Abstract
Many patients with prostate cancer have disease recurrence following surgical removal of tumors and fail to respond to androgen ablation therapy. Despite the existence of a number of clinical/pathological factors, it is not possible to predict which patients will fall into this category. The results of our previous studies demonstrated that the HOXB13 homeodomain protein plays a key role in the development of prostate cancer and the progression of this malignancy. In addition, HOXB13 has been reported to predict estrogen-resistant breast cancer tumors. The purpose of this study was to investigate whether HOXB13 could be used as a molecular marker to predict prostate cancer recurrence. To examine the role of HOXB13 as a molecular marker with clinical/pathological data, the expression of HOXB13 was compared using immunohistochemistry in 57 organ-confined prostate cancer tumors obtained by radical prostatectomy. There was no significant correlation between the expression of HOXB13 and most clinical/pathological parameters, including tumor margin, invasion, pathological stage and risk level. The HOXB13 expression levels correlated with the Gleason score and there was a positive correlation with the pre-operative prostate specific antigen (PSA) levels. Accordingly, the tumor specimens from 4 patients who ultimately had biochemical failure (PSA >0.2 ng/ml), all showed a high expression of HOXB13, while their risk levels were either intermediate or high. This is the first study to report that HOXB13, together with other clinical/pathological factors, can be used as a molecular marker to predict the progression of prostate cancer.
Keywords: HOX, HOXB13, prostate cancer, cancer recurrence, diagnostic marker
Introduction
Prostate cancer (PCa) may be present, but undetectable, for several decades before finally being diagnosed by a clinician. Despite improvements in screening and early diagnosis, approximately one third of men whose cancer is detected early will still develop PCa metastases. For men with metastatic PCa, there are few alternatives and the front-line treatment is androgen ablation or surgical castration resulting in a favorable clinical response and a dramatic regression of PCa as a result of apoptotic cell death. Most of these patients, however, will experience a recurrence within 5 years and no effective alternative therapies are currently available. Therefore, there is an urgent need to develop progression-associated markers for PCa that will allow for a more aggressive treatment approach in patients likely to develop recurrent tumors and that may also facilitate the treatment of patients unlikely to have a recurrence.
Homeobox proteins are generally considered as modulators for growth and differentiation, and their loss or gain of expression is increasingly reported in human tumors (1). Most notably, NKX3.1, a prostate-specific non-HOX homeodomain protein, functions as a tumor suppressor and its loss has been observed in early-phase prostate tumors, while its overexpression has also been reported in advanced PCa (2–4). HOXB13 homeobox protein shares a similar function to NKX3.1, and there is evidence to suggest the link between the role of HOXB13 and the malignant progression of PCa. Notably, the highly prostate-specific expression pattern of HOXB13 is involved in the proper formation and maintenance of this hormone-dependent organ (5–8). HOXB13 loss-of-function mice have been shown to manifest abnormal prostate (9). HOXB13 suppresses androgen-stimulated androgen receptor (AR) activity through interaction with AR and subsequently inhibits the growth-regulatory function of AR (7,10). However, HOXB13 is overexpressed in most androgen-refractory PCas (11). In breast cancers possessing similar biological behavior to PCa, the overexpression of HOXB13 predicts tamoxifen-resistant tumors (12). In the present study, we investigated the value of HOXB13 as a molecular marker for PCa progression and malignancy.
Materials and methods
Patient cohort
Paraffin-embedded human primary PCas were acquired from patients at the Chonnam National University Hospital between 1995 and 2007. Written informed consent was obtained from all patients. Specimens from 57 PCa patients were obtained by radical prostatectomy with various treatment regimes, as shown in Table I. Pre- and post-operative prostate specific antigen (PSA) levels were available for all patients. Biochemical failure was defined when PSA levels bounced back to >0.2 ng/ml following radical prostatectomy. Benign prostate tissues obtained from radical prostatectomy were used for the baseline expression of HOXB13.
Table I.
Prostate cancer patient cohort (n=57).
| Age (median; range) | 62 (47–71) |
| Pre-operative PSA (ng/ml) | |
| <10 | 31 |
| 10–20 | 12 |
| >20 | 14 |
| Pathological stage | |
| Stage 2 | 36 |
| Stage 3 | 21 |
| Invasion | |
| Extraprostatic invasion | 14 |
| Seminal vesicle invasion | 7 |
| Pelvic lymph node invasion | 31 |
| Angiolymphatic invasion | 4 |
| Perineural invasion | 36 |
| Hormone refractory disease | 7 |
| Gleason score | |
| ≤6 | 9 |
| 7 | 30 |
| ≥8 | 18 |
| Surgical margins | |
| Negative | 43 |
| Positive | 14 |
| Biochemical failurea | |
| Relapse | 4 |
| No relapse | 44 |
Nine patients were excluded due to sustained PSA levels following surgery.
PSA, prostate specific antigen.
Immunohistochemistry and scoring
Immunostaining was performed according to the method described by Komuves et al (13). Tissues were deparaffinized followed by microwave antigen retrieval in citrate buffer. Endogenous peroxidase activity was destroyed by treating tissue sections with 3% H2O2. After non-specific reactivity was sequentially blocked by avidin-biotin blocking reagent and 10% normal goat serum, tissues were incubated with anti-HOXB13 antibodies and then with donkey anti-rabbit IgG (Fab fragment) conjugated to peroxidase (Jackson Laboratories, Bar Harbor, ME, USA). Anti-HOXB13 antibodies were produced under contractual agreement by Sigma Genosys (St. Louis, MO, USA), as described by Komuves et al (13). Antibodies were purified by affinity chromatography against immobilized HOXB13 synthetic peptides. In a previous study, we showed that these antibodies were specific to HOXB13 (11). Colorimetric signals were detected using diaminobenzidine (DAB). Sections were counterstained with hematoxylin for microscopic evaluation. Anti-AR antibodies (Upstate) were used to confirm tissue integrity. For the negative control slide, non-immune rabbit IgG was used. Immunostained slides were evaluated by 2 different investigators, including a pathologist, blinded to the patient clinical features. The intensity of HOXB13 staining was classified into 1 of 4 grades (0, absent; 1, weak; 2, intermediate; and 3, strong). This approach is considered reliable and reproducible by several groups (14–16). Tumors with <5% of HOXB13 expression were considered negative, irrespective of the intensity (5,17).
Statistical analysis
Statistical analysis was performed using SPSS software 17.0 (SPSS Inc., Chicago, IL, USA). The Spearman’s correlation co-efficienct test (two-tailed) was used to estimate the correlation with clinicopathological factors.
Results
In the present study, we evaluated HOXB13 as a molecular marker to increase the preditability of pre-existing clinicopathological parameters. We evaluated HOXB13 expression in 57 tumors using anti-HOXB13 antibodies. As shown in Fig. 1A, HOXB13 was mildly expressed in normal prostatic epithelial cells (left panel), while there were both HOXB13-underexpressed tumors (middle panel) and HOXB13-overexpressed tumors (right panel). HOXB13 appeared to be overexpressed in more invasive tumor cells. The expression of HOXB13 was also verified in PCa culture cells by western blot analysis (Fig. 1B), whose expression patterns were identical to the HOXB13 transcript, as previously shown (10). We assessed the correlation between HOXB13 expression and clinicopathological parameters available for the patients: age, pre-operative PSA levels, tumor margin, seminar vesicle invasion, pelvic lymph node invasion, angiolymphatic invasion, perineural invasion, Gleason score, pathological stage and risk level, using the two-tailed Spearman’s correlation test (Table II). HOXB13 expression was not correlated with age, tumor margin, invasion or pathological stage. HOXB13 expression was positively correlated with pre-operative PSA levels (r=0.298, p=0.064). There was a significant positive correlation between HOXB13 staining intensity and the Gleason score (r=0.286, p=0.031). There was also a significant difference in HOXB13 expression with a Gleason score between 7 and 9 (Student’s t-test, p=0.017). However, no correlation was observed between HOXB13 and grouping of the Gleason score into 6, 3+4, 4+3, 8 and 9 (r=0.167, p=0.261).
Figure 1.
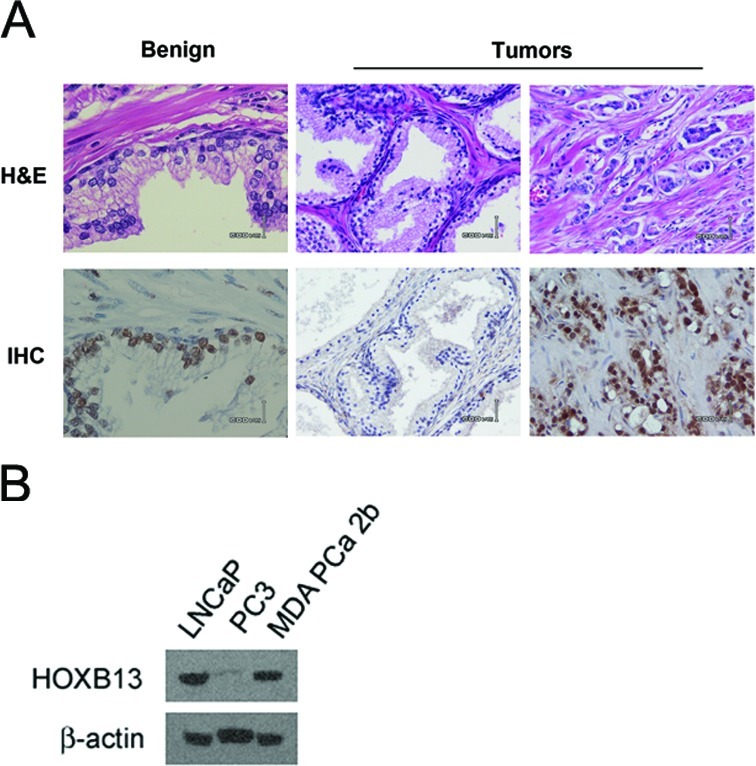
Expression of HOXB13 in prostate cancer. (A) Immunolocalized HOXB13 expression shown in a tumor specimen from radical prostatectomy. Bar indicates 600 μm. (B) HOXB13 expression as demonstrated by western blot analysis in selected prostate cancer cells.
Table II.
Correlation between HOXB13 expression and clinical parameters.
| Parameters | r | p |
|---|---|---|
| Age | −0.086 | 0.299 |
| Pre-operative PSA | 0.298 | 0.064 |
| Tumor margin | 0.188 | 0.162 |
| Extraprostatic invasion | 0 | 1 |
| Seminal vesicle invasion | 0.163 | 0.227 |
| Pelvic lymph node invasion | −0.064 | 0.634 |
| Angiolymphatic invasion | 0.079 | 0.558 |
| Perineural invasion | 0.085 | 0.529 |
| Gleason score | 0.286 | 0.031 |
| Stage | 0.105 | 0.236 |
| Risk level | 0.074 | 0.596 |
r = correlation coefficient by Spearman’s correlation test (two-tailed). r>0.20 are indicated in bold.
PSA, prostate specific antigen.
To further validate HOXB13 as a marker of progression, patients were divided according to risk groups, as recommended by the Clinical Practice Guidelines in Oncology 2009 from the National Comprehensive Cancer Network (NCCN): high risk, PSA >20 ng/ml or Gleason score ≥8 or ≥T3a; intermediate risk, PSA 10–20 ng/ml or Gleason score 7 or T2b and T2c; low risk, PSA<10 ng/ml and Gleason score 2–6 and T1-T2a. There was no correlation between HOXB13 and risk level (r=0.074, p=0.596). Table III summarizes the detailed clinical information of 4 patients with ultimate biochemical failure. Although 3 patients were considered as high risk and 1 as intermediate risk, all 4 patients showed the highest expression levels of HOXB13 (score 3). The patient with intermediate risk level was relatively young (50 years of age) and showed no extraprostatic and seminar vesicle invasion with clean surgical margin. These results suggest that the evaluation of HOXB13 expression in addition to pre-existing clinicopathological factors, including Gleason score and pre-operative PSA, may increase the predictability of patients at risk of biochemical failure.
Table III.
HOXB13 score in patients with biochemical failure.
| Patients | ||||
|---|---|---|---|---|
|
|
||||
| #1 | #2 | #3 | #4 | |
| Age (years) | 66 | 69 | 67 | 50 |
| Pre-operative PSA | 9.128 | 20.797 | 11.6 | 9.12 |
| Gleason score | ||||
| Primary | 4 | 5 | 3 | 4 |
| Secondary | 4 | 4 | 4 | 3 |
| TNM | pT2cNX | pT3cN0 | pT3bN0 | pT2cNX |
| Distant metastasis | No | No | No | No |
| Invasion | ||||
| Locala | No | Yes | Yes | No |
| Intratumorb | No | Yes | Yes | Yes |
| Surgical margin | − | + | + | − |
| Treatment | ||||
| Hormone | Yes | Yes | Yes | Yes |
| Radiation | No | Yes | No | Yes |
| Chemical | No | No | No | No |
| Risk level | High | High | High | Intermediate |
| HOXB13 score | 3 | 3 | 3 | 3 |
Extraprostatic and seminal vesicle invasion.
Angiolymphatic and perineural invasion.
PSA, prostate specific antigen.
Discussion
PCa may be present, but undetectable, for several decades before being diagnosed by a clinician. The long latency period of PCa results in the lack of overt symptoms in most men. The multifocal nature of PCa also makes it difficult to diagnose and treat PCa patients. Despite improvements in screening and early diagnosis, a small fraction of cells may remain undetectable and develop metastases. Patients with these tumors become refractory to hormonal ablation, resulting in rapid tumor progression and ultimately death. Therefore, there is an urgent need to define the fraction of PCa cells that differ with respect to clinical and pathological outcome. Based on several clinicopathological features, including the pre-operative PSA levels, pathological stage and tumor differentiation status, patients are stratified into subgroups, according to outcome after surgery, that are widely used to guide clinical decision making (18). However, PSA-based cancer screening has limited discriminatory capabilities. The identification of progression-associated markers for PCa will allow for a more aggressive treatment approach in patients likely to develop recurrent tumors, and may also facilitate the treatment of those patients unlikely to have a recurrence. Furthermore, the markers used to identify the aggressive from the indolent disease could potentially be used as therapeutic targets for aggressive disease management.
In this study, we demonstrate that the expression of HOXB13 is positively correlated with the Gleason score, and, to a lesser extent, with pre-operative PSA levels. HOXB13 has previously been shown to be overexpressed in hormone-refractory breast tumors as opposed to hormone-responsive tumors, further demonstrating that HOXB13 is a useful prognostic marker of hormone-refractory breast cancers (12). In addition, the forced expression of HOXB13 promoted the growth of MCF10A estrogen receptor (ER)-negative breast cells along with increased migration and invasion. In PCa, we also observed that HOXB13 is overexpressed in most androgen-refractory tumors and its expression confers a positive growth signal on PCa cells in the absence of androgen (11). Increasing numbers of studies are being published, linking HOXB13 with several solid tumors, including breast, cervical and skin cancer (12,13,19–22). Therefore, HOXB13 may be used as a marker to discriminate between aggressive and indolent prostate tumor cells as: i) HOXB13 is highly prostate-specific and is involved in the proper formation and maintenance of this hormone-dominant organ (7,9,23); ii) alteration of HOXB13 expression modulates the growth of PCa cells; iii) HOXB13 is generally overexpressed in androgen-refractory PCa, in which circumstance PCa cells grow better (11). Our current study also demonstrates that HOXB13 is positively correlated with cell differentiation, as shown by the Gleason score, suggesting that HOXB13 may be required for undifferentiated cells to survive in a low or no androgen environment.
Risk level is established by several factors, including pre-operative PSA levels, the Gleason score and T stage. In this study, HOXB13 expression was not correlated with risk level; however, samples from all 4 patients whose PSA levels bounced back to >0.2 ng/ml after radical prostatectomy showed the highest expression levels of HOXB13. One patient was considered as an intermediate-risk group. These results suggest that HOXB13 may be of significant prognostic value (supporting the incumbent Gleason score) for predicting recurrent PCa patients. However, this study was restricted due to the limited availability of recurrent tumors. Therefore, a larger scale study needs to be conducted to test its clinical value.
This study demonstrates that HOXB13 may be a potentially valuable molecular marker of recurrent PCa. HOXB13 was positively correlated with the Gleason score and pre-operative PSA levels; moreover, HOXB13 increased the predictive value of the incumbent Gleason score by predicting the progress of a patient with an intermediate recurrent risk. HOXB13 may be a good addition to pre-existing clinicopathological parameters, including the Gleason score, tumor margin, T stage and other genetic markers, to identify high-risk patients. Therefore, HOXB13 may aid in the development of a more aggressive treatment for patients with a high risk of recurrent PCa.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (no. 0720120), the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-313-E00353), and the Korea Science & Engineering Foundation through the Medical Research Center for Gene Regulation (2011-0030734) at Chonnam National University.
References
- 1.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen C, Bubendorf L, Voeller HJ, et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 2000;60:6111–6115. [PubMed] [Google Scholar]
- 4.Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, et al. Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis. Cancer Res. 2002;62:2999–3004. [PubMed] [Google Scholar]
- 5.Feber A, Clark J, Goodwin G, et al. Amplification and overexpression of E2F3 in human bladder cancer. Oncogene. 2004;23:1627–1630. doi: 10.1038/sj.onc.1207274. [DOI] [PubMed] [Google Scholar]
- 6.Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- 7.Sreenath T, Orosz A, Fujita K, Bieberich CJ. Androgen-independent expression of hoxb-13 in the mouse prostate. Prostate. 1999;41:203–207. doi: 10.1002/(sici)1097-0045(19991101)41:3<203::aid-pros8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi Y, Hamada J, Murakawa K, et al. Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp Cell Res. 2004;293:144–153. doi: 10.1016/j.yexcr.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Economides KD, Capecchi MR. Hoxb13 is required for normal differentiation and secretory function of the ventral prostate. Development. 2003;130:2061–2069. doi: 10.1242/dev.00432. [DOI] [PubMed] [Google Scholar]
- 10.Jung C, Kim RS, Zhang HJ, Lee SJ, Jeng MH. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res. 2004;64:9185–9192. doi: 10.1158/0008-5472.CAN-04-1330. [DOI] [PubMed] [Google Scholar]
- 11.Kim YR, Oh KJ, Park RY, et al. HOXB13 promotes androgen independent growth of LNCaP prostate cancer cells by the activation of E2F signaling. Mol Cancer. 2010;9:124. doi: 10.1186/1476-4598-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma XJ, Wang Z, Ryan PD, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5:607–616. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Komuves LG, Ma XK, Stelnicki E, Rozenfeld S, Oda Y, Largman C. HOXB13 homeodomain protein is cytoplasmic throughout fetal skin development. Dev Dyn. 2003;227:192–202. doi: 10.1002/dvdy.10290. [DOI] [PubMed] [Google Scholar]
- 14.Dhanasekaran SM, Barrette TR, Ghosh D, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes DR, Sanda MG, Otte AP, Chinnaiyan AM, Rubin MA. Multiplex biomarker approach for determining risk of prostate-specific antigen-defined recurrence of prostate cancer. J Natl Cancer Inst. 2003;95:661–668. doi: 10.1093/jnci/95.9.661. [DOI] [PubMed] [Google Scholar]
- 16.Rubin MA, Zhou M, Dhanasekaran SM, et al. alpha-Methylacyl coenzyme A racemase as a tissue biomarker for prostate cancer. JAMA. 2002;287:1662–1670. doi: 10.1001/jama.287.13.1662. [DOI] [PubMed] [Google Scholar]
- 17.Foster CS, Falconer A, Dodson AR, et al. Transcription factor E2F3 overexpressed in prostate cancer independently predicts clinical outcome. Oncogene. 2004;23:5871–5879. doi: 10.1038/sj.onc.1207800. [DOI] [PubMed] [Google Scholar]
- 18.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–1451. [PubMed] [Google Scholar]
- 19.Zhao Y, Yamashita T, Ishikawa M. Regulation of tumor invasion by HOXB13 gene overexpressed in human endometrial cancer. Oncol Rep. 2005;13:721–726. [PubMed] [Google Scholar]
- 20.Edwards S, Campbell C, Flohr P, et al. Expression analysis onto microarrays of randomly selected cDNA clones highlights HOXB13 as a marker of human prostate cancer. Br J Cancer. 2005;92:376–381. doi: 10.1038/sj.bjc.6602261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid JF, Lusa L, De Cecco L, et al. Limits of predictive models using microarray data for breast cancer clinical treatment outcome. J Natl Cancer Inst. 2005;97:927–930. doi: 10.1093/jnci/dji153. [DOI] [PubMed] [Google Scholar]
- 22.Okuda H, Toyota M, Ishida W, et al. Epigenetic inactivation of the candidate tumor suppressor gene HOXB13 in human renal cell carcinoma. Oncogene. 2006;25:1733–1742. doi: 10.1038/sj.onc.1209200. [DOI] [PubMed] [Google Scholar]
- 23.Economides KD, Zeltser L, Capecchi MR. Hoxb13 mutations cause overgrowth of caudal spinal cordand tail vertebrae. Dev Biol. 2003;256:317–330. doi: 10.1016/s0012-1606(02)00137-9. [DOI] [PubMed] [Google Scholar]


