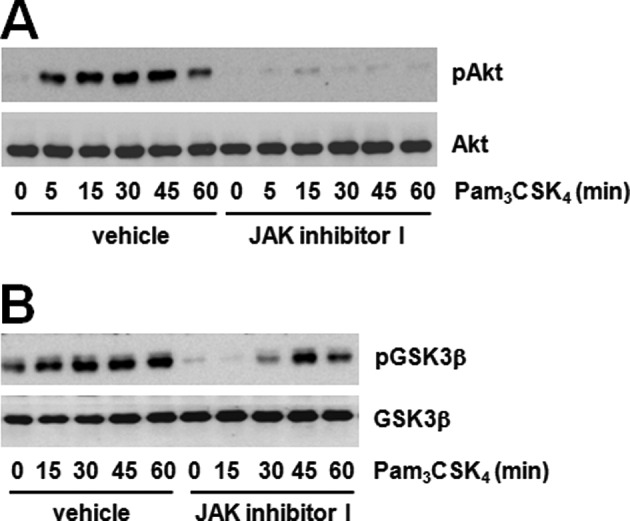
JAK inhibitor attenuates Pam3CSK4-mediated phosphorylation of Akt and GSK3β. (A) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) in the presence or absence of JAK inhibitor I (10 μM) for the indicated duration of times. Akt phosphorylation was determined by western blotting using α-phospho-Akt antibody (S473) and normalized to Akt total protein. (B) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) in the presence or absence of JAK inhibitor I (10 μM) for the indicated duration of times. GSK3β phosphorylation was determined by western blotting using α-phospho-GSK3β antibody (S9) and normalized to GSK3β total protein.
Figure 4.
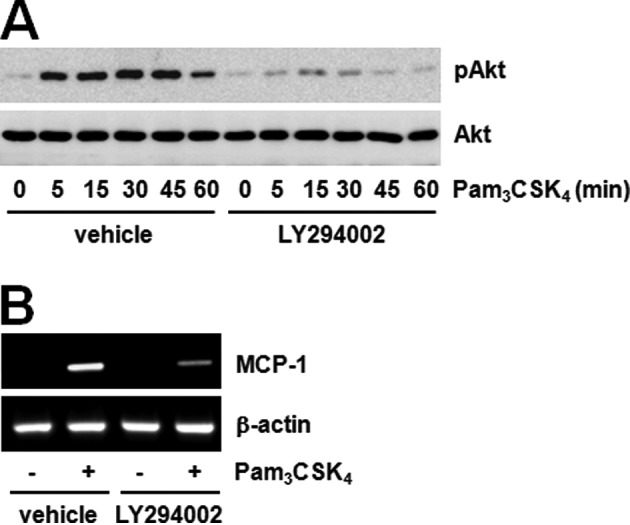
Akt is an effecter of TLR2-mediated MCP-1 expression. (A) Raw264.7 cells were stimulated with Pam3CSK4 (100 ng/ml) in the presence or absence of LY294002 (10 μM) for the indicated duration of times. Akt phosphorylation was determined by western blotting using α-phospho-Akt antibody (S473) and normalized to Akt total protein. (B) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) for 6 h in the presence or absence of LY294002 (10 μM). MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control.
PI3K and Akt have been identified as kinases involved in the ability of TLRs to mediate the regulation of GSK3β activity (32). Pam3CSK4 increased GSK3β phosphorylation, decreased activation and pre-treatment with the GSK3β inhibitor LiCl potentiated GSK3β phosphorylation by stimulating Pam3CSK4 (Fig. 5A). Additionally, LiCl pre-treatment amplified MCP-1 expression by stimulation of Pam3CSK4 (Fig. 5B). We then confirmed the relationship between Akt and GSK3β. The PI3K-Akt inhibitor blocked the TLR2-mediated GSK3β phosphorylation (Fig. 5C). These results suggest that the JAK2-Akt-GSK3β pathway contributes to TLR2-mediated MCP-1 expression.
Figure 5.
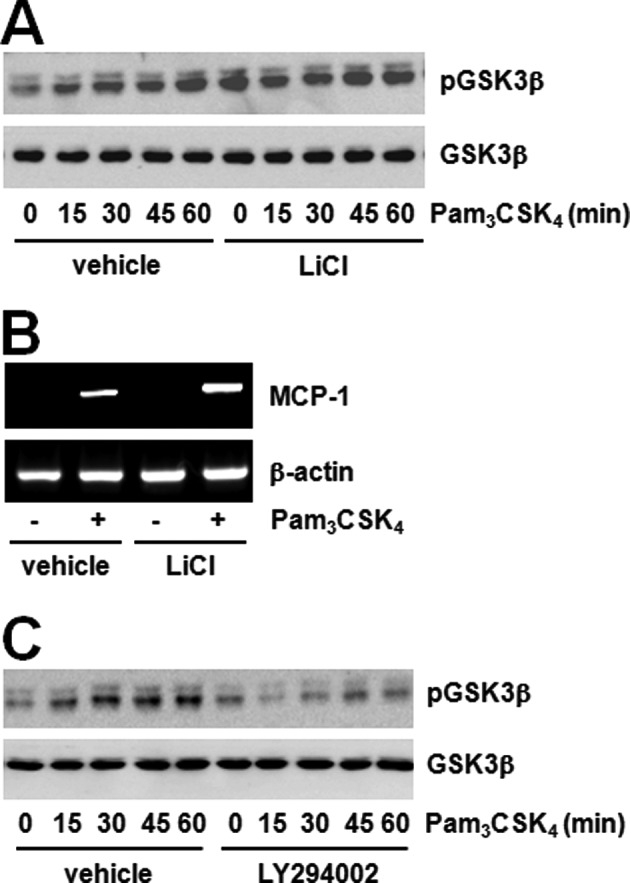
GSK3β is a downstream molecule for Akt in TLR2-mediated MCP-1 expression. (A) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) in the presence or absence of LiCl (20 mM) for the indicated duration of times. GSK3β phosphorylation was determined by western blotting using α-phospho-GSK3β antibody (S9) and normalized to GSK3β total protein. (B) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) for 6 h in the presence or absence of LiCl (20 mM). MCP-1 mRNA expression was determined by RT-PCR and normalized to β-actin control. (C) Raw264.7 cells were treated with Pam3CSK4 (100 ng/ml) in the presence or absence of LY294002 (10 μM) for the indicated duration of times. GSK3β phosphorylation was determined by western blotting using α-phospho-GSK3β antibody (S9) and normalized to GSK3β total protein.
Overall, our results demonstrate a MyD88-independent pathway in TLR2 signaling, thus providing a different mechanism other than the already known MyD88-dependent pathway to regulate MCP-1 expression, and thereby causing foam cell formation and atherosclerosis. Additionally, TLR2-mediated GSK3β induced MCP-1 production in a negative manner through the JAK2-Akt signaling pathway.
Acknowledgements
This study was supported by the Yeungnam University research grants in 2010 (210-A-380-054).
Abbreviations
- TLRs
toll-like receptors
- MCP-1
monocyte chemoattractant protein-1
- JAK2
janus kinase 2
- GSK3β
glycogen synthase kinase-3β
- MyD88
myeloid differentiation primary response gene 88
- MAPKs
mitogen-activated protein kinases
- BMDM
bone marrow-derived macrophage
- TRIF
TIR domain-containing adapter inducing IFN-β
References
- 1.Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704–720. doi: 10.1136/gut.2008.156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 3.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Kang JY, Nan X, Jin MS, et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity. 2009;31:873–884. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2008;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Ukai T, Yumoto H, et al. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196:146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwahata S, Fujita S, Orihara K, et al. High expression level of Toll-like receptor 2 on monocytes is an important risk factor for arteriosclerotic disease. Atherosclerosis. 2010;209:248–254. doi: 10.1016/j.atherosclerosis.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 10.Monaco C, Terrando N, Midwood KS. Toll-like receptor signaling: common pathways that drive cardiovascular disease and rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:500–511. doi: 10.1002/acr.20382. [DOI] [PubMed] [Google Scholar]
- 11.Mullick AE, Soldau K, Kiosses WB, Bell TA, III, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abe Y, Kawakami A, Osaka M, et al. Apolipoprotein CIII induces monocyte chemoattractant protein-1 and interleukin 6 expression via Toll-like receptor 2 pathway in mouse adipocytes. Arterioscler Thromb Vasc Biol. 2010;30:2242–2248. doi: 10.1161/ATVBAHA.110.210427. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Cao F, Castrillo A, Tontonoz P, Re F, Byrne GI. Chlamydia pneumoniae-induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun. 2007;75:753–759. doi: 10.1128/IAI.01386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 15.Koenen RR, Weber C. Therapeutic targeting of chemokine interactions in atherosclerosis. Nat Rev Drug Discov. 2010;9:141–153. doi: 10.1038/nrd3048. [DOI] [PubMed] [Google Scholar]
- 16.Ni CW, Wang DL, Lien SC, Cheng JJ, Chao YJ, Hsieh HJ. Activation of PKC-epsilon and ERK1/2 participates in shear-induced endothelial MCP-1 expression that is repressed by nitric oxide. J Cell Physiol. 2003;195:428–434. doi: 10.1002/jcp.10259. [DOI] [PubMed] [Google Scholar]
- 17.Arndt PG, Suzuki N, Avdi NJ, Malcolm KC, Worthen GS. Lipopolysaccharide-induced c-Jun NH2-terminal kinase activation in human neutrophils: role of phosphatidylinositol 3-Kinase and Syk-mediated pathways. J Biol Chem. 2004;279:10883–10891. doi: 10.1074/jbc.M309901200. [DOI] [PubMed] [Google Scholar]
- 18.Park DW, Baek K, Kim JR, et al. Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med. 2009;41:171–179. doi: 10.3858/emm.2009.41.3.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai CS, Lin FY, Chen YH, et al. Cilostazol attenuates MCP-1 and MMP-9 expression in vivo in LPS-administrated balloon-injured rabbit aorta and in vitro in LPS-treated monocytic THP-1 cells. J Cell Biochem. 2008;103:54–66. doi: 10.1002/jcb.21388. [DOI] [PubMed] [Google Scholar]
- 20.Lee JG, Lee SH, Park DW, et al. Toll-like receptor 9-stimulated monocyte chemoattractant protein-1 is mediated via JNK-cytosolic phospholipase A2-ROS signaling. Cell Signal. 2008;20:105–111. doi: 10.1016/j.cellsig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714–721. [PubMed] [Google Scholar]
- 22.Ahmad R, El Bassam S, Cordeiro P, Menezes J. Requirement of TLR2-mediated signaling for the induction of IL-15 gene expression in human monocytic cells by HSV-1. Blood. 2008;112:2360–2368. doi: 10.1182/blood-2008-02-137711. [DOI] [PubMed] [Google Scholar]
- 23.Park JE, Kim YI, Yi AK. Protein kinase D1 is essential for MyD88-dependent TLR signaling pathway. J Immunol. 2009;182:6316–6327. doi: 10.4049/jimmunol.0804239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Sorrentino R, Shimada K, et al. Chlamydia pneumoniae-induced foam cell formation requires MyD88-dependent and -independent signaling and is reciprocally modulated by liver X receptor activation. J Immunol. 2008;181:7186–7193. doi: 10.4049/jimmunol.181.10.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okugawa S, Ota Y, Kitazawa T, et al. Janus kinase 2 is involved in lipopolysaccharide-induced activation of macrophages. Am J Physiol Cell Physiol. 2003;285:C399–C408. doi: 10.1152/ajpcell.00026.2003. [DOI] [PubMed] [Google Scholar]
- 26.Kamezaki K, Shimoda K, Numata A, Matsuda T, Nakayama K, Harada M. The role of Tyk2, Stat1 and Stat4 in LPS-induced endotoxin signals. Int Immunol. 2004;16:1173–1179. doi: 10.1093/intimm/dxh118. [DOI] [PubMed] [Google Scholar]
- 27.Rebouissou S, Amessou M, Couchy G, et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature. 2009;457:200–204. doi: 10.1038/nature07475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosell R, Bertran-Alamillo J, Molina MA, Taron M. IL-6/gp130/STAT3 signaling axis in cancer and the presence of in-frame gp130 somatic deletions in inflammatory hepatocellular tumors. Future Oncol. 2009;5:305–308. doi: 10.2217/fon.09.3. [DOI] [PubMed] [Google Scholar]
- 29.Lee IT, Lee CW, Tung WH, et al. Cooperation of TLR2 with MyD88, PI3K, and Rac1 in lipoteichoic acid-induced cPLA2/COX-2-dependent airway inflammatory responses. Am J Pathol. 2010;176:1671–1684. doi: 10.2353/ajpath.2010.090714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown GT, McIntyre TM. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1beta-rich microparticles. J Immunol. 2011;186:5489–5496. doi: 10.4049/jimmunol.1001623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirata N, Yanagawa Y, Iwabuchi K, Onoe K. Selective regulation of interleukin-10 production via Janus kinase pathway in murine conventional dendritic cells. Cell Immunol. 2009;258:9–17. doi: 10.1016/j.cellimm.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]


